In a recently published article by Eslam M et al.1 they amaze us and at the same time make us question how effective is the nomenclature currently being used for non-alcoholic fatty liver disease (NAFLD) approach by physicians worldwide. The authors achieve to sow this doubt through a well-founded explanation that describes the great variability that exists among patients with NAFLD based on several factors such as age, gender, ethnicity, genetic predisposition, metabolic-associated diseases, gut microbiota composition, among others, which in turn generates different phenotypes of this condition leading to a degree of complexity so high that it ends up hindering the management of this selected group of patients.
In essence, they propose a strategy through a new diagnostic criterion that is based in inclusion rather than exclusion criteria for the diagnosis of NAFLD and the implementation of metabolic-associated fatty liver disease (MAFLD) as a more specific term that describes in a more realistic way the basis of this condition compared to the original term coined in 19802. But, if this proposal is implemented, will it really achieve an improvement in the approach of this clinical entity?
First, we agree that the current NAFLD nomenclature has important implications in the prevalence, diagnosis, staging and therefore in the management of these patients. The fact that it is a diagnosis of exclusion obligates medical personnel to know and rule out all the etiological causes that can develop fatty liver, implying a greater challenge for physicians and a greater expenditure to the health sector3. In 2018, a cost analysis study showed that the cost of care per newly diagnosed NAFLD patient with private insurance was $7,804 annually and $3,789 for long-term management, compared to the $2,298 annually spend by patients with similar metabolic conditions without NAFLD4. In addition, the diagnostic difficulty that NAFLD currently represents, and the lack of effective pharmacological treatments, imply an exponential increase in the progression of patients to more advanced stages of the disease. In this sense, patients with advanced stages of NAFLD have a higher annual hospitalization rate (2.51-4.95) compared to non-progressors (1.75) (p<0.05) and represent a higher cost to government health systems with an approximate expense of € 10, 291- € 35, 910 annually for each patient vs. the € 3, 818 spent by non-progressors (p<0.05)5. If we also add to consideration the discouraging predictions that have been analyzed for the near future6, we can realize the very important need to implement new strategies to avoid both clinical and economic consequences expected for the following years.
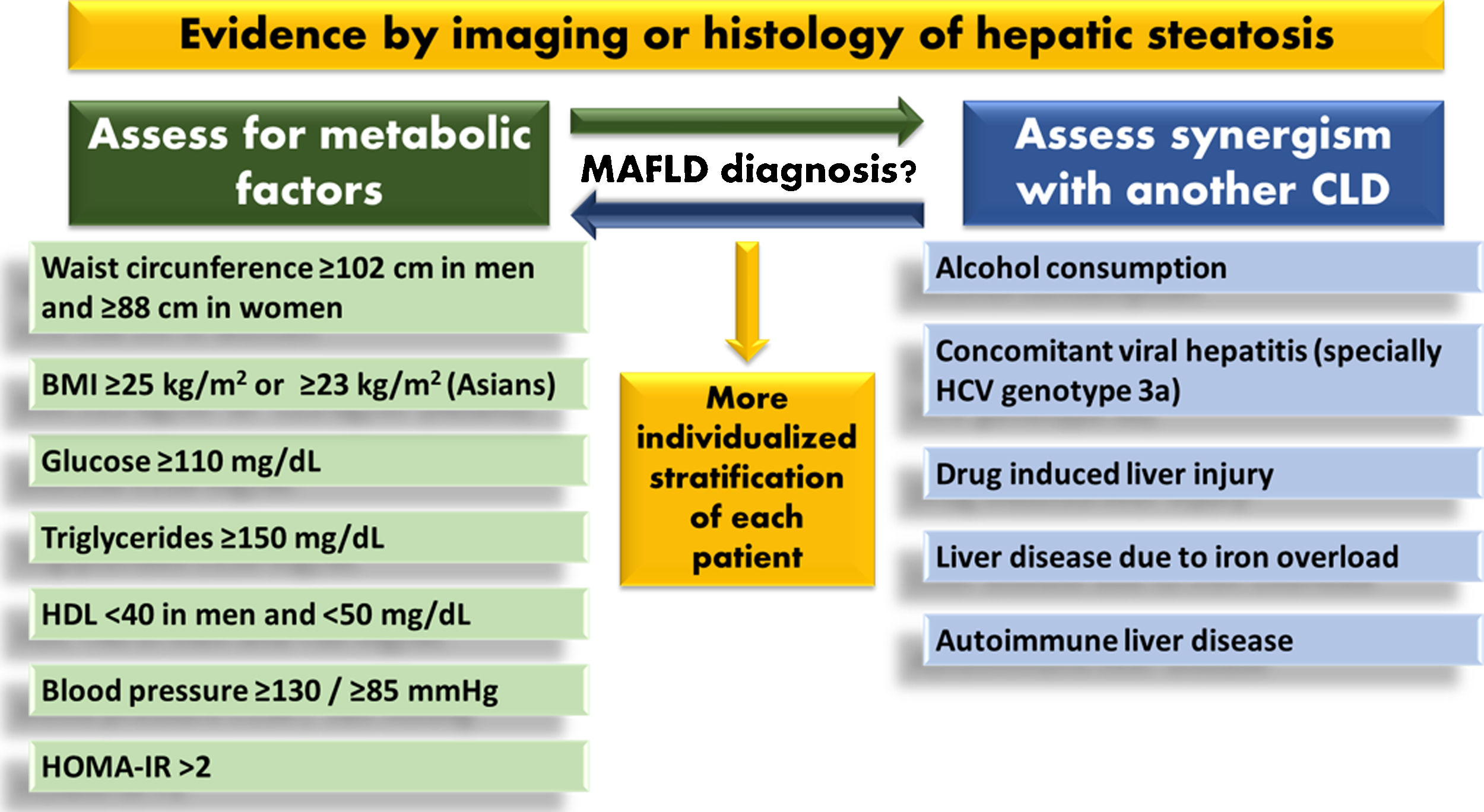
Furthermore, by performing an intentional assessment of the multiple hits associated with the development of NAFLD within the diagnostic approach, we could be able to delimit in a more precise manner the clinical spectrum of NAFLD and reduce the costs associated with this disease. In general, as in other chronic liver diseases, we can say that the most important risk factors are genetic and environmental. However, we must bear in mind that these factors are dynamic and therefore, the same variable analyzed may have different spectra to consider. A clear example of this dynamism within the genetic factors can be seen in our population. Hispanic ethnicity is considered a risk factor for the development of NAFLD, nevertheless, within the same Hispanics there are different groups in a subcontinent and even in a same country that confer varying degrees of susceptibility to a specific disease7 highlighting the importance of assessing each patient in a more personalized way. With respect to environmental factors, something similar can be found, especially with diet composition. The climatic conditions and socio-economical customs that surround a population predispose to the type of diet they consume, and the risk that this will represent for the development of a disease. For NAFLD, it is well known that a diet mainly consisting of saturated fatty acid and simple sugars will predispose to the development of this condition, while a diet rich in fiber and natural antioxidants will serve as a protective factor8. Since, Western countries are more attached to a high-caloric “fast food” diet, we can understand the highest prevalence of NAFLD seen in those countries 9. As a result, an interrelation between each of these genetic and environmental dynamic factors will achieve an endless number of phenotypes of the disease with different clinical, biochemical and histological characteristics that explains in an important manner the heterogeneity shown in the outcomes of the studies focused on this issue10 and the need to take into consideration these factors for the stratification of this condition.
In a similar way, the current NAFLD nomenclature demands the exclusion of other causes of fatty liver, but can they not coexist? If we look at autoimmune liver diseases (AILD), we can see how sometimes two entities can coexist calling them overlap syndrome. This condition presents a completely different evolution from the rest of the AILD demanding a distinct approach11. In the case of NAFLD, current evidence has demonstrated the synergism that can be achieved with other chronic liver diseases modifying the natural history of the disease and therefore its prognosis. Alcohol appears to be an independent factor of progression in both viral hepatitis12 and NAFLD13 regardless of the level of consumption. An increase of 20g / day in alcohol consumption at baseline is associated with a 17% increase in the incidence of fatty liver in the general population13. If we consider that the vast majority of patients have an underestimate of 30-40% in their alcohol consumption14, how many patients diagnosed with NAFLD will not have an alcoholic component that makes synergism and alters the evolution of this disease? On the other hand, viral hepatitis has also been shown to have a synergism with metabolic factors in the development of fatty liver (mainly hepatitis C virus genotype 3a) altering in a same way the chronicity of the disease15.
By having new diagnostic criteria, which allows the detection and assessment of the multiple hits commonly found in NAFLD, and facilitates the evaluation of the synergistic effect of more than one CLD in a single patient, could result in an important landmark in the approach of this condition, and also, allow a more specific selection of patients for clinical trials decreasing the probability of bias and therefore obtaining more reliable outcomes.
Finally, we now have to question what could it be the new criteria for the diagnosis of MAFLD. The authors propose that the diagnosis of this disease should be based on the presence of metabolic dysfunction, so we could assume similar criteria to those found in metabolic syndrome (Fig. 1), but in this moment is unclear how to define precisely all the potential phenotypes in MAFLD. In addition, prospective clinical trials that assess the diagnostic accuracy and cost / effectiveness of the criteria proposed would be required. It would be interesting if the authors of this novel paper can amaze us with some retrospective analysis of the large databases of patients with NAFLD that they have, to observe if an inclusive diagnosis with well-defined variables allows the reliable detection of previously studied patients.
This new term is highly attractive for a better perception for the patients and third payers. As the author's states, this is an umbrella for a heterogeneous disease. Consequently, this big umbrella brings more complexity, and many questions that need to be solved in the near future. Particularly looking for an intrinsic divorce between real-life and clinical trials scenarios.
In conclusion, the strategy proposed by Eslam M et al.1 could be the onset of a new direction towards the approach of NAFLD. A subtle but well-founded change in the nomenclature that allows a diagnosis of inclusion could be very useful to achieve a better comprehension by physicians of this condition and help to diminish the great heterogeneity that currently exists in clinical trials focused on NAFLD. At the moment, a great step has been taken towards a new era of comprehension of one of the most important chronic liver diseases in the actuality.
Conflict of interestThe authors have no conflict of interest to declare.
Funding sourceThis work was supported partially by Medica Sur Clinical Foundation.
Authors contributionN.M-S. the concept and design of the study; A.V-R., A. V-B, N. C-T, and M. U. data acquisition; A.V-R. and N. C-T drafted the manuscript. All authors critically revised the manuscript and approved the final version to be published.
None.