Recently, an attractive study entitled “Lactate-dehydrogenase associated with mortality in hospitalized patients with COVID-19 in Mexico: a multi-centre retrospective cohort study” was published in Annals of Hepatology [1]. In this study, the authors investigated the risk factors associated with mortality in hospitalized patients with coronavirus disease 2019 (COVID-19). Undoubtedly, this is a study with essential clinical guiding value. However, the following concerns need to be noted.
First of all, it is apparent that the purpose of this study is to explore the risk factors related to mortality in hospitalized patients with COVID-19 and several potential variables were screened in this study. However, some variables that are easily obtained in clinical practice and have been clarified to be related to mortality in COVID-19 patients should not be neglected. For instance, previous studies have exhibited that obesity is closely related to the prognosis and disease severity in COVID-19 patients [2,3], and it is recommended that clinicians should pay enough attention to obese patients in order to improve the short- and long-term prognosis. On the other hand, considering that weight and height are currently used to define obesity [4], and these two variables can be easily extracted from medical records. Therefore, the influence of obesity on the prognosis of COVID-19 patients should not be unnoticed. It is recommended that obesity should be adjusted as a potential confounding variable in the multivariate analysis to ensure the reliability of the final conclusions.
Second, it is common knowledge that the severity of the disease is associated with the prognosis. The higher the severity of the disease, the worse the prognosis. Similarly, this regular pattern also applies to COVID patients. Nowadays, the severity of COVID could be divided into the following four types: mild, moderate, severe, and critical [5]. Additionally, prior study has demonstrated that the severity of disease in COVID patients is associated with a higher risk of mortality [6]. Unfortunately, this study only described the recruitment of COVID patients, yet does not clarify the severity of the patients’ disease, which could easily lead to misinterpretations. One possible hypothesis is that deaths mainly occur in severe/critical patients, and are caused by the severity of the disease, not by lactate dehydrogenase level.
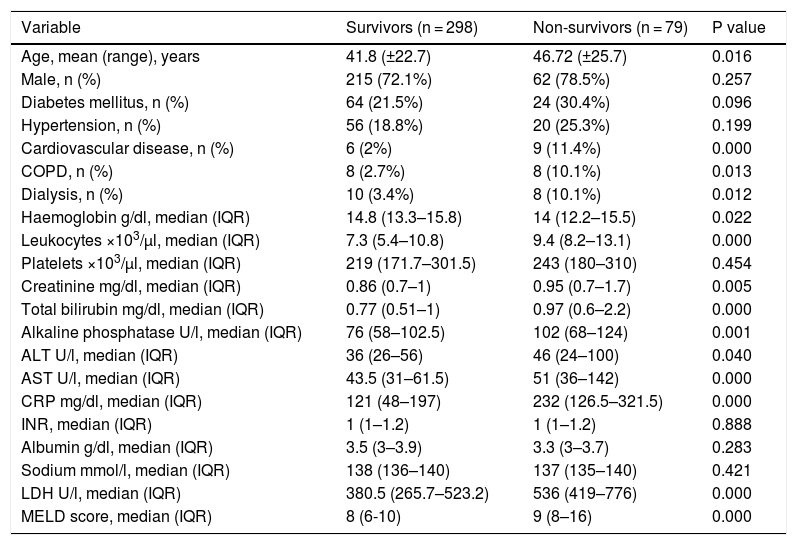
Third, it is undeniable that treatment strategies are related to the prognosis of COVID patients, and appropriate treatment strategies can remarkably improve the prognosis of patients [7]. Nevertheless, this study did not describe in detail the treatment drugs and strategies between the two groups. How many patients need to be treated in the intensive care unit (ICU)? How many patients have received mechanical ventilation treatment? Obviously, Table 1 describes that 3.4% of patients in the survivor group have a history of dialysis, while 10.1% of the patients in the non-survivor group have a history of dialysis (P = 0.012). Nonetheless, it is indistinct that how many patients receive continuous renal replacement therapy (CRRT) in ICU for patients with a history of dialysis?
Demographic characteristics and laboratory findings on admission.
| Variable | Survivors (n = 298) | Non-survivors (n = 79) | P value |
|---|---|---|---|
| Age, mean (range), years | 41.8 (±22.7) | 46.72 (±25.7) | 0.016 |
| Male, n (%) | 215 (72.1%) | 62 (78.5%) | 0.257 |
| Diabetes mellitus, n (%) | 64 (21.5%) | 24 (30.4%) | 0.096 |
| Hypertension, n (%) | 56 (18.8%) | 20 (25.3%) | 0.199 |
| Cardiovascular disease, n (%) | 6 (2%) | 9 (11.4%) | 0.000 |
| COPD, n (%) | 8 (2.7%) | 8 (10.1%) | 0.013 |
| Dialysis, n (%) | 10 (3.4%) | 8 (10.1%) | 0.012 |
| Haemoglobin g/dl, median (IQR) | 14.8 (13.3–15.8) | 14 (12.2–15.5) | 0.022 |
| Leukocytes ×103/µl, median (IQR) | 7.3 (5.4–10.8) | 9.4 (8.2–13.1) | 0.000 |
| Platelets ×103/µl, median (IQR) | 219 (171.7–301.5) | 243 (180–310) | 0.454 |
| Creatinine mg/dl, median (IQR) | 0.86 (0.7–1) | 0.95 (0.7–1.7) | 0.005 |
| Total bilirubin mg/dl, median (IQR) | 0.77 (0.51–1) | 0.97 (0.6–2.2) | 0.000 |
| Alkaline phosphatase U/l, median (IQR) | 76 (58–102.5) | 102 (68–124) | 0.001 |
| ALT U/l, median (IQR) | 36 (26–56) | 46 (24–100) | 0.040 |
| AST U/l, median (IQR) | 43.5 (31–61.5) | 51 (36–142) | 0.000 |
| CRP mg/dl, median (IQR) | 121 (48–197) | 232 (126.5–321.5) | 0.000 |
| INR, median (IQR) | 1 (1–1.2) | 1 (1–1.2) | 0.888 |
| Albumin g/dl, median (IQR) | 3.5 (3–3.9) | 3.3 (3–3.7) | 0.283 |
| Sodium mmol/l, median (IQR) | 138 (136–140) | 137 (135–140) | 0.421 |
| LDH U/l, median (IQR) | 380.5 (265.7–523.2) | 536 (419–776) | 0.000 |
| MELD score, median (IQR) | 8 (6-10) | 9 (8–16) | 0.000 |
COPD (Chronic Obstructive Pulmonary Disease), ALT (alanine aminotransferase), AST (aspartate aminotransferase), CRP (C reactive protein), INR (international normalized ratio), LDH (lactate dehydrogenase), MELD (Model of End-Stage Liver Disease).
None.
Conflict of interestThe authors have no conflicts of interest to declare.