Gallbladder disease is a common disease with high prevalence. Majority of gallbladder disease is due to gallstone. Though genetics are believed to play a role in its pathogenesis, the contribution of environmental pressures in early life to the development of this disease in adulthood has not been ever investigated. This study aimed to clarify the risk of maternal smoking exposure in association with gallbladder disease in adulthood. The interaction of maternal smoking and own smoking during adulthood on this association was studied as well.
Patients and methodsA total of 286,731 eligible participants from the UK Biobank population-based cohort were included. Multivariable Cox regression analysis were used to examine the HR and 95% CI with adjustment for covariates.
ResultDuring a median of 8.8 years follow-up, 7110 incident cases of gallbladder disease including 6800 (95.6%) gallstone were identified. Maternal smoking was associated with increased risk of incident total gallbladder disease (HR = 1.13; 95%CI: 1.06 - 1.21; P = 0.0002) as well as gallstones (HR = 1.13; 95%CI: 1.06 -1.21; P = 0.0003) in adulthood. Compared with those who were neither exposed to maternal smoking nor own smoking, subjects adherence to no smoking during adulthood but having maternal smoking exposure still had increased risk of total gallbladder disease (HR = 1.21; 95%CI: 1.1-1.34, P=0.0001) and gallstones (HR = 1.21; 95%CI: 1.1-1.35, P=0.0001).
ConclusionThe present study using large prospective cohort data from UK Biobank, for the first time, demonstrated maternal smoking exposure bringing elevated risk of incident total gallbladder disease/gallstone in adulthood.
Gallbladder disease, mainly due to gallstones, is reaching highest prevalence in the age of 60 for man and 50 for woman [1]. It is common in the population with prevalence reaching 10-30% in US and European countries ([2,3]). Over 25 million Americans required hospitalization each year because of gallstone disease, and more than 700,000 cholecystectomies were performed annually that cost approximately 6 billion dollars in US ([4,5]). Other western countries are suffered from this costly disease as well ([6,7]).
It is known that genetic susceptible play a role in gallstone formation. The Swedish Twin study demonstrated that genetic factors contributed 25% to the risks of gallstone disease [8]. Accumulated epidemiological data indicated other risk factors such as age, dyslipidemia, obesity, over-nutrition diet, etc. contributing to gallstone disease ([9,10]). The pathophysiological mechanisms leading to gallstone formation were linked with imbalance of hepatic cholesterol and bile acids metabolism resulting in supersaturation of cholesterol in gallbladder bile ([11,12]).
Developmental Origins of Health and Disease (DOHaD) is a recent attractive concept in establishing certain environmental pressures in early life leading to maladaptation of developing organism resulting in homeostatic systems changes, which increases the risk of chronic diseases in later of life [13]. Exposure of hazards in early life such as maternal smoking during pregnancy has proved to be associated with various diseases [14]. A recent meta-analysis demonstrated that maternal smoking in pregnancy increased the risk of overweight or obesity for offspring in childhood [15]. Another meta-analysis found that children who exposed to maternal smoking had 50% higher risk of later overweight [16]. Furthermore, higher systolic and diastolic blood pressure were found in children whose mothers smoked during pregnancy ([17,18]). Nevertheless, heavy smoking of mothers during pregnancy raised risks of gestational diabetes mellitus in daughters [19]. All these results pointed out a possible link between maternal smoking in pregnancy and risk of metabolic disorders and related diseases in offspring. We therefore, hypothesize that exposure of maternal smoking during pregnancy may affect the development of gallbladder/gallstone disease in later of life. However, to our knowledge, none data was available on this so far.
UK Biobank is a large population-based cohort study conducted across the United Kingdom between 2007 and 2010. All the participants have been follow-up prospectively for the incidence of certain diseases [20]. Using data in UK Biobank cohort, we were able to investigate the association between maternal smoking in pregnancy and the risk of incident gallbladder disease in adulthood. Meanwhile, the combined effect of maternal and own smoking on this association was examined.
2Material and methods2.1UK Biobank ParticipantsAfter sending out more than 9.2 million invitation letters to potential participants, UK Biobank recruited around 500, 000 participants aged 40 to 70 years via 22 assessment centers across the United Kingdom. Extensive information of participants was collected through baseline questionnaires, interviews, and physical measurements [20]. All participants provided written informed consent before enrolment in the cohort [21]. UK Biobank received ethical approval from the North West Multi-center Research Ethics Committee [22]. The details of questionnaire data collection and current validation efforts could be accessed in elsewhere [23,24].
2.2Ascertainment of incident gallbladder disease and gallstoneThe diagnosis information of gallbladder disease was accessed from the hospital inpatient records in either the main or secondary position. The diagnosis was made by the treating physicians based on strict clinical criteria. We identified gallbladder disease cases through their code of the International Classification of Diseases version-10 (ICD-10) as K80-82, or code of the Office of Population Censuses and Surveys Classification of Interventions and Procedures, version 4 (OPCS-4) as J181-269. Participants with ICD-10 code as K80 or OPCS-4 code as J181-189 were further coded as gallstone. The patients of gallbladder include all diagnoses of disorders in gallbladder as well as cholecystectomy. Any cases with gallbladder cancer was not included in the study.
2.3Assessment of exposure of maternal smoking and covariatesThe information of maternal smoking was collected through self-reported touch-screen questionnaire at baseline. The question asked: ‘’Did your mother smoke regularly around the time when you were born? ‘’ with response options “Yes,” “No,” “Do not know,” and “Prefer not to answer.” Participants who answered “Do not know” or “Prefer not to answer” were set to missing. In UK biobank, pack-years of own smoking was calculated by formula as following: pack-years of own smoking = number of cigarettes per day / 20 * (age stopped smoking - age start smoking). Then, we included additional information in subsequent analysis as potential covariates: age (years), sex (male/female), ethnicity (white and other), birth place (England/ Wales/ Scotland/ Northern Ireland/ Republic of Ireland/ Elsewhere), birth weight (kg), breastfeeding (yes/no), part of a multiple birth (yes/no), Townsend Deprivation Index (continuous), college or university degree (yes/no), body mass index, (BMI, in kg/m2), physical activity (metabolic equivalents [MET], and total MET-h/week), smoke status (current/past/never), alcohol intake frequency (daily or almost daily/three or four times a week/once or twice a week/one to three times a month/special occasions only/never), vegetables and fruits intake (serving/day), red meat consumption more than three times a week (yes/no), vitamin and miner supplements (yes/no). We further adjusted for the variables as use of menopause and hormone-replacement therapy (yes/no) for female participants.
2.4Statistical analysisParticipants contributed person-years form baseline until the date of diagnosis of gallbladder disease, death, loss to follow-up or the end of the study period. Multivariable Cox regression analysis were used to examine the HR and 95% CI with age as the underlying time scale, and further stratified by sex. Multivariable analysis adjusted baseline age, sex, race, and other early life events including birth place, birth weight, breastfeeding, and part of a multiple birth in model 1, and further adjusted Townsend deprivation index, college or university degree, BMI, physical activity (MET-min/week), smoke status, alcohol intake frequency, vegetables and fruits intake, red meat intake, vitamin and miner supplements, female-special factors (menopause and hormone-replacement therapy (HRT)) in model 2. As reported in previous articles, those models included possible intermediate variables that influences the outcomes ([1,25]).
We further classified participants as exposed to maternal smoking or not and further stratified according to categories of pack-years of own smoking (more than 20/up to 20/only exposure to second-hand smoking/no exposure). The HRs and 95%CIs were calculated between the mutually exclusive exposure groups of maternal smoking and pack-years of own smoking with incident gallbladder disease using the same adjustments as model 2. Multiplicative interactions between maternal smoking and pack-years of own smoking for incident gallbladder disease events by comparing the −2 log likelihood of the multivariate adjusted models with and without the cross-product interaction term were further performed.
In subgroup analysis, we conducted analysis stratified according to baseline characteristics of participants: birth year (40s and before/50s/60s), breastfeeding (yes/no), part of a multiple birth (yes/no), BMI (<25/>=25), alcohol intake frequency (more than three or four times a week/less than three or four times a week/never). As a sensitivity analysis, we included the participants with normal birth weight (2.5-4kg) only. All analysis mentioned above adjusted for the covariates in model 2.
Two tailed P values less than 0.05 were considered as statistically significant. All analyses were conducted in SAS version 9.4.
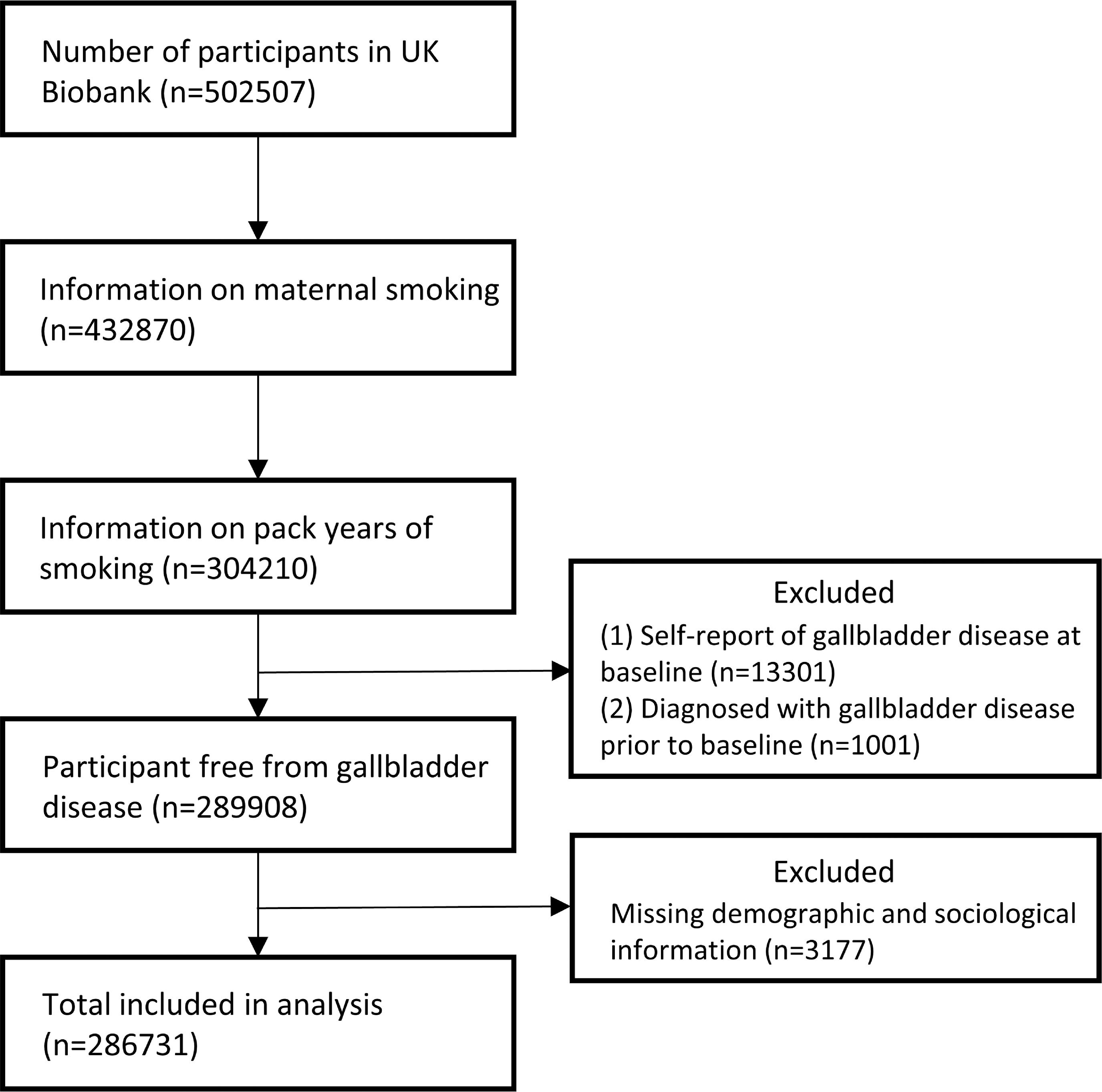
3Results3.1Characteristics of the populationThe flow chart of subject inclusion process in this study was shown in Fig. 1. A total of 502,507 participants had data available in the UK Biobank. We limited our analysis in participants with complete information in both maternal smoking and pack-years of own smoking (N=304,210). Therefore, participants with self-reported gallbladder disease at baseline (N=13,301) or diagnosis of gallbladder disease before baseline (N=1,001) were excluded. Additionally, we excluded participants with missing data on covariates (N=3,177). In the end, a total of 286,731 eligible participants were included in subsequent analysis. Of these, 90,203 had maternal smoking exposure in which 47.5% were female and the mean age was 55.85, while 196,528 were without maternal smoking exposure in which 47.5% were female and the mean age was 56.43. Most of participants were white (93.7%). Details of baseline characteristics are presented in Table 1. During a median of 8.8 years (quartiles: 8.19, 9.44) of follow-up, 7110 incident cases of gallbladder disease were identified, including 6800 (95.6%) gallstone. Therefore, we focused on gallstone and total gallbladder disease in the next analysis.
Baseline characteristics of study participants according to gallbladder disease.
Values are means ± SDs for continuous variables and percentages for categorical variables unless otherwise specified. BMI, body mass index. MET, Metabolic Equivalent Task.
All parameters have no statistically significant differences between groups.
Participants exposed to maternal smoking showed higher risk for the incidence of total gallbladder disease (HR = 1.24; 95%CI: 1.16-1.33; P <0.0001) as well as gallstones (HR = 1.24; 95%CI: 1.16-1.33; P <0.0001) in adulthood adjusted for the covariates in model 1 (Table 2). When further adjusting for covariates such as Townsend deprivation index, college or university degree, BMI, physical activity, smoke status, alcohol intake frequency, vegetables and fruits intake, red meat intake, use of vitamin and miner supplements, female-special factors (menopause and hormone-replacement therapy (HRT) in model 2, maternal smoking was still associated with high risks for incidence of gallbladder disease (HR = 1.13; 95%CI: 1.06-1.21; P =0.0002) and gallstones (HR = 1.13; 95%CI: 1.06-1.21; P =0.0003). With multivariable adjusted model, the associations between maternal smoking and gallbladder disease and gallstones were found only in females (total gallbladder disease: HR = 1.13; 95%CI: 1.05-1•21; P =0.002; gallstones: HR = 1.14; 95%CI: 1.05-1.23; P =0.002). However, a positive but insignificant association was also observed in males (total gallbladder disease: HR = 1.12; 95%CI: 0.99-1.27; P =0.08; gallstones: HR = 1.12; 95%CI: 0.98-1.27; P =0.1).
Association between maternal smoking during pregnancy and gallbladder diseases in genders.
Ref, the reference set for analysis. Values are hazard ratios (95% confidence intervals) unless stated otherwise.
Model 1 adjust for baseline age, sex, race, and other early life events including birth place, birth weight, breastfeeding, and part of a multiple birth;
Model 2: Model 1 plus Townsend deprivation index, college or university degree, BMI, physical activity (MET-min/week), smoke status, alcohol intake frequency, vegetables and fruits intake, red meat intake, vitamin and miner supplements, female-special factors (menopause and hormone-replacement therapy (HRT)).
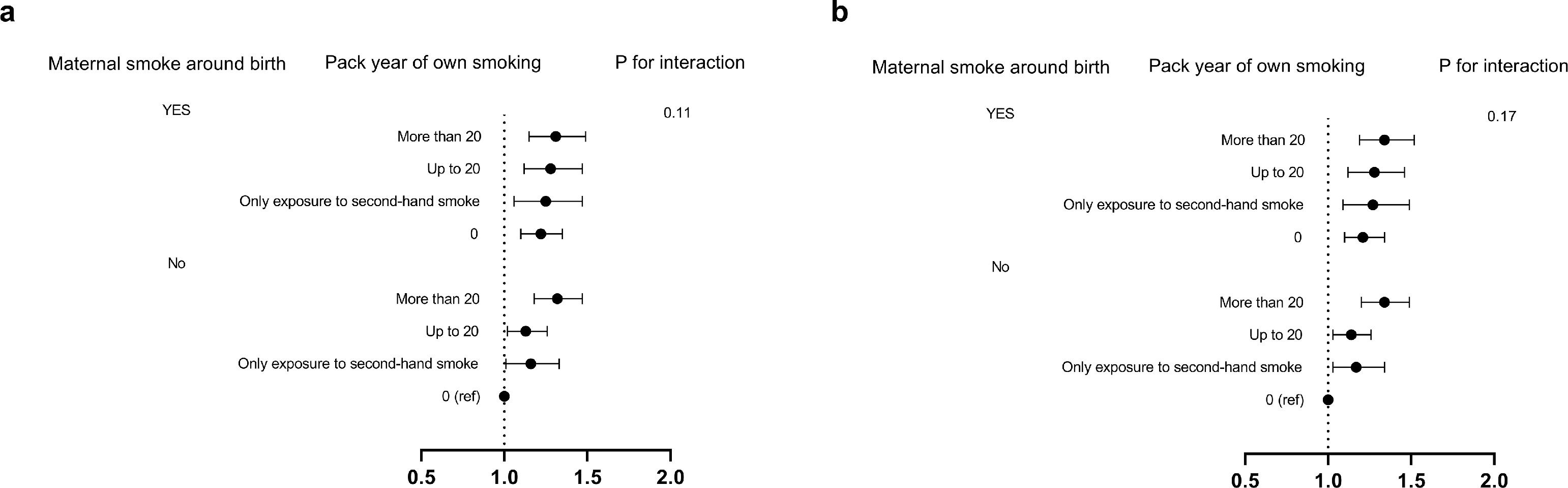
We further divided the participants according exposure to maternal smoking during pregnancy and own smoking during adulthood (Fig. 2). By comparing with those who were neither exposed to maternal smoking nor were own smoking, we found that subjects who were not own smoking but having maternal smoking exposure still had increased risks of total gallbladder disease (HR = 1.21; 95%CI: 1.1-1.34, P=0.0001) and gallstones (HR = 1.21; 95%CI: 1.1-1.35, P=0.0001). Within no maternal smoking exposure group, the highest risk for each gallbladder disease event was observed at a dose of over 20 pack-years of own smoking (Fig. 2). However, there were no significant interactions between maternal smoking and own smoking of any outcome (P interaction = 0.17 for total gallbladder disease and P interaction = 0.11 for gallstones). These data suggested that maternal smoking before birth was per se associated with high of incident gallbladder disease events irrespective of own smoking.
Multiple-imputation analysis of maternal and pack-years of own smoking in relation to incident gallbladder disease / gallstone events in later of life. The vertical line indicates the reference value of 1. The horizontal lines represent 95% CIs. The tests for interaction were performed using likelihood ratio tests. a. for gallstone, and b. for gallbladder disease.
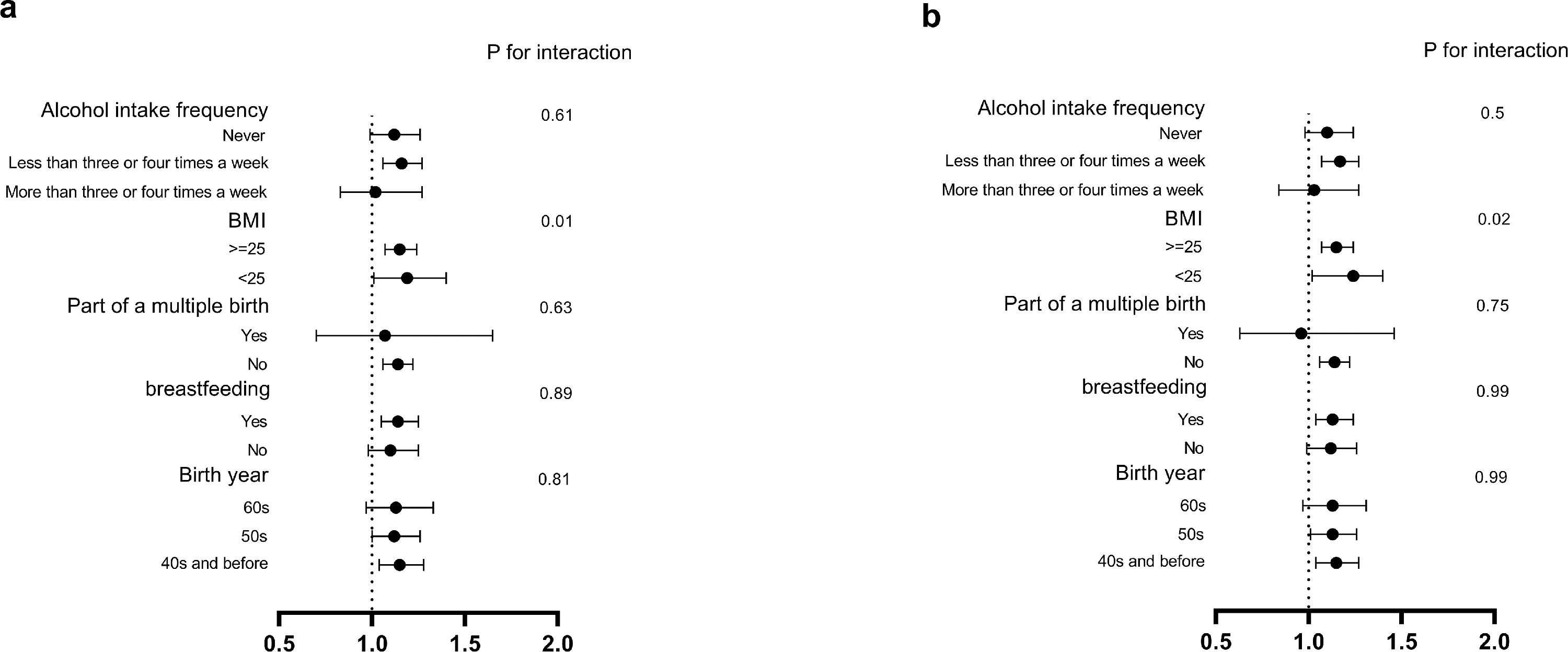
Next, we examined the consistency of association between maternal smoking and incident gallbladder disease events in adulthood among different subgroups of participants (Fig. 3). The subgroup analysis showed that, when stratified by BMI, slightly increased risk was observed in BMI lower than 25 group (P interaction = 0.02 for total gallbladder disease and P interaction = 0.01 for gallstones). Across the stratified groups according to birth year, breastfeeding, part of a multiple birth and alcohol intake frequency, the positive association was generally similar (all P interaction > 0.05).
Subgroup analysis of the association between maternal smoking and risk of incident gallbladder disease / gallstone events in adulthood according to potential baseline risk factors. The vertical line indicates the reference value of 1. The horizontal lines represent 95% CIs. The tests for interaction were performed using likelihood ratio test. a. for gallstone, and b. for gallbladder disease.
At last, we performed the sensitivity analysis (Table 3), and excluded 38,366 (13.38%) participants with abnormal birthweight. The risk estimates of incident gallbladder disease events did not materially change after excluding the participants with abnormal birth weight.
Association between maternal smoking during pregnancy and gallbladder diseases in different gender excluding participants with abnormal birth weight (n= 126065).
Ref, the reference set for analysis. Values are hazard ratios (95% confidence intervals) unless stated otherwise.
Model 1 adjust for baseline age, sex, race, and other early life events including birth place, birth weight, breastfeeding, and part of a multiple birth;
Model 2: Model 1 plus Townsend deprivation index, college or university degree, BMI, physical activity (MET-min/week), smoke status, alcohol intake frequency, vegetables and fruits intake, red meat intake, vitamin and miner supplements, female-special factors (menopause and hormone-replacement therapy (HRT)).
In this prospective study, we showed exposure of maternal smoking was associated with increased risk of incident gallbladder disease events in adulthood using data from a large cohort of UK biobank. Exposure of maternal smoking was associated with 13% higher risk of total gallbladder disease and 13% higher risk of gallstones. We also showed maternal smoking exposure was per se associated with gallbladder disease / gallstone independent of own smoking in adulthood. The results in this study implied that the DOHaD concept, which indicating that environmental pressures in early life such as maternal smoking exposure leading to chronic disease in adult, was also applicable for gallbladder disease.
A recent survey of 2015 showed that in 1/4 of the Europe countries, more than 12.5% of women still smoked during pregnancy with the highest percentages in Valencia, Spain (18.3%), followed by Wales (17.3%), France (16.3%), and Northern Ireland (14.3%) [26]. A recent meta-analysis including 39 studies reported that percentages of maternal smoking in pregnancy ranged from 5.5% to 38.7% worldwide [15]. There are numerous studies established and recognized the short-term effects of maternal smoking during pregnancy on several outcomes, including preterm birth, fetal growth restriction, low birth weight and stillbirth [14,27]. In recent year, many researchers have been focused on the long-term effects of maternal smoking on chronic diseases of offspring in adulthood [28]. Several studies suggested that exposure of maternal smoking was associated with an increased risk of obesity in adulthood [25,29,30]. The Nurses’ Mothers’ Cohort study indicated that the daughter whose mother heavily smoked during pregnancy had 98% higher risk of gestational diabetes mellitus [19]. Our results provided novel evidences highlighting the risk of maternal smoking in association with incident gallbladder disease / gallstone events in adulthood.
Early life environmental signals induce adverse health effects on later in life, which has long been considered as the predisposing factors to non-communicable diseases (NCDs) [31]. This concept is known as the Developmental Origins of Health and Disease (DOHaD), though the actual mechanisms of DOHaD remain unclear [32,33]. Epigenetics including DNA methylation, histone modifications and the non-coding RNAs, which can be modified by environmental hazards, is suggested as possible mediators of DOHaD [34,35]. Prenatal smoking exposure in mice was shown to change insulin-like growth factor-1 promoter methylation status in offspring across all developmental stages [36]. Cotinine is the primary metabolite of nicotine. It could be detected in foetal organs, placentas and maternal plasma from smoking pregnant woman with the mean concentrations of 61ng/g, 108ng/g and 99ng/g respectively [37]. Analysis of 7,000 newborns from 13 cohorts identified more than 6000 differentially methylated CpG sites after maternal smoking exposure. These DNA changes could persist until adolescence [38]. The metabolic changes induced by maternal smoking exposure through epigenetics might be one potential mechanism accounting for the risk of maternal smoking in association with gallbladder disease/gallstone in adulthood.
The association of own smoking with increased risk of gallbladder disease had been previously studied. However, the results were unexpectedly contradictory in various studies [39–42]. A recent meta-analysis of 10 prospective studies indicated that the risk of gallbladder disease increased 11% per 10 cigarettes per day [43]. Many studies focused on the interaction between maternal smoking and own smoking [44–46]. Combined exposure of maternal and own smoking resulted in reduction in lung function and increase of incident hospitalization/death from chronic obstructive pulmonary disease [45,46]. However, none of the study had considered the potential interaction between maternal smoking and own smoking of gallbladder disease. After dividing the populations according to maternal smoking exposure and own smoking during adulthood, we could not observe any interaction between maternal smoking and own smoking. The results in the present study, in one hand, confirmed that own smoking increased risk of incident gallbladder disease / gallstone events in adulthood even among those who did not expose to maternal smoking. On the other hand, maternal smoking exposure was enough to increase the risk of adult gallbladder disease / gallstone events independent of adult own smoking. These findings highlighted the importance of control of maternal smoking as well. The biological mechanisms of adult own smoking on risk of gallbladder disease could more be ascribed to direct effects by the ingredients as nicotine or heavy metals in cigarettes on metabolic dysfunction in cholesterol or bile acids leading to supersaturation of biliary cholesterol ([47,48]).
In our subgroups results, the associations between maternal smoking and higher risk of gallbladder disease and gallstones were found only in females. Females are more susceptible to gallstones due to oestrogen which enhances synthesis and secretion of cholesterol [49]. Drug use such as progesterone and the somatostatinIn would promote similar process [1]. In addition, pregnancy is a wellrecognized risk factor for gallbladder disease ([50,51]). We also found that low BMI is a significant risk factor for both gallstones and gallbladder disease. It is reported that individuals with rapid weight loss which happen after extreme dieting or bariatric surgery have increased risk of biliary symptoms and cholecystectomy [52–54]. Except for sex and BMI, we found that moderate alcohol consumption and birth year earlier than 1940 had higher risk of gallbladder disease and gallstones.
To our knowledge, this is the first study providing evidences for the risk of maternal smoking to incident gallbladder disease events in adulthood using large prospective cohort data from UK Biobank. The large size of this study allowed us to consider adequate covariates including early life factors such as birth weight, breastfeeding and multiple birth. Our study also has several limitations. First, the data of maternal smoking and other covariates were self-reported, the recall bias was unavoidable. Data on maternal smoking was reported base on regular or not but not the quantity of cigarettes per day, which prevented us from further analysis of dose-effect of maternal smoking. Second, the information of childhood nutrition and exposure, as well as genes or family history of gallbladder disease was unavailable, though these information might play roles in pathogenesis of gallstone formation.
5ConclusionExposure of maternal smoking was associated with high risk of incident gallbladder disease / gallstone events in later of life. This association was more prominent among female and population with low BMI. Our study highlights the important effect of control of smoking during pregnancy on protecting adulthood chronic disease in offspring.
Author contributionsLW, LJ, SW: acquisition of data, analysis and interpretation of data, and drafting the manuscript; XC, XJ: analysis and interpretation of data and drafting the manuscript, JZ and GA: forming the conception and design of the study and interpretation of data, and drafting and editing the manuscript. All authors have the final approval of the version to be submitted. JZ and GA are authors responsible for the integrity of the work as a whole.
This work was supported by grants from the National Key Research and Development Program (2018YFC1004203, 2019YFA0802701), the National Science Fund for Outstanding Young Scholars (81722040), and the National Natural Science Foundation of China (91839102, 91943301, 81770626).