Abstracts of the 2022 Annual Meeting of the ALEH
Más datosTyrosine kinases (TKs) are a family of proteins with a critical role in controlling cancer phenotypes, and many TK inhibitors (TKI) as anti-cancer agents are available. Mandatory black box warning has been issued for some TKI since 2012, and DILI is the most frequent adverse event quoted. This study aimed to describe the most crucial aspects of DILI linked to TKI in the SLATINDILI registry.
Materials and MethodsWe revised data concerning liver injury related to any TKI in the SLATINIDLI registry and consigned epidemiological information, latency, implied drug, biochemical, severity, and evolution.
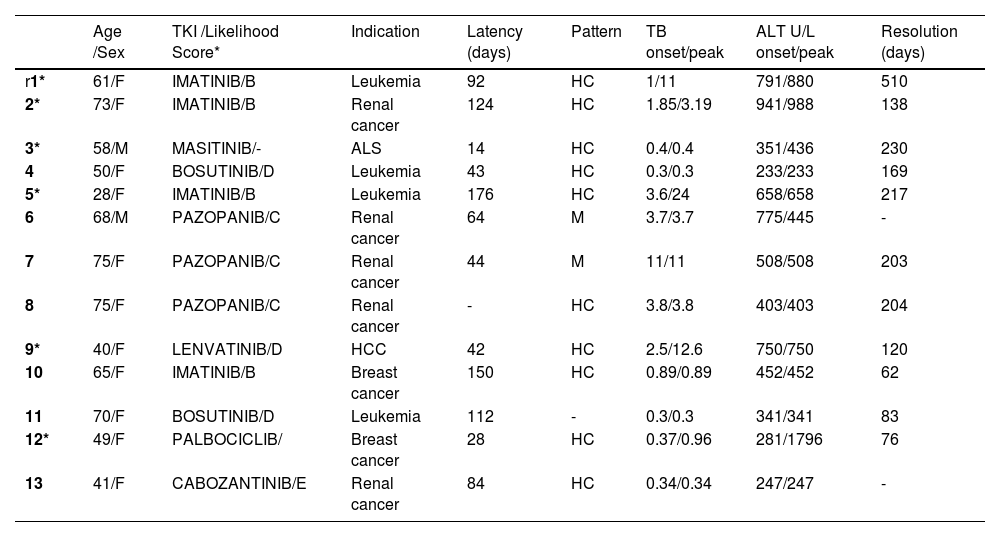
ResultsFrom thirteen cases identified, imatinib and pazopanib represented four and three cases each. The mean age was 58 years, and eleven were female. Median latency was 64 days, with median ALT and ALP at the onset of 452 U/L (range 233-941) and 199 U/L (range 85-1621), respectively; a hepatocellular pattern was seen in ten cases. Autoimmune/Allergic features were present in seven patients. Resolution of liver injury occurred on an average of 183 days. No death was consigned. Liver function tests (LFTs) worsened during an initial period (>7 days) after drug withdrawal in six patients (cases 1,2,3,5,9 and 12), and two of them were treated with corticoids. Table 1 resumes data.
ConclusionsHepatocellular acute liver injury with/without jaundice is the most common presentation of DILI linked to TKI. Clinicians should be aware that LFTs may worsen after drug withdrawal and monitor these patients before making a treatment decision.
Age /Sex
Table
*Likelihood of association with DILI, based upon the known potential of the drug to cause such injury. HCC hepatocellular carcinoma; ALS amyotrophic lateral sclerosis; HC hepatocellular pattern; M mixed pattern; M male; F: female.