Background & aims. Hyperferritinemia (HF) is frequently present in patients with metabolic syndrome (MS). MS associated with HF is named dysmetabolic hyperferritinemia (DH). There are some publications that propose that DH is associated with a raised liver iron concentration (LIC). We studied the LIC in patients referred for HF to a secondary hospital to determine if there are differences between patients with or without MS.
Material and methods. We conducted a prospective study of 132 consecutive patients with HF from January to December 2010. The MS was defined by the International Diabetes Federation criteria (2005). LIC was determined by Magnetic resonance imaging (MRI).
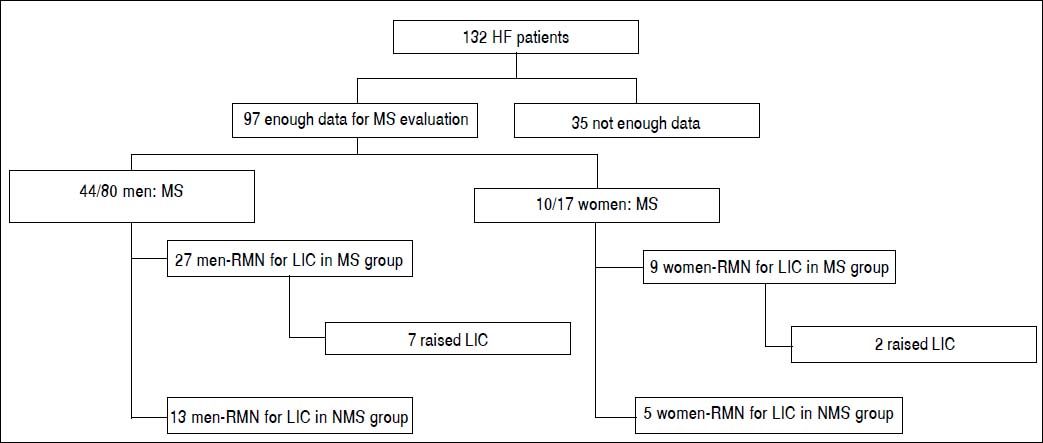
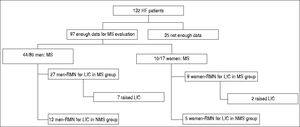
Results. The number of patients for which there was enough data to determine MS was 97, out of which 54 had MS and 43 had no MS (NMS). In 54/97 patients, MRI for LIC determination was performed. From the MS group, 44 were men (27 underwent MRI) and 10 women (9 MRI). The mean LIC was 27.83 ± 20.90 μmol/g for the MS group. In the NMS group, 36 were men (13 MRI), and 7 women (5 MRI). In 18 patients from the NMS group, LIC was determined by MRI. The mean LIC was 33.16 ± 19.61 μmol/g in the NMS group. We compared the mean values of LIC from both groups (MS vs. NMS) and no significant differences were found (p = 0.067).
Conclusion. Patients with DH present a mean LIC within normal values and their values do not differ from those of patients with HF but without MS.
The prevalence of metabolic syndrome (MS) is around 25% in the adult population of Western countries.1,2 Patients with otherwise unexplained liver iron overload have MS with a high frequency.3 Hyperferritinemia (HF) is frequently present in patients with MS. This clinical entity has been named dysmetabolic hyperferritinemia (DH) or dysmetabolic hepatic iron overload.3 DH may or may not be associated with raised liver iron concentration. There are some publications that support that HF is associated with a raised liver iron concentration (LIC) in these patients, but some doubts persist.3,4 Because MS is an inflammatory condition, hepcidin levels may be raised in DH, in which case one would not expect liver iron overload. The studies about this syndrome have selected patients with liver iron overload.3
However, the prevalence of liver iron overload in unselected patients with HF and metabolic syndrome has rarely been studied.3,4
Recently, Chen, et al.3 found that DH was associated with normal transferrin saturation, mild hepatic iron overload, and elevated hepcidin, which generally corresponds with a normal LIC. This group used Gandon’s magnetic resonance imaging (MRI) method5 for LIC calculation, a method that has a tendency to overestimate LIC. Brudevold, et al.,4 on the other hand, found only one case with iron overload on liver biopsy out of 18 patients with DH (from a study of 40 patients with HF).
Our aim was to study the LIC with an accurate MRI determination method6,7 in patients referred for HF to a secondary hospital in the Basque Country, Spain, and to determine if there are differences between patients with DH or without MS.
Material and MethodsPatientsThis was a prospective study including patients from January to December 2010. The study was conducted in Mendaro Hospital, Deba Valley, a secondary hospital in the Basque Health Service (Osakidetza), with a total catchment of 70,000 people. Other objectives of the study have recently been published.6
The study protocol conforms to the 1975 ethical guidelines of the Declaration of Helsinki, with the understanding and the consent of the patients. The study was approved by the ethical committee of the Mendaro Hospital.
Inclusion criteria: consecutive outpatients referred for HF (serum ferritin (SF) > 200 μg/L in women, > 300 μg/L in men), in accordance with the WHO criteria.8
Exclusion criteria: systemic inflammation, infections, and renal or neoplastic diseases (excluded by clinical, laboratory and radiologic methods); patients younger than 18 years.
Patients were studied and treated according to best practice.
Methods• Radiology. MRI images were recorded and read by the same investigator (JMA). The investigator was blinded. MRI images were obtained with a 1.5-Tesla system (Philips Intera, Osatek, Donostia). The MRI technique used (SIR method) was that proposed by Alustiza, et at.7 We systematically performed T1-weighted in-phase and opposed-phase imaging to discard liver steatosis. All the iron quantification sequences were in-phase sequences to make sure that the fat did not interfere in signal intensity measurements.9 The LIC calculated by this model has a high correlation with biochemical measurements obtained by atomic spectrometry in liver biopsy samples (r = 0.931).7 The LIC was considered as: normal (< 36 μmol/g), iron overload (IO) (31-80 μmol/g), and high iron overload (HIO) (> 80 /μmol/g).7
• Laboratory measurements. SF, serum iron (Fe) and transferrin saturation index (TSI) were obtained from blood samples taken during fasting from all the patients included. All were found to have raised SF values of > 300 μg/L – the laboratory normal range for SF is 15-200 μg/L in women and 30-300 μg/L in men.6,8 The ranges considered to be normal for Fe and TSI were 50-145 μg/dL and 15-45% respectively.6
The serum values of glucose, cholesterol, HDL-cholesterol and triglycerides were obtained from the same blood samples.
• Definition of metabolic syndrome (MS). We employed the established International Diabetes Foundation criteria for MS,10 namely:
- °
Waist circumference ≥ 94 cm in men; ≥ 80 cm in women, and two of the following factors:
- °
Triglycerides ≥ 150 mg/dL or treatment for this dyslipidaemia.
- °
HDL < 40 mg/dL men, < 50 mg/dL women or treatment for this dyslipidaemia.
- °
Glucose ≥ 100 mg/dL or type 2 diabetes.
- °
Hypertension: systolic pressure ≥ 130 mmHg; diastolic pressure ≥ 85 mmHg, or treatment for arterial hypertension.
• Statistics. SPSS 15.0 software (SSPS Inc., Chicago, IL, USA) was used to perform the appropriate statistical analyses. Mean values with range and standard deviation were calculated for continuous variables and frequencies and percentages for categorical variables.
To compare LIC mean values in patients with MS and without MS, we used Pearson’s χ2 test. In all the analyses, a p < 0.05 value was considered statistically significant.
ResultsPatientsNot all the results were available for all patients (Figure 1). Out of 132 patients, we have enough data to determine the MS presence in 97 patients. Of these, 44/80 men (55%) and 10/17 women (59%) presented MS. In the 54 cases with LIC as determined by MRI, 36 (21 men, 9 women) were in the MS group and 18 were in the non-MS (NMS) group (13 men and 5 women). The mean age was 55.18 ± 11.94 years (range 32-15).
RadiologyIn 54/91 patients, MRI for LIC determination was performed. From the MS group of 54 patients, 44 were men (21 had MRI) and 10 were women (9 MRI). Therefore, we had LIC results from 36 patients (21 men, 9 women). In this group, 9/36 patients had raised LIC and 21 had normal LIC (Table 1). The mean LIC was 21.63 ± 20.18 μmol/ g (range: 5-80) in men, 28.44 ± 18.31 /μmol/g (range: 5-10) in women, and 21.83 ± 20.90 /μmol/g (range: 5-80) for the entire MS group. In 43/91 patients, MS was not diagnosed (NMS): 36 were men (13 MRI), and 1 were women (5 MRI). In 18 patients (13 men, 5 women) from the NSM group, LIC was determined by MRI. Eight of 19 patients had raised LIC and 11 had normal LIC. The mean LIC was 30.84 ± 23.79 μmol/g (range: 5-55) in men, 39.2 ± 23.79 μmol/g (range: 17-79) in women, and 33.16 ± 19.61 μmol/g (range: 5-79) for the entire NMS group. We compared the mean values of LIC from both groups (MS vs. NMS) by Pearson’s χ2 test. No significant differences were found (p = 0.067).
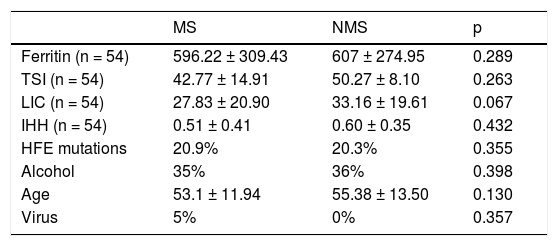
HFE mutations (hemochromatosis predisposing mutations),6 alcohol consumption (> 40 g/day), age (years) and virus serology (HBV, HCV) in both groups (MS: metabolic syndrome; NMS: no metabolic syndrome) from the 97 patients studied with enough data for MS study.
| MS | NMS | p | |
|---|---|---|---|
| Ferritin (n = 54) | 596.22 ± 309.43 | 607 ± 274.95 | 0.289 |
| TSI (n = 54) | 42.77 ± 14.91 | 50.27 ± 8.10 | 0.263 |
| LIC (n = 54) | 27.83 ± 20.90 | 33.16 ± 19.61 | 0.067 |
| IHH (n = 54) | 0.51 ± 0.41 | 0.60 ± 0.35 | 0.432 |
| HFE mutations | 20.9% | 20.3% | 0.355 |
| Alcohol | 35% | 36% | 0.398 |
| Age | 53.1 ± 11.94 | 55.38 ± 13.50 | 0.130 |
| Virus | 5% | 0% | 0.357 |
TSI: transferrin saturation index. LIC: liver iron concentration. IHH: iron hepatic index, (LIC/age) from the studied group of 54 patients with MRI for LIC.
No patient had HIO (> 80 μmol/g) in the liver. Hepatic iron index (LIC/age) values were lower than 1.5 (normal < 1.5) in all the patients.3,7
Clinical and laboratory dataMS10 was detected in 44/80 men (55%) and 10/17 women (59%), which corresponded overall to 54/97 (55.67%) of the HF patients. There were no significant differences between the two groups with respect to HFE mutations, alcohol, age or virus serology (Table 1). No liver biopsies were taken during the study period. An alcohol consumption cut-off of > 40 g/day was applied.
In all the patients with determined LIC, SF and TSI (Table 1) values were compared: SF mean values were 610.04 ± 298.51 μg/L (range: 312-1239) in men, 554.77 ± 355.95 μg/L (range: 206-1388) in women, and 596.22 ± 309.43 μg/L (range: 206-1388) for the entire MS group (n = 54). In the NMS group, the mean SF was 662.54 ± 284 μg/L (range: 314-1054) in men, 462.6 ± 207.91 μg/L (range: 240-675) in women, and 607 ± 274.95 μg/L (range: 240-1,054) for the entire group. TSI (%) mean was 40.26 ± 12.09 (range: 12.1-66.8) in men, 49.11 ± 20.77 (range: 25.1-83.6) in women, and 42.77 ± 14.91 (range: 12.1-83.6) in the entire MS group. TSI in the NMS group was 48.92 ± 8.88 (range: 34.5-68.9) in men, 53.8 ± 4.60 (range: 48-60.3) in women, and 50.27 ± 8.10 (range: 34.5-68.9) for the entire group. There were no statistically significant differences between the two groups (MS vs. NMS) in the mean SF and TSI values (p < 0.05).
DiscussionThe presence of hepatic iron overload has been described in various metabolic conditions, such as insulin resistance, non-alcoholic steatohepatitis, liver steatosis (non-alcoholic fatty liver disease), and MS alterations.11–15 Iron has been proposed as a promising target for the treatment of these diseases.14 In a randomized trial, Valenti, et al.16 performed iron depletion by phlebotomy in patients with non-alcoholic fatty liver disease and hyperferritinemia. They conclude that iron depletion is associated with a higher rate of histologic liver damage improvement than lifestyle changes alone and with liver enzyme amelioration. On the other hand, Adams, et al.17 have demonstrated in a prospective, randomized and controlled trial that reduction in ferritin by phlebotomy does not improve liver enzymes, hepatic fat, or insuline resistance in patients with non-alcoholic fatty liver disease.
In 2006, Brissot and de Bels18 defined dysmetabolic hepatosiderosis as a frequent syndrome characterised by multiple metabolic abnormalities (increased body mass index, high blood pressure, hyperlipidaemia, non-insulindependent diabetes, and hyperuricaemia), HF, normal transferrin saturation, mild hepatic iron excess, and mixed iron deposition in liver biopsy.
More recently, the term DH has been used for patients with MS and associated HF.3 DH does not always present raised LIC.3,4 Only a proportion of MS patients (14.5%) seem at risk of iron overload, presenting HF,19 but the real prevalence of liver iron overload in these patients remains unknown. The studies that have been performed to define DH have always recruited selected patients on the basis of known liver iron overload.3 Recently DH has been pointed out as a possible target for phlebotomy treatment.20
There have been two previous studies that have evaluated the presence of iron overload in the liver with non-selected patients. In both of them, MS was diagnosed fulfilling the International Diabetes Foundation criteria.10 In the study by Chen, et at.,3 1/10 evaluated patients had “mild” hepatic iron overload, but with values up to 120 μmol/g. Three had normal LIC values. They used MRI (SIR method) for LIC determination with the method from the Rennes University.21 This method has a tendency to overestimate LIC;5,21,22 perhaps this study was not a very accurate work and probably the real LIC values were lower than that published.5,22
In a study from Norway, Brudevold, et at.4 studied 18 patients that fulfilled the IDF criteria for MS. They concluded that liver steatosis and insulin resistance, but not increased iron load, is found in patients with HF. All liver biopsies in patients with MS criteria showed steatosis, with only one showing an iron content of grade 2 (Rowe method). The rest were normal (grade 0-1) for iron content. Hepatic iron overload was defined as ≥ 10 μmol/g dry weight (grade 2+ to 4+ by the Rowe method). From 40 HF patients, liver biopsy was performed in 29, with 15 he-mosiderin grade 0, ten grade 2, and three grade 2.
In our study, we evaluated 91 patients with HF using clinical and laboratory data to determine if they suffered from MS. In 54 patients with HF, they presented MS criteria,10 DH, and MRI for LIC was performed in 36 (21 men, 9 women) patients. We compared the LIC of DH group with the patients without MS (NMS) and HF (43 patients, 18 MRI, 13/18 men). There were no differences between the two groups, and the LIC mean values were normal.
The greatest limitation of our study is the relatively small patient population and male preponderance. The strengths of the study are that it presents an unselected patient population, i.e. patients with hyperferritinaemia referred to a secondary hospital without known raised liver iron concentration, and that it represents the highest number of patients to date with DH (36 cases). In previous unselected studies, 10 and 18 patients were included.3,4
Recently, Freixenet, et at.23 confirmed that men with HF and diabetes in the Mediterranean area do not have a higher iron overload than those without diabetes, which was measured through serum ferritin, the transferrin saturation index, and serum transferrin receptor. In our study, we did not find statistical differences between the two groups MS and NMS for serum ferritin and TSI values.
ConclusionIn conclusion, we can say from this study that patients with DH have normal LIC and LIC values in patients with DH have no significant differences with patients with HF and not MS in our study. This must be studied in a larger series to confirm the results.
Abbreviations- •
DH: dysmetabolic hyperferritinemia.
- •
Fe: serum iron.
- •
HF: hyperferritinemia.
- •
HIO: high iron overload.
- •
IO: iron overload.
- •
LIC: liver iron concentration.
- •
MRI: magnetic resonance imaging.
- •
MS: metabolic syndrome.
- •
NMS: no metabolic syndrome.
- •
SF: serum ferritin.
- •
TSI: transferrin saturation index.
The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
Author’s ContributionsAll authors met the criteria for authorship as stablished by the International Committee of Medical Journal Editors. The manuscript represents honest work and all authors are able to verify the validity of the results reported.
CongressPresented as abstract at the Liver International meeting (EASL) 2015; Vienna, Austria, 22-26 April / 2015.