Background. Clinical and endoscopic features of cirrhotic patients with nonvariceal upper gastrointestinal bleeding (NVUGIB) have been rarely reported and clinical outcomes and predictors of mortality have not been evaluated.
Aims. 1) To describe the clinical features; 2) To define the clinical outcomes; and 3) To identify the predictors of in-hospital mortality of cirrhotic patients with NVUGIB.
Methods. One hundred sixty cirrhotic patients with NVUGIB were prospectively studied. Clinical features, endoscopic findings, clinical outcomes and in-hospital mortality rate were studied. Predictors of death were identified by means of univariate and multiple logistic regression analysis.
Results. The mean age was 56.5 ± 14.4, male gender prevailed. Alcohol was the most frequent etiology. Hemodynamic instability was reported in 29.4%. Mean serum hemoglobin was 9.5 ± 3.3 g/dL and blood transfusions were required in 59.4%. Gastroduodenal ulcers were the most frequent source of bleeding (50.6%). In endoscopy “high-risk” bleeding stigmata (HRBS) at the ulcer base were found in 53.1%. All patients with HRBS received endoscopic treatment. Rebleeding occurred in 3 patients (1.9%) and mortality was of 13.8%. By univariate analysis: Cryptogenic etiology, BUN, hypoalbuminemia, active bleeding at ulcer base, and endoscopic treatment were predictors of mortality. However, only cryptogenic etiology, hypoalbuminemia and active bleeding at ulcer base were independent predictors of death in multivariate analysis.
Conclusions. Gastroduodenal ulcers as a source of NVUGIB are frequent in cirrhotic patients. They were severe; half of them had HRBS, and required frequently endos-copic treatment. In-hospital mortality of these patients seemed to be greater than that of non-cirrhotic patients, and it was significantly related to cryptogenic etiology of cirrhosis, renal dysfunction, severe hepatic failure, and active bleeding ulcers on admission to the hospital.
Upper gastrointestinal bleeding is a common life-threatening condition in which mortality rate is from 4 to 15%.1–5 Upper gastrointestinal bleeding has been classified according to the presence of a variceal or nonvariceal source of bleeding. In cirrhotic patients, variceal bleeding has been very extensively studied.6–8 Nonetheless, 30 to 40% of cirrhotic patients who bleed may have nonvariceal upper gastrointestinal bleeding (NVUGIB), and it is frequently caused by gastroduodenal ulcers.9,10 Although NVUGIB is not uncommon in cirrhotic patients, clinical and endoscopic features of patients with this complication have been very rarely reported,6,7,11,12 and clinical outcomes and mortality have not been evaluated in specifically conducted investigations. As chronic liver disease has a noxious impact in patients with NVUGIB,2,13,14 the knowledge of the clinical outcomes and predictors of death of these patients becomes imperative.
AimThe aims of this study were:
- •
To describe the clinical features and endoscopic findings.
- •
To define the clinical outcomes.
- •
To identify the predictors of in-hospital death of cirrhotic patients with NVUGIB.
All consecutive adult patients with NVUGIB (age ≥ 18 years) admitted from January 2000 to February 2009 were prospectively registered. Those with confirmed diagnosis of liver cirrhosis were included in the study. Data regarding demographic variables, clinical features, bleeding characteristics, endoscopic findings, type of hemostatic endoscopic therapy, and clinical outcomes during hospitalization were collected. Follow up was performed by daily visits until discharge or death.
PatientsPatients with hemodynamic instability were managed with crystalloid solutions and blood transfusions. The treatment using acid-blocking drugs such as proton pump inhibitors (PPI) and/or H2 receptor antagonists (H2RA) was prescribed according to one of the following protocols: ranitidine 50 mg IV every 8 h and/or omeprazole 40 mg IV every 12 h given either by bolus or an 8 mg/h continuous infusion for three days and then continued orally. The number of blood units transfused was indicated according to individual requirements. All endos-copic procedures were performed by professors or gastroenterology fellows involved in the project. Patients with variceal bleeding and those who did not complete the in-hospital follow-up period were excluded for analysis.
Liver diseaseThe diagnosis of cirrhosis was confirmed by liver biopsy or by clinical, radiologic and laboratory criteria. Child-Pugh status and etiology of liver disease were determined. Alcohol-related liver disease was defined as daily alcohol consumption > 80 g in men and > 40 g in women for at least 10 years with negative viral, metabolic, and autoimmune markers.15 Diagnosis of hepatitis C and B viruses-related liver disease was determined with specific viral markers (HBsAg or anti-HCV). Autoimmune liver disease was diagnosed with specific autoimmune markers (anti-nuclear antibodies, anti-smooth muscle antibodies, or liver-kidney antimicrosomal antibodies);16 while non-alcoholic fatty liver disease (NAFLD) was diagnosed clinically and radiologically in the absence of significant alcohol consumption and in association with metabolic syndrome or by histological examination.17,18
Study variablesVariables included demographic data, clinical manifestations of bleeding, history of gastrointestinal bleeding, the use of tobacco, the use of non-ste-roidal anti-inflammatory drugs (NSAID), and alcohol intake. Comorbidities, other than cirrhosis, such as diabetes mellitus, cardiovascular disease, chronic kidney failure, chronic pulmonary disease, and malignancy were registered. Clinical characteristics such as melena, bright red hematemesis, coffee-ground hematemesis, hematochezia, and hemodynamic instability (expressed by a heart rate > 100 beats/min, hypotension with a systolic pressure < 90 mmHg and/or diastolic value < 60 mmHg) were also recorded. Laboratory data included hemoglobin, hematocrit, blood urea nitrogen, serum albumin, and prothrombin time.
EsophagogastroduodenoscopyTiming from admission to the realization of the endoscopy was measured, and bleeding source was identified and patients were stratified according to the Rockall classification.19 In patients with ulcers, stigmata of recent hemorrhage (SRH) at ulcer base were described according to the Forrest classification.20 Active bleeding, visible vessel, or adherent lots were classified as “high risk” bleeding stigmata (HRBS). Endoscopic treatment was performed only in patients with HRBS. When blood was found in the stomach, but no specific lesion was visualized, it was classified as unidentified source of bleeding. Investigation of gastric biopsies for Helicobacter pylori was not routinely performed, but frequencies of realization and detection were reported.
OutcomesMain outcomes included rebleeding, surgical treatment, and in-hospital mortality. These parameters were defined as follows:
- •
Rebleeding. It was defined as the presence of fresh blood hematemesis, melena, or both associated with hypovolemic shock or a decrease in serum hemoglobin level of > 2 g/dL after successful endoscopic or pharmacological treatment and hemodynamic stability of at least 24 hrs. Bleeding recurrence was confirmed by endoscopy in all cases.
- •
Surgical treatment. It was defined as the requirement of surgical treatment for bleeding after endoscopic or pharmacological treatment failure.
- •
In-hospital mortality. Deaths occurred during hospital stay. Cause of death was also determined.
Descriptive statistics were used to describe findings, using relative frequencies, medians, interquartile ranges, and 95% confidence intervals for categorical variables and means and standard deviations for continuous variables. Intergroup comparisons were made by Student’s t, Chi-square, and Mann-Whitney tests. Variables were analyzed using multivariate Cox proportional hazard model to determine independent predictive factors of mortality.21 Only the variables that were significant in univariate analysis were analyzed in multivariate analysis. The results were expressed as odds ratios (OR) with 95% confidence intervals. A p-value ≤ 0.05 was considered statistically significant. All statistical analyses were done using the statistical package SPSS v17.0 (Chicago, Illinois, USA).
ResultsDuring the study period, 2,217 patients with upper gastrointestinal bleeding were admitted. Bleeding was due to esophageal or gastric varices in 1,140 (51.3%) patients and it was related to NVU-GIB in 1,077 (48.7%) patients. Among patients with NVUGIB, 160 (14.8%) had confirmed diagnosis of cirrhosis; therefore, they were included in the study.
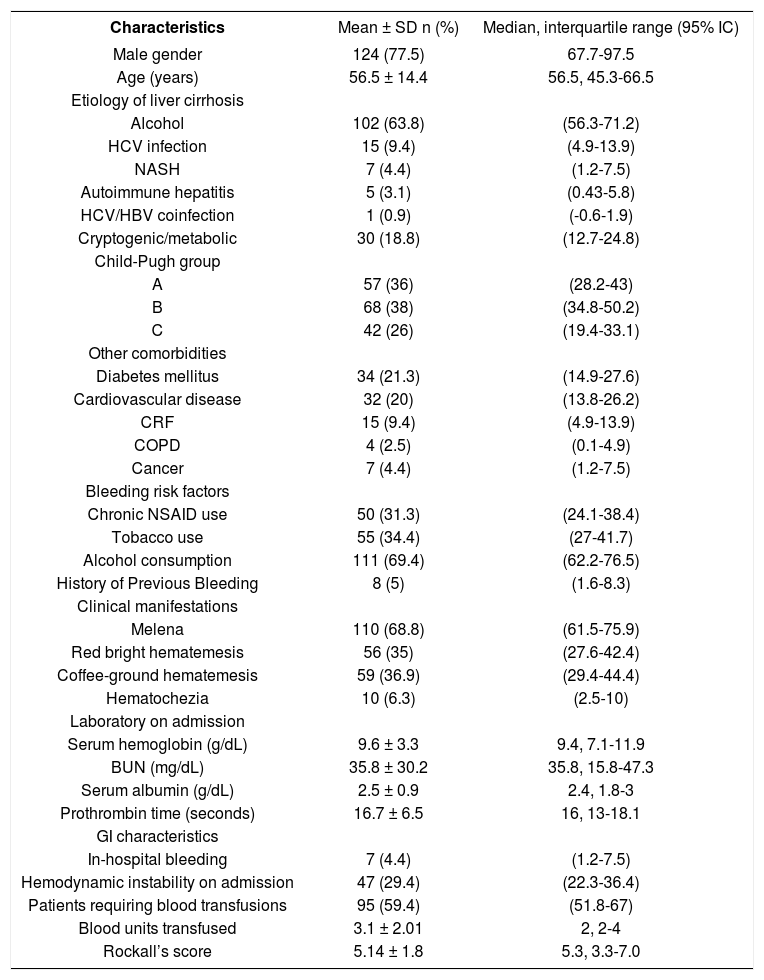
Demographic and clinical characteristicsThe mean age of patients was 56.5 years old with a male predominance (77.5%). Alcohol-induced liver disease was the most frequent etiology (63.8%) and Child-Pugh A and B status were the most frequent (36 and 38%) respectively. The mean number of co morbidities (other than cirrhosis) per patient was 0.5 ± 0.3, and 61 patients (38.1%) had one or more co-morbidities. One third of the patients had a risk factor associated with peptic ulcer disease including NSAID use and smoking. Melaena was the most frequent clinical finding (68.8%) and hemodynamic instability on admission was found in one third of cases. Mean serum hemoglobin level on admission was 9.6 ± 3.3 g/dL, and 95 patients (59.4%) required blood transfusions, with a mean number of 3.1 ± 2.01 blood units per patient (Table 1).
Clinical characteristics of patients with nonvariceal upper gastrointestinal bleeding and liver cirrhosis (n = 160).
| Characteristics | Mean ± SD n (%) | Median, interquartile range (95% IC) |
|---|---|---|
| Male gender | 124 (77.5) | 67.7-97.5 |
| Age (years) | 56.5 ± 14.4 | 56.5, 45.3-66.5 |
| Etiology of liver cirrhosis | ||
| Alcohol | 102 (63.8) | (56.3-71.2) |
| HCV infection | 15 (9.4) | (4.9-13.9) |
| NASH | 7 (4.4) | (1.2-7.5) |
| Autoimmune hepatitis | 5 (3.1) | (0.43-5.8) |
| HCV/HBV coinfection | 1 (0.9) | (-0.6-1.9) |
| Cryptogenic/metabolic | 30 (18.8) | (12.7-24.8) |
| Child-Pugh group | ||
| A | 57 (36) | (28.2-43) |
| B | 68 (38) | (34.8-50.2) |
| C | 42 (26) | (19.4-33.1) |
| Other comorbidities | ||
| Diabetes mellitus | 34 (21.3) | (14.9-27.6) |
| Cardiovascular disease | 32 (20) | (13.8-26.2) |
| CRF | 15 (9.4) | (4.9-13.9) |
| COPD | 4 (2.5) | (0.1-4.9) |
| Cancer | 7 (4.4) | (1.2-7.5) |
| Bleeding risk factors | ||
| Chronic NSAID use | 50 (31.3) | (24.1-38.4) |
| Tobacco use | 55 (34.4) | (27-41.7) |
| Alcohol consumption | 111 (69.4) | (62.2-76.5) |
| History of Previous Bleeding | 8 (5) | (1.6-8.3) |
| Clinical manifestations | ||
| Melena | 110 (68.8) | (61.5-75.9) |
| Red bright hematemesis | 56 (35) | (27.6-42.4) |
| Coffee-ground hematemesis | 59 (36.9) | (29.4-44.4) |
| Hematochezia | 10 (6.3) | (2.5-10) |
| Laboratory on admission | ||
| Serum hemoglobin (g/dL) | 9.6 ± 3.3 | 9.4, 7.1-11.9 |
| BUN (mg/dL) | 35.8 ± 30.2 | 35.8, 15.8-47.3 |
| Serum albumin (g/dL) | 2.5 ± 0.9 | 2.4, 1.8-3 |
| Prothrombin time (seconds) | 16.7 ± 6.5 | 16, 13-18.1 |
| GI characteristics | ||
| In-hospital bleeding | 7 (4.4) | (1.2-7.5) |
| Hemodynamic instability on admission | 47 (29.4) | (22.3-36.4) |
| Patients requiring blood transfusions | 95 (59.4) | (51.8-67) |
| Blood units transfused | 3.1 ± 2.01 | 2, 2-4 |
| Rockall’s score | 5.14 ± 1.8 | 5.3, 3.3-7.0 |
CRF: Chronic renal failure. COPD: Chronic obstructive pulmonary disease.
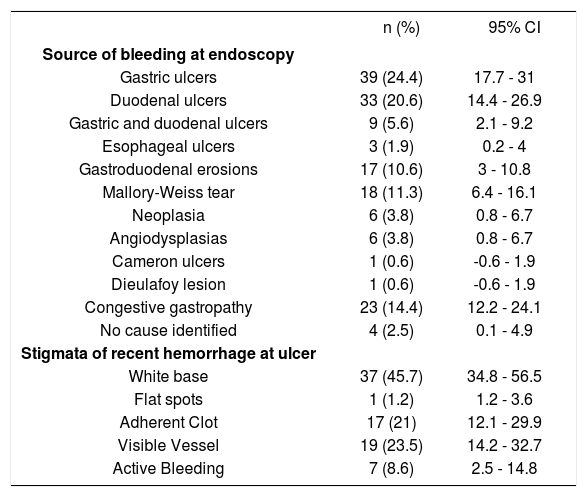
Endoscopy was performed in 73.1% of patients during the first 24 h from admission. Eighty one patients (50.6%) had ulcers in the stomach or duodenum (gastric ulcers in 24.4%, duodenal ulcers in 20.6%, and combined gastroduodenal ulcers in 5.6%). These lesions were the most frequent source of bleeding. In 23 patients (14.4%), blood was found in the stomach, but only congestive gastropathy was found; moreover, no cause of bleeding was identified in 4 patients (2.5%). In 43 patients (53%) with gas-troduodenal ulcers, a HRBS at ulcer base (active bleeding in 8.6%, visible vessel in 23.5%, and adherent clot in 21%) was observed at endoscopy. Nevertheless, white base at the ulcer was the most frequently observed stigmata (45.7%) (Table 2). Mean value in the Rockall scoring system was 5.14 ± 1.8 at admission, and 51.5% of patients had a value ≥ 5.
Endoscopic findings of patients with nonvariceal upper gastrointestinal bleeding and liver cirrhosis (n = 160).
| n (%) | 95% CI | |
|---|---|---|
| Source of bleeding at endoscopy | ||
| Gastric ulcers | 39 (24.4) | 17.7 - 31 |
| Duodenal ulcers | 33 (20.6) | 14.4 - 26.9 |
| Gastric and duodenal ulcers | 9 (5.6) | 2.1 - 9.2 |
| Esophageal ulcers | 3 (1.9) | 0.2 - 4 |
| Gastroduodenal erosions | 17 (10.6) | 3 - 10.8 |
| Mallory-Weiss tear | 18 (11.3) | 6.4 - 16.1 |
| Neoplasia | 6 (3.8) | 0.8 - 6.7 |
| Angiodysplasias | 6 (3.8) | 0.8 - 6.7 |
| Cameron ulcers | 1 (0.6) | -0.6 - 1.9 |
| Dieulafoy lesion | 1 (0.6) | -0.6 - 1.9 |
| Congestive gastropathy | 23 (14.4) | 12.2 - 24.1 |
| No cause identified | 4 (2.5) | 0.1 - 4.9 |
| Stigmata of recent hemorrhage at ulcer | ||
| White base | 37 (45.7) | 34.8 - 56.5 |
| Flat spots | 1 (1.2) | 1.2 - 3.6 |
| Adherent Clot | 17 (21) | 12.1 - 29.9 |
| Visible Vessel | 19 (23.5) | 14.2 - 32.7 |
| Active Bleeding | 7 (8.6) | 2.5 - 14.8 |
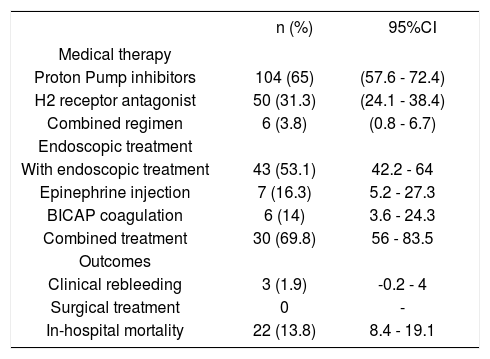
All patients received anti acid-secretion therapy, mostly proton pump inhibitors (65%) followed by H2 receptor antagonist (31.2%) (Table 3).
Medical, endoscopic treatment and outcomes of patients with nonvariceal upper gastrointestinal bleeding and liver cirrhosis (n = 160).
| n (%) | 95%CI | |
|---|---|---|
| Medical therapy | ||
| Proton Pump inhibitors | 104 (65) | (57.6 - 72.4) |
| H2 receptor antagonist | 50 (31.3) | (24.1 - 38.4) |
| Combined regimen | 6 (3.8) | (0.8 - 6.7) |
| Endoscopic treatment | ||
| With endoscopic treatment | 43 (53.1) | 42.2 - 64 |
| Epinephrine injection | 7 (16.3) | 5.2 - 27.3 |
| BICAP coagulation | 6 (14) | 3.6 - 24.3 |
| Combined treatment | 30 (69.8) | 56 - 83.5 |
| Outcomes | ||
| Clinical rebleeding | 3 (1.9) | -0.2 - 4 |
| Surgical treatment | 0 | - |
| In-hospital mortality | 22 (13.8) | 8.4 - 19.1 |
BICAP: Bipolar coagulation probe.
Endoscopic treatment was performed in all patients with HRBS ulcers; combined therapy (BICAP coagulation plus epinephrine injections) was used in most cases (Table 3).
Investigation of gastric biopsies for Helicobacter pylori was performed in only 67.9%, and its detection rate was of 20%.
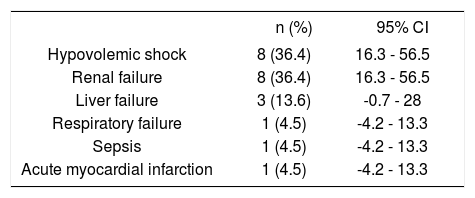
Clinical outcomesRebleeding occurred in 3 patients (1.9%); surgical treatment was not performed in this series. Overall in-hospital mortality occurred in 22 patients (13.8%) (Table 3). Causes of death are depicted in table 4. It was due to hypovolemic shock in only 8 patients (36.4%), and it was not related to bleeding causes in 14 patients (63.6%). Renal failure was the most frequent non-related to bleeding cause of death (8 patients, 36.4%), followed by liver failure in three patients (13.6%), and respiratory failure, sepsis due to intestinal perforation, and acute myocardial infarction in each one. The mean length of follow up was 5.7 days (range 1-50 days) in all patients.
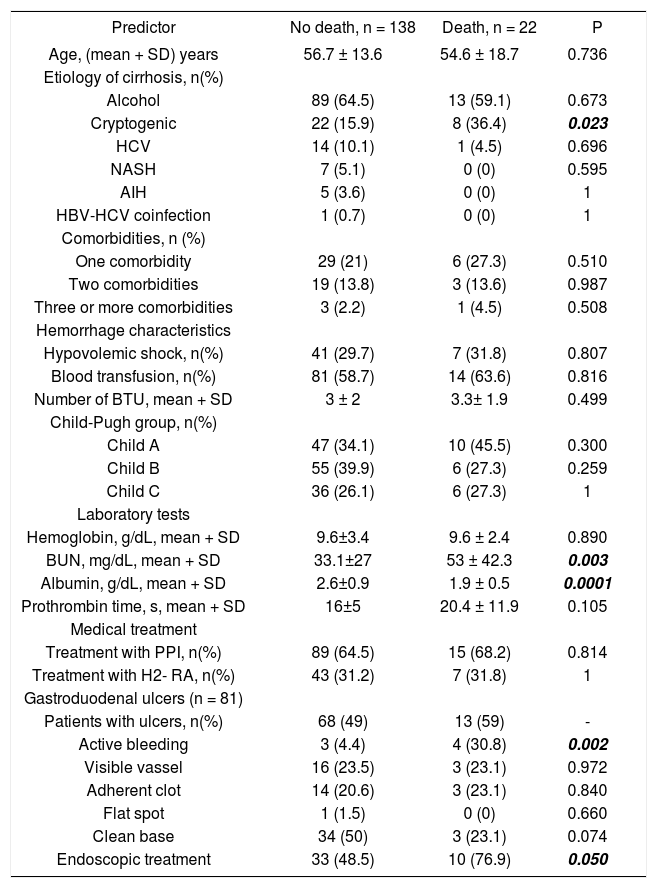
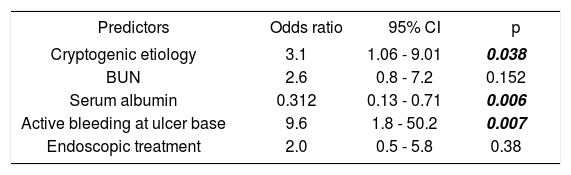
Univariate and multivariate analysisThe following variables were statistically significant in the univariate analysis: Cryptogenic etiology of cirrhosis (p = 0.023), BUN (p = 0.003), serum albumin levels (p = 0.0001), active bleeding at ulcer base (p = 0.002), and endoscopic treatment (p = 0.05). However, only the following parameters were independent predictors of in-hospital death in multivariate analysis: cryptogenic etiology of cirrhosis (OR 3.1; 95% CI: 1.06-9.01, p = 0.038), serum albumin levels (OR 0.31; 95% CI: 0.13-0.71, p = 0.006), and active bleeding at ulcer base (OR 9.6; 95% CI: 1.8-50.2, p = 0.007) (Tables 5 and 6).
Predictors of in-hospital mortality at univariate analysis.
| Predictor | No death, n = 138 | Death, n = 22 | P |
|---|---|---|---|
| Age, (mean + SD) years | 56.7 ± 13.6 | 54.6 ± 18.7 | 0.736 |
| Etiology of cirrhosis, n(%) | |||
| Alcohol | 89 (64.5) | 13 (59.1) | 0.673 |
| Cryptogenic | 22 (15.9) | 8 (36.4) | 0.023 |
| HCV | 14 (10.1) | 1 (4.5) | 0.696 |
| NASH | 7 (5.1) | 0 (0) | 0.595 |
| AIH | 5 (3.6) | 0 (0) | 1 |
| HBV-HCV coinfection | 1 (0.7) | 0 (0) | 1 |
| Comorbidities, n (%) | |||
| One comorbidity | 29 (21) | 6 (27.3) | 0.510 |
| Two comorbidities | 19 (13.8) | 3 (13.6) | 0.987 |
| Three or more comorbidities | 3 (2.2) | 1 (4.5) | 0.508 |
| Hemorrhage characteristics | |||
| Hypovolemic shock, n(%) | 41 (29.7) | 7 (31.8) | 0.807 |
| Blood transfusion, n(%) | 81 (58.7) | 14 (63.6) | 0.816 |
| Number of BTU, mean + SD | 3 ± 2 | 3.3± 1.9 | 0.499 |
| Child-Pugh group, n(%) | |||
| Child A | 47 (34.1) | 10 (45.5) | 0.300 |
| Child B | 55 (39.9) | 6 (27.3) | 0.259 |
| Child C | 36 (26.1) | 6 (27.3) | 1 |
| Laboratory tests | |||
| Hemoglobin, g/dL, mean + SD | 9.6±3.4 | 9.6 ± 2.4 | 0.890 |
| BUN, mg/dL, mean + SD | 33.1±27 | 53 ± 42.3 | 0.003 |
| Albumin, g/dL, mean + SD | 2.6±0.9 | 1.9 ± 0.5 | 0.0001 |
| Prothrombin time, s, mean + SD | 16±5 | 20.4 ± 11.9 | 0.105 |
| Medical treatment | |||
| Treatment with PPI, n(%) | 89 (64.5) | 15 (68.2) | 0.814 |
| Treatment with H2- RA, n(%) | 43 (31.2) | 7 (31.8) | 1 |
| Gastroduodenal ulcers (n = 81) | |||
| Patients with ulcers, n(%) | 68 (49) | 13 (59) | - |
| Active bleeding | 3 (4.4) | 4 (30.8) | 0.002 |
| Visible vassel | 16 (23.5) | 3 (23.1) | 0.972 |
| Adherent clot | 14 (20.6) | 3 (23.1) | 0.840 |
| Flat spot | 1 (1.5) | 0 (0) | 0.660 |
| Clean base | 34 (50) | 3 (23.1) | 0.074 |
| Endoscopic treatment | 33 (48.5) | 10 (76.9) | 0.050 |
AIH: Autoimmune hepatitis. BTU: Blood transfused units. PPI: Proton pump inhibitors. H2-RA: H2 receptors antagonists.
Independent predictors of in-hospital mortality (Logistic regression analysis).
| Predictors | Odds ratio | 95% CI | p |
|---|---|---|---|
| Cryptogenic etiology | 3.1 | 1.06 - 9.01 | 0.038 |
| BUN | 2.6 | 0.8 - 7.2 | 0.152 |
| Serum albumin | 0.312 | 0.13 - 0.71 | 0.006 |
| Active bleeding at ulcer base | 9.6 | 1.8 - 50.2 | 0.007 |
| Endoscopic treatment | 2.0 | 0.5 - 5.8 | 0.38 |
This study is relevant for the following reasons:
- •
The NVUGIB is not uncommon in cirrhotic patients, as it may occur in 30 to 40% of all upper gastrointestinal hemorrhages observed in them,9,10 so that the knowledge of the clinical characteristics and the identification of predictors of death of patients with this complication are of practical interest to hepatologists.
- •
Currently, there are not any published studies in which the clinical and endoscopic characteristics of cirrhotic patients with NVUGIB are specifically described, and predictors of death of these patients have not been evaluated. Most studies focus on the characteristics of the variceal bleeding,22–25 although there are few reports in which nonvariceal and variceal bleeding are analyzed together.6,11,12,26
- •
Because liver cirrhosis is itself a clinically labile condition, NVUGIB certainly has particular implications in the clinical evolution of cirrhotic patients as compared with the non cirrhotic ones.12
The results of our study show significant differences between our patients and the non cirrhotic patients presenting NVUGIB reported in the literature. Our patients were younger and consuming less NSAIDs. The mechanisms that cause ulcers in the stomach and duodenum in cirrhotics appear to be different from those in non-cirrhotic patients. While advanced age, chronic intake of NSAIDs, and infection with Helicobacter pylori are the most important in the latter patients;27 consumption of alcohol and portal hypertension may play a predominant role in cirrhotics.28 Although the frequency of gastroduodenal ulcers in our patients was similar to that reported in non-cirrho-tics,2,29 the frequency of “high-risk” bleeding stigmata at the ulcer base was higher (53 vs. 44%),29 so that the need for endoscopic treatment was also higher (100 vs. 74%).2 In this context, ulcers in cirrhotic patients may have an increased risk of bleeding due to the coagulation disorders and the thrombo-cytopenia that are frequently observed in these patients.30
However, the rebleeding rate observed in our patients was lower than that reported in non-cirrhotic patients (1.9 vs. 3.2%).2 We believe that this low frequency of rebleeding may be due to the fact that 100% of our patients with HRBS at ulcer base were treated endoscopically as compared with 79% of patients in the study mentioned.2 In the other side, most of our patients -who were treated endos-copically- received a combined therapy of epinephrine injection and coagulation with BICAP (69.8 vs. 8.3 and 34%).2,29 Currently, it has been demonstrated that combination therapy is significantly better than either adrenaline injections or coagulation alone for reducing the recurrence of peptic ulcer bleeding.31
On the contrary, in-hospital mortality was significantly higher in our study than that reported in non-cirrhotic patients from two studies (13.8 vs. 5.4 and 4.5%).2,29 Only one-third of the deaths were directly related to the bleeding episode, while the remaining deaths were associated with kidney disorders and liver failure. This may suggest that comorbidities alone or in combination with hemorrhage are importantly implicated with the decease of these patients. A similar finding has been described in non-cirrhotics in a recently published study with 10,428 patients with NVUGIB. It was observed that in only 29.2% of cases, death was associated with causes directly related to the bleeding episode. In the remaining cases, comorbidities had a fundamental role in the occurrence of death.4
Based on the results of our study, predictors of in-hospital death in cirrhotic patients with NVUGIB proved to be substantially different from those observed in non-cirrhotics. The following predictors have been observed in the non-cirrhotic patients: advanced age, the severity of the bleeding episode, number of comorbidities, functional class, and inhospital bleeding.2,12,29,32
According to univariate analysis, in our patients the predictors of death were: the cryptogenic etiology of cirrhosis, renal impairment (manifested by elevated BUN), impaired liver function (reflected by hypoalbuminemia), the severity of the ulcer (manifested by active bleeding on admission), and the need for endoscopic treatment. However, only cryptogenic etiology, hypoalbuminemia, and active bleeding detected by endoscopy were independent predictors of in-hospital death.
These data suggest that patients who died could have had aggravated metabolic disorders that affect the liver and kidney function as it could be type-2 diabetes mellitus and metabolic syndrome. This coincides with what was reported recently by our group where we demonstrated that DM increased the mortality in cirrhotic patients. Death in these patients was significantly associated with cryptogenic etiology, renal damage, and higher degree of liver failure.33
Moreover, the present study also reveals that the dead patients had more severe gastroduodenal ulcers, which had higher frequency of active bleeding. The evidence of the efficacy of PPIs for bleeding ulcers in cirrhotic patients is poor. Moreover, there are convincing papers suggesting that acid secretion is reduced in patients with liver cirrhosis.34 This could suggest that particularly in these patients, endoscopic treatments are essential as a complement to PPIs in patients with HRBS at endoscopy.
Our study has some limitations: it lacks a control group of non-cirrhotic patients. Often, we compare our results with those published in studies performed by other authors. However, we also use data from our group, which were published recently.32 On the other hand, the collection of patient data was not exhaustive because certain laboratory tests were not registered (such as blood glucose, serum creatinin, blood platelet count, etc.), with which we could better explain the abnormalities related with the death of our patients. However, the results of this pioneering study are a stimulus to carry out comparative studies in which a higher number of predictors can be analyzed in a larger number of patients.
ConclusionOur results show that cirrhotic patients with NVUGIB may develop gastroduodenal ulcers as often as non-cirrhotic patients. However, ulcers in cirrhotics may be more severe and less frequently associated with chronic intake of NSAIDs, may have more often “high-risk” bleeding stigmata, and may require more frequently endoscopic treatment. Inhospital mortality in these patients seems to be greater than that of non-cirrhotic patients, and it is significantly related to cryptogenic etiology of liver cirrhosis, as well as to the presence of renal dysfunction, severe hepatic failure, and active bleeding ulcers on admission to hospital.