Aim: The Child Pugh and MELD are good methods for predicting mortality in patients with chronic liver disease. We investigated their performance as risk factors for failure to control bleeding, in-hospital overall mortality and death related to esophageal variceal bleeding episodes. Methods: From a previous collected database, 212 cirrhotic patients with variceal bleeding admitted to our hospital were studied. The predictive capability of Child Pugh and MELD scores were compared using c statistics. Results: The Child-Pugh and MELD scores showed marginal capability for predicting failure to control bleeding (the area under receiver operating characteristics curve (AUROC) values were < 0.70 for both). The AUROC values for predicting inhospital overall mortality of Child-Pugh and MELD score were similar: 0.809 (CI 95%, 0.710 - 0.907) and 0.88 (CI 95% 0.77-0.99,) respectively. There was no significant difference between them (p > 0.05). The AU-ROC value of MELD for predicting mortality related to variceal bleeding was higher than the Child-Pugh score: 0.905 (CI 95% 0.801-1.00) vs 0.794 (CI 95% 0.676 - 0.913) respectively (p < 0.05). Conclusions: MELD and Child-Pugh were not efficacious scores for predicting failure to control bleeding. The Child-Pugh and MELD scores had similar capability for predicting in-hospital overall mortality. Nevertheless, MELD was significantly better than Child-Pugh score for predicting in-hospital mortality related to variceal bleeding.
Esophageal variceal bleeding (EVB) in cirrhotic patients is one of the most severe complications and has a mortality rate of 13% to 30 %.1-3 Patients with advanced liver disease are at higher risk to develop complications or to die because of hypovolemic shock, infections and liver failure.4,5
Diverse methods have been used for predicting complications and mortality rates in cirrhotic patients over the last decades. One of the most used is the Child-Pugh (CP) score. It was developed in 1973 as a modification of the Child-Turcotte’s score, by substituting the nutrition status variable for prothrombin time.3
One limitation of the CP score is the use of two clinical parameters such as ascites and hepatic encephalopathy. The subjective assessment for grading these parameters can affect the reliability of the score.
Recently, a Model for End Stage of Liver Disease (MELD) was designed in order to determine the complication risks in patients with transjugular intrahepatic portosystemic shunts.4 This method comprises three biochemical parameters: serum bilirrubin, prothrombin time and serum creatinine. MELD was also used in cirrhotic patients with various clinical conditions, including renal failure, gallbladder surgery, liver transplant and acute alcoholic hepatitis. It has proved its utility as an independent indicator of survival.8-12 Because MELD is more accurate than CP score for predicting short term (3 months) mortality, it was promptly adopted by the UNOS to allocate liver grafts for transplantation.13,14
Studies assessing the efficiency of CP and MELD score in predicting mortality of any cause in cirrhotic patients with EVB have also been published.15-17 Nevertheless, to our knowledge the utility of CP and MELD score for predicting 5-day failure to control bleeding and mortality related to variceal bleeding episodes has not been evaluated.
Patients and methodsAll cirrhotic patients from a database with a diagnosis of an acute EVB episode admitted to our hospital between 2003 and 2006 were studied. The inclusion criteria were: adult patients with diagnosis of hepatic cirrhosis of any etiology (diagnosed by liver biopsy, clinical manifestations and/or imaging studies) and acute EVB (hematemesis and/or melena occurred during a period of 24 hrs before admission due to endoscopically confirmed esophageal varices). We excluded non-cirrhotic patients with EVB or cirrhotic patients with any other source of bleeding such as gastric varices, congestive gastropathy or ectopic varices and as well as patients with hepatocellular carcinoma. Variables such as age, gender, comorbid entities, etiology of cirrhosis and type of haemostatic treatment were recorded for each patient. The variceal size was defined by the Paquet classification.18
Liver function at admission was assessed using the CP and MELD scores. The CP score was determined using the classical parameters: ascites, encephalopathy, serum albumin, total bilirubin and prothrombin time. The patients were classified as CP stages A (5-6 points), B (7-9 points) and C (10 or more points).6 The MELD was calculated using the following formula: 3.8 log e (serum bilirubin mg/dL) + 11.2 log e (INR) + 9.6 log e (serum creatinine mg/dL) + 6.4.6
During hospitalization the following variables were determined: a) 5-day failure to control bleeding: which was defined according to Baveno IV19 as fresh hematemesis > 2 hrs after performing an endoscopic treatment or the administration of vasoactive drugs, 3 g drop in Hb and death in a time frame of 120 hours; b) overall mortality: which was defined as death due to any cause during the hospitalization and c) mortality related to an EVB episode: which was defined as death due to cardiovascular failure and/or hemodynamic instability as a result of uncontrollable hemorrhage during admission or rebleeding episode (fresh hematemesis > 2 hrs after initial treatment or a 3 g drop in Hb) occurring any time during the hospitalization despite new endoscopic of pharmacologic treatment.
Statistical analysisThe continuous variables were expressed as mean, range and standard deviation. For relative proportions the 95% confidence intervals were calculated. The performance of CP and MELD scores for predicting the rates of failure to control bleeding, overall mortality and mortality related to EVB was evaluated by measurement of their discriminative ability, estimated by the concordance c-statistic (area under the Receiving Operating Characteristics (AUROC) curves. An AUROC value of > 0.70 was considered as clinically relevant. Statistical differences between both scores were assessed comparing the AUROC values by means of the Z test. An alpha value of < 0.05 was considered statistical significant. The program SPSS v 13.0 was used for the statistical analysis.
ResultsClinical and biochemical characteristics of patients (Table I)Two hundred twelve patients were included into the study: 145 were male (68%) with a mean age of 53 years old (range 27 - 91 years). The most frequent etiology of cirrhosis was alcohol abuse (73%). Thirty five patients (17%) showed renal impairment manifested by serum creatinine > 1.5 mg/dL. Only one patient had a history of chronic renal failure non treated with dialysis.
Clinical and biochemical features of patients.
| Patients, N | 212 |
| Gender (M/F) | 145/65 |
| Age, mean ± SD | 53 ± 12 |
| Etiology of cirrhosis, n (%) | |
| Alcohol | 154 (73) |
| Virus B/C | 15 (7) |
| Autoimmune | 7 (3) |
| Other | 38 (17) |
| Biochemical tests, mean ± SD | |
| Hemoglobin, g/DL | 8.7 ± 2.8 |
| Total bilirubin, mg/DL | 2.5 ± 3.2 |
| Serum creatinine, mg/DL | 1.1 ± 1 |
| INR, mg/DL | 1.9 ± 2.1 |
| Child-Pugh stage, n (%) | |
| A | 38 (18) |
| B | 108 (51) |
| C | 66 (31) |
| Child-Pugh score, mean ± SD | 9 ± 3 |
| MELD, mean ± SD | 15 ± 8 |
N: number of patients; M: male; F: female; SD: standard deviation; INR: international normalized ratio.
Using the CP score 38 patients (18%) were on stage A, 108 patients (51%) on stage B ad 66 (31%) on stage C. The Meld mean value was 15 + 7.
Endoscopic findings and treatment of bleeding (Table II)The esophageal variceal size was determined as Grade I in one patient (0.5%), Grade II in 90 (42.5%), Grade III in 104 (49%) and Grade IV in 17 patients (8%). There were 155 patients (73%) with recent stigmata of variceal bleeding, 39 had active variceal bleeding (oozing or spurting from a varix) and 18 had blood in the stomach without any other source of bleeding.
All patients received endoscopic treatment: variceal band ligation was used in 202 patients (95.5%), sclerotherapy (with Polidocanol 1.5% in 9 (4%) patients and one patient was treated with both procedures (0.5%). In 19 patients a vasoactive drug (octreotide) was administered intravenously. Antibiotic prophylaxis (with IV or PO ciprofloxacin or norfloxacin) was prescribed to all patients since hospital admission and during a period of at least seven days.
The mean length of hospital stay was 5.1 days (range 1 to 32 days).
Capability of CP and MELD to predict 5-day failure to control bleeding, overall mortality and mortality related to EVB.
a) Control bleeding: seventeen (8%) patients presented failure to control bleeding. Eleven of these patients died during the first five days after admission. In 8 of them death was considered related to the bleeding event (Table II)
Endoscopic findings, treatment of bleeding and outcomes of patients.
| Endoscopic findings, n (%) | |
| Active bleeding | 39 (18) |
| White nipple sign | 155 (74) |
| Blood in stomach and NOSB | 18 (8) |
| Treatment of bleeding, n(%) | |
| Endoscopic treatment | 100 (100) |
| Band ligation | 202 (95.5) |
| Sclerotherapy | 9 (4) |
| Combined | 1 (0.5) |
| Pharmacological therapy, n (%) | 19 (9) |
| Units of blood transfused, mean ± SD | 2.2 ± 1.7 |
| Outcomes of patients, n (%) | |
| 5-day failure to control bleeding, | 17 (8) |
| Overall deaths | 17 (8) |
| Deaths due to variceal bleeding | 10 (5) |
NOSB: no other source of bleeding
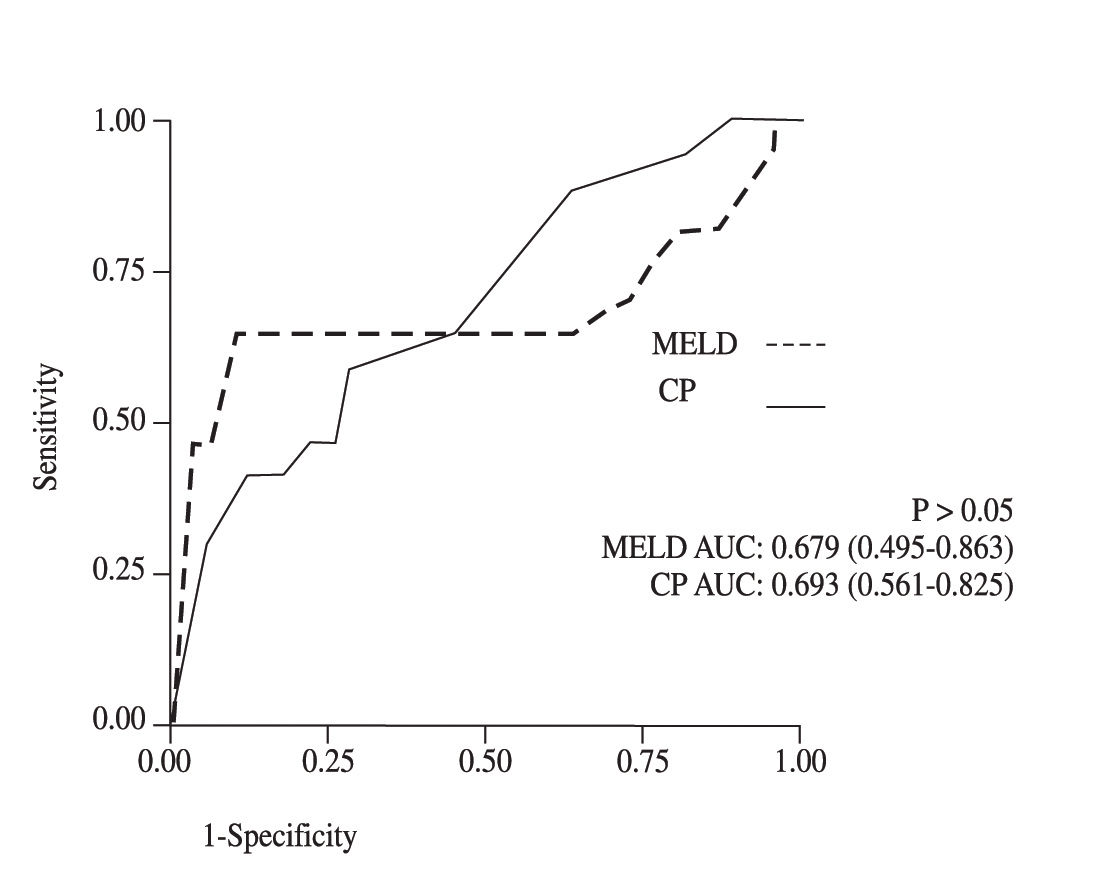
The AUROC values for failure to control bleeding were: for CP 0.69 (CI 95% 0.56-0.82) and for MELD 0.67 (CI 95% 0.49-0.86). No significant statistical differences were observed between both methods (p > 0.05) (Figure 1)
b) Overall mortality: Seventeen patients died (8%). Three were on CP stage B and 14 on CP stage C.
Death was attributed to the EVB episode in 10 patients; to liver failure in 5 patients (three of hepatic coma and 2 of hepatorenal syndrome type 1), sepsis in one and another patient because of pulmonary complications (Table II)
Concerning in-hospital overall mortality rate, the AU-ROC values of CP and MELD scores were 0.80 (CI 95%, 0.71 - 0.90) and 0.88 (CI 95% 0.77-0.99) respectively. Both methods significantly predicted mortality, but they had similar values and no statistical significant difference was found (p < 0.05) (Figure 2)
c) Mortality due to EBV: From the 10 patients whose death was attributed to the EVB episode 4 died at admission and 6 died during hospitalization. Finally, the AUROC values for CP and MELD scores in regard to mortality rate induced by the EVB episode were: 0.79 (CI 95% 0. 0.67 - 0.91) and 0.90 (CI 95% 0.80-1.0) respectively. Although, both scores are good predictive tools, MELD was superior to CP score (p < 0.05) (Figure 3)
DiscussionThe MELD score was originally designed with the objective to predict mortality risk in patients with transjugular intrahepatic portosystemic shunts (TIPSS).7 After this, MELD was evaluated as prognostic score for mortality in patients with chronic liver disease in different clinical situations.8-12 In the other side, the CP score is simpler and easier to calculate at bedside than MELD. Similar to the original Child’s grading, neither the division of the grades nor the scoring system of CP score were statistically validated.6 Although CP score has proved consistency and reproducibility in many studies, it has serious limitations: 1) two variables are clinical such as ascites and encephalopathy, therefore imprecision in their subjective assessment may occur; 2) The continuous variables in the CP score are categorized using arbitrary cut-off points. Therefore, this grading system does not distinguish some subgroups inside each stage («mild» grade C from «severe» grade C, etc.).6 Thus, the combination of CP score with other clinical and biochemical parameters such as serum creatinine, serum sodium and encephalopathy has been proposed in order to increase its sensibility.6
Although MELD has fewer variables than CP score, its calculation is more complicated. It may require the use of a computed system or internet services. Notwithstanding the advantage of this method is the use of objective variables particularly serum creatinine which is a very important prognostic risk parameter for mortality in patients with chronic liver disease.
Recently, some studies have compared CP and MELD score in cirrhotic patients on waiting lists for liver transplantation; in patients with TIPSS and in cirrhotic patients with and without complications.7-14 In all of them, CP and MELD scores had similar capability for predicting mortality, except in patients on waiting lists for liver transplantation, in whom MELD had substantially better predictive power.
The results of our study were similar to those reported in previous studies in which the CP and MELD scores were assessed in patients with EVB for in-hospital mortality rates due to any cause.15,16 Nevertheless, we found that MELD was better than the CP score for predicting in-hospital mortality related to the EVB episode. As far as we know this issue has not been previously evaluated.15-17,19
Several factors may be evoked for explaining the better performance of MELD for predicting mortality related to EVB that was observed in our study: 1) the patients who died may have had renal impairment before the occurrence of the bleeding episode, 2) More severe bleeding may have given rise to acute renal failure due to hemodynamic instability and hypovolemic shock in some patients particularly those who died, 3) liver function was probably more deteriorated in patients who died. That was probably reflected by higher values of serum bilirubin, serum creatinine and prothrombin time.
On the other side, it is important to underline that our in-hospital mortality rate (8%) was lower than the one observed in other series particularly in Chalassani and Carbonell studies. These authors reported a mortality rate of 14.2% and 14.5% respectively.2,3 These differences may be explained by some factors: 1) in the Chalassani study only 64% of the patients were treated with antibiotic prophylaxis. It is well known, that the use of antibiotic prophylaxis in patients with EVB reduces the in-hospital mortality rate by 9%;21 2) in the Carbonell study, the patients sample size was small (83 patients) and patients with gastric varices and hepatocellular carcinoma were included.2 Recently, the mortality rate during the first 7 days of hospital admission was reported to be of 9.7% which is similar to our study.22 Unfortunately, this study was published only in abstract form and more specific details were unavailable.
Finally, in our study MELD and CP scores were not efficacious tools for predicting 5-day failure to control bleeding since their AUROC values were < than 0.70. The usefulness of MELD score for predicting failure to control bleeding may be further evaluated in future trials.
Certainly, our study does have several limitations: 1) it is derived from a database; 2) we did not assess the influence of some variables in the outcome of patients such as spontaneous bacterial peritonitis, sepsis, and hyponatremia. However, none of these variables are taken into account in CP and MELD scores; 3) we did not include patients with gastric variceal bleeding, ectopic varices or hepatocellular carcinoma.
In conclusion, our results showed that the CP and MELD scores are not efficacious methods for predicting failure to control bleeding. Conversely they are equally efficient tools for predicting in-hospital overall mortality. Nevertheless, MELD is significantly better than CP score for predicting mortality related to an acute EVB episode. Prospective clinical trials are needed to confirm this later finding.
AcknowledgementsWe would like to thanks Doctors-Rafael Castaneda-Sepulveda, Miguel Mar-Ruiz, Jorge Leal-Salazar, Eduardo Mendoza-Fuerte and Laura Cortez-Sanabria for the help on this work.