Data on the prevalence of non-alcoholic fatty liver disease (NAFLD) in subgroups of the United States (US) population are limited. This study was conducted to estimate NAFLD prevalence overall and by subgroups, and prevalence of NAFLD with advanced fibrosis.
Materials and MethodsUsing the National Health and Nutrition Examination Survey (NHANES) 2011-2018 data, a cross-sectional study was conducted. NAFLD was defined as having a US Fatty Liver Index (USFLI) ≥ 30 in the absence of other causes of liver disease, including excessive alcohol intake, chronic hepatitis B, and chronic hepatitis C. Likelihood for having advanced fibrosis was determined by the calculated NAFLD fibrosis score (NFS; high ≥ 0.676; low < −1.445) and fibrosis-4 index (FIB-4; high ≥ 2.67; low < 1.30).
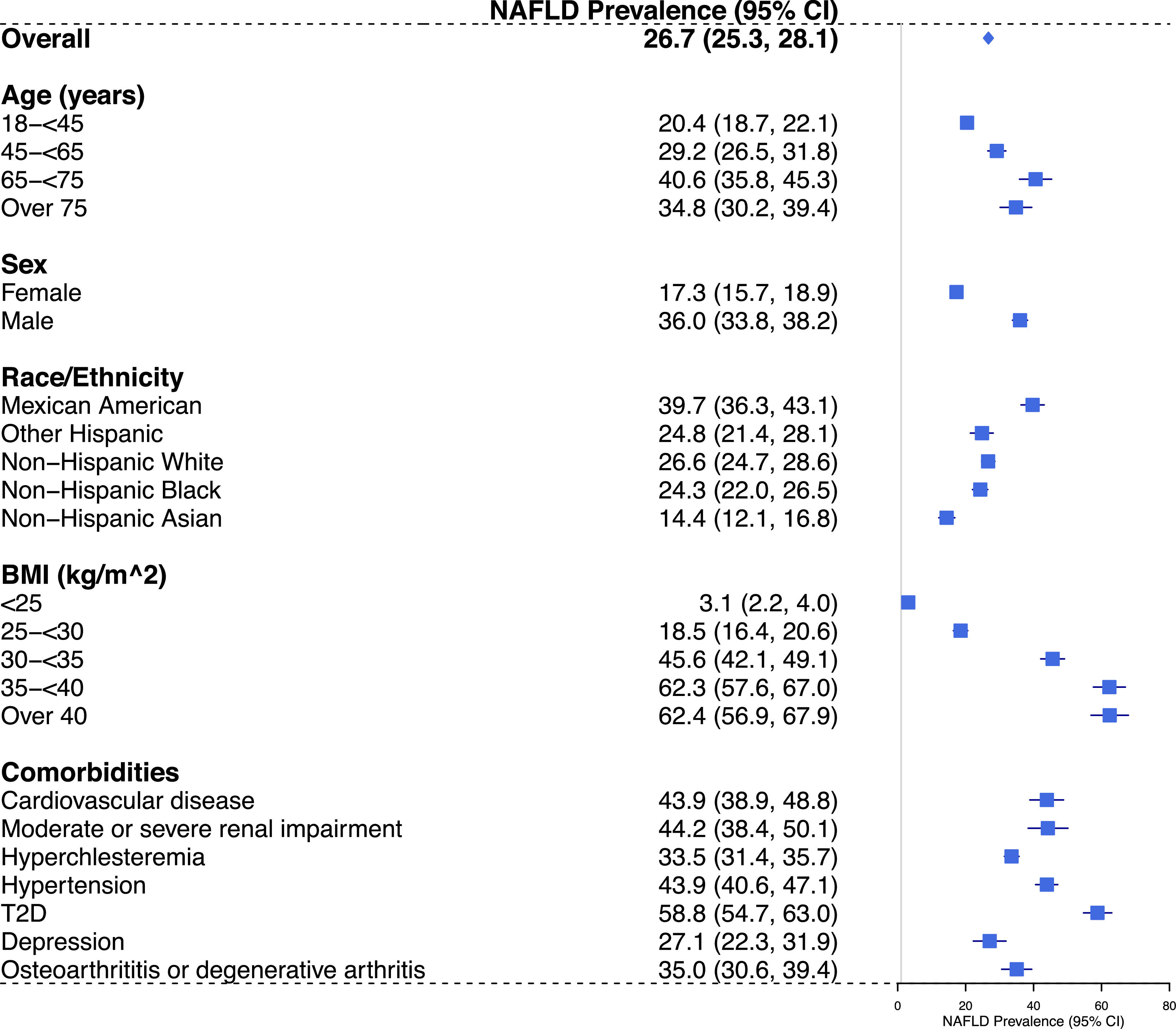
ResultsThe weighted national prevalence of NAFLD in US adults was 26.7% (95% confidence interval: 25.3%-28.1%). Prevalence was higher among those aged ≥ 65 years, males, Mexican Americans, with BMI ≥ 35 kg/m2 (class 2 and 3 obesity) and with type 2 diabetes (T2D). Of those meeting the USFLI criterion for NAFLD, 18.1% and 3.7% were determined as having a high probability of advanced fibrosis based on NFS ≥ 0.676 and FIB-4 ≥ 2.67 cut-off values, respectively.
ConclusionsThis study supports an increased prevalence of NAFLD in specific subpopulations (aged ≥ 65 years, males, Mexican Americans, obese population, and patients with T2D). The observed difference in the prevalence of advanced fibrosis as estimated by NFS and FIB-4 highlights the challenge of choosing optimal cut-off values.
Nonalcoholic fatty liver disease (NAFLD) is a condition of increased accumulation of fat in hepatocytes (steatosis) that is typically a consequence of obesity-associated insulin resistance and strongly associated with metabolic syndrome [1]. It is the fastest growing cause of liver transplantation in the United States (US), affecting approximately 25% of the global population [2]. In 2020, NAFLD nomenclature was proposed to be updated to metabolic dysfunction associated steatotic liver disease (MASLD), which better captured the etiology of disease [3].
NAFLD patients are typically asymptomatic and, therefore, are more likely to be identified initially based on risk factors (obesity, metabolic syndromes, type 2 diabetes (T2D)) [4] and/or abnormal liver tests without alternate explanation [5,6]. Several indices have been used to predict NAFLD such as Fatty Liver Index (FLI), which was originated in Italy and can be calculated with triglycerides, body mass index (BMI), gamma-glutamyl transpeptidase (GGT), and waist circumference (FLI = (e0.953×log_e (triglycerides) + 0.139 × BMI + 0.718 × log_e (GGT) + 0.053 × waist circumference −15.745) / (1 + e0.953 × log_e (triglycerides) + 0.139 × BMI + 0.718 × log_e (GGT) + 0.053 × waist circumference −15.745) × 100) [7], and it has been validated in population-based studies [8–12]. Another index, the US Fatty Liver Index (USFLI), has also been widely used. In addition to age, GGT, waist circumference, fasting glucose, and fasting insulin, this index contains ethnicity component in the calculation, making it more reliable than FLI in predicting NAFLD in the US that has multi-ethnic makeup of the population [13]. Using the USFLI, the estimated NAFLD prevalence increased from 18% in 1988-1991 to 29% in 1999-2000, based on the National Health and Nutrition Examination Survey (NHANES) data in the US, then has remained relatively stable at approximately 30% ever since [13–16]. However, data on the NAFLD prevalence in subgroups of the population are limited.
Fibrosis stages associated with NAFLD include no fibrosis (F0), perisinusoidal or portal fibrosis (F1), sinusoidal or periportal without bridging (F2), bridging fibrosis (F3), and cirrhosis (F4). F3 and F4 are considered advanced fibrosis, which is the major predictor of liver-related outcomes among NAFLD patients [17–19]. Liver biopsy, considered as a gold standard for determining fibrosis, is associated with complications, high cost, and feasibility issues, and therefore not routinely used for screening purposes in clinical practice. Noninvasive scoring systems have been developed to identify persons with advanced fibrosis using routinely collected data in clinical setting [20–23]. Among those, NAFLD Fibrosis Score (NFS) and Fibrosis-4 (FIB-4) index are the most validated. NFS is calculated using age, BMI, aspartate transaminase (AST)/alanine transaminase (ALT) ratio, platelet count, hyperglycemia, and albumin, whereas FIB-4 is based only on age, AST, ALT and platelet count. The scores employ two thresholds (cut-off value) to exclude or include advanced fibrosis. One threshold emphasizes high sensitivity (FIB: < 1.30; NFS: < -1.445), while the other emphasizes high specificity (FIB-4: ≥ 2.67; NFS: ≥ 0.676) [22,24–28]. Both of them have been endorsed by the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) to determine the need for additional diagnostic testing in NAFLD patients [24,29]. As such, both NFS and FIB-4 were chosen as the non-invasive assessment for determining the likelihood or probability for advanced fibrosis in this study.
The aim of this study was to provide an updated national estimate of the prevalence of NAFLD and NAFLD with advanced fibrosis in US adults, and subgroup prevalence of NAFLD based on age, sex, race/ethnicity, BMI, and absence/presence of comorbidities.
2Materials and Methods2.1Data sourceNHANES is a nationwide survey conducted in the U.S. by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) [30]. Details on sampling design have been fully described elsewhere (http://www.cdc.gov/nchs/nhanes.htm).
2.2Study populationThe NHANES 2011-2018 data were used for this study. Participants aged 18 years or older, with complete data on demographics, laboratory, and clinical information required for this study were included. Pregnant participants at the time of health examination were excluded.
2.3NAFLD and likelihood for having advanced fibrosis (F3 or F4)NAFLD was defined as having an USFLI ≥ 30 in the absence of other causes of liver disease, such as excessive alcohol intake (reported average standard drinks per day > 2 for males and > 1 for females), chronic hepatitis B (HBsAg +), chronic hepatitis C (HCV RNA +). The USFLI was calculated according to the formula below [13].
USFLI = [e−0.8073 × non-Hispanic black + 0.3458 × Mexican-American + 0.0093 × age + 0.6151×log_e (gamma-glutamyl transferase) + 0.0249 × waist circumference +1.1792 × log_e (insulin) + 0.8242 × log_e (glucose) − 14.7812]/ [1 + e−0.8073 × non-Hispanic black + 0.3458 × Mexican-American + 0.0093 × age + 0.6151×log_e (gamma-glutamyl transferase) + 0.0249 × waist circumference +1.1792 × log_e (insulin) + 0.8242 × log_e (glucose) − 14.7812] × 100
Likelihood for having advanced fibrosis (F3 or F4) was determined by NFS and FIB-4.
The NFS was calculated according to the formula below [22]. NAFLD patients were classified into three groups based on NFS, including those with low probability for advanced fibrosis (NFS < −1.445), intermediate probability (NFS −1.445 to <0.676), and high probability (NFS ≥ 0.676) [22].
NFS = [− 1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glycemia or diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet (× 109/L) − 0.66 × albumin (g/dL)].
FIB-4 score was calculated based the formula below [31]. Similar to NFS, NAFLD patients were classified into three groups by, including those with low (FIB-4 < 1.30), intermediate (FIB-4 1.30 to < 2.67) and high (FIB-4 ≥ 2.67) probability for advanced fibrosis [31].
FIB-4 = [age (years) × AST (U/L)]/[platelet [109/L] × (ALT [U/L])1/2].
2.4Demographics and comorbiditiesGeneral demographic characteristics collected during the interview included age (18 to < 45, 45 to < 65, 65 to < 75, and ≥ 75 years), sex, and race/ethnicity (Mexican American, non-Hispanic white, non-Hispanic black, non-Hispanic Asian, other Hispanic, or others). BMI was recorded during the physical examination. Seven chronic conditions were included in the analyses for their prevalence and previously established association with NASH: T2D [32], cardiovascular diseases (CVD) [33], depression [34–36], hypertension, hypercholesterolemia, moderate or severe renal impairment [37], and osteoarthritis, based laboratory results and questionnaire responses.
2.5Statistical analysisDescriptive statistics were reported as frequency and proportion for categorical variables that defined the subgroups of interest. The prevalence of NAFLD was calculated among the overall population, as well as within each pre-specified subgroup, along with corresponding 95% confidence intervals (CI). Weight was incorporated in the analyses to account for the complex survey design (including oversampling), survey nonresponse, and post-stratification in order to ensure that calculated estimates are representative of the U.S. population [38]. Statistical analyses were conducted using SAS, version 9.4.
2.6Ethical statementsWritten informed consent was obtained from each patient included in the study and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the National Center for Health Statistics Ethics Review Board (Protocol #2018-01).
3ResultsThe NHANES 2011-2018 sample included a total of 7,429 adult non-pregnant participants who had complete information on the variables of interest for this analysis, yielding a national projected population estimate of 84.3 million. Based on the national estimates, half of the study population (50.4%) were male, 67.6% were white, 37.3 were obese (BMI ≥ 30kg/m2) and 11.8% had T2D (Table 1).
Characteristics of the study population by NAFLD status, NHANES 2011-2018.
| Characteristics | Total study population (N=7,429) | NAFLD status | ||||
|---|---|---|---|---|---|---|
| Yes (USFLI ≥ 30) (N=2,048) | No (USFLI < 30) (N=5,381) | |||||
| n | Weighted % (SE) | n | Weighted % (SE) | n | Weighted % (SE) | |
| Age (years) | ||||||
| 18-<45 | 3,465 | 47.1 (0.8) | 708 | 36.0 (1.5) | 2,757 | 51.2 (0.9) |
| 45-<65 | 2,452 | 35.0 (0.8) | 769 | 38.2 (1.6) | 1,683 | 33.8 (0.9) |
| 65-<75 | 922 | 11.7 (0.5) | 369 | 17.8 (1.2) | 553 | 9.5 (0.6) |
| ≥75 | 590 | 6.2 (0.3) | 202 | 8.0 (0.7) | 388 | 5.5 (0.3) |
| Sex | ||||||
| Male | 3,712 | 50.4 (0.8) | 1,333 | 67.9 (1.4) | 2,379 | 44.0 (0.9) |
| Race/ethnicity | ||||||
| Mexican American | 1,031 | 8.6 (0.3) | 424 | 12.8 (0.7) | 607 | 7.1 (0.3) |
| Other Hispanic | 795 | 6.0 (0.3) | 221 | 5.6 (0.4) | 574 | 6.2 (0.3) |
| Non-Hispanic White | 2,892 | 67.6 (0.6) | 795 | 67.3 (1.2) | 2,097 | 67.6 (0.7) |
| Non-Hispanic Black | 1,563 | 9.8 (0.3) | 411 | 8.3 (0.5) | 1,152 | 10.2 (0.3) |
| Non-Hispanic Asian | 887 | 4.7 (0.2) | 135 | 2.5 (0.2) | 752 | 5.5 (0.2) |
| Other | 261 | 3.3 (0.3) | 62 | 2.8 (0.5) | 199 | 3.5 (0.4) |
| BMI | ||||||
| <25 | 2,312 | 30.4 (0.7) | 92 | 3.5 (0.5) | 2,220 | 40.2 (0.9) |
| 25-<30 | 2,358 | 32.3 (0.8) | 507 | 22.4 (1.2) | 1,851 | 35.9 (0.9) |
| 30-<35 | 1,502 | 20.8 (0.7) | 695 | 35.4 (1.5) | 807 | 15.4 (0.7) |
| 35-<40 | 716 | 9.4 (0.5) | 420 | 22.0 (1.3) | 296 | 4.9 (0.4) |
| ≥40 | 541 | 7.1 (0.4) | 334 | 16.7 (1.1) | 207 | 3.7 (0.3) |
| Comorbidities | ||||||
| Cardiovascular diseases | 766 | 9.0 (0.4) | 333 | 14.9 (1.1) | 433 | 6.9 (0.4) |
| Depression | 606 | 7.2 (0.4) | 182 | 7.3 (0.8) | 424 | 7.2 (0.5) |
| Moderate or severe renal impairment | 471 | 8.8 (0.4) | 195 | 9.5 (0.8) | 276 | 5.3 (0.4) |
| T2D | 1,147 | 11.8 (0.5) | 645 | 25.9 (1.3) | 502 | 6.6 (0.4) |
| Hypercholesterolemia | 3,709 | 51.0 (0.8) | 1,287 | 64.0 (1.5) | 2,422 | 46.2 (0.9) |
| Hypertension | 2,045 | 23.3 (0.7) | 815 | 38.2 (1.5) | 1,230 | 17.8 (0.7) |
| Osteoarthritis or degenerative arthritis | 812 | 12.6 (0.6) | 291 | 16.5 (1.2) | 424 | 11.2 (0.6) |
NAFLD, Non-Alcoholic Fatty Liver Disease; NHANES, National Health and Nutrition Examination Survey; SE, standard error; USFLI, The United States Fatty Liver Index; BMI, Body Mass Index; T2D, type 2 diabetes.
Of 2,048 adults meeting the USFLI criterion for NAFLD, 67.9% were male, 25.8% were aged ≥ 65 years, 74.1% were obese (BMI ≥ 30 kg/m2) and 67.3% were non-Hispanic white. Common comorbid conditions included hypercholesterolemia (64.0%), hypertension (38.2%), T2D (25.9%), and cardiovascular disease (14.9%) (Table 1). Compared with subjects not meeting USFLI criterion for NAFLD, NAFLD patients were more likely to be male, older, Mexican American, obese and have comorbid conditions, such as T2D, CVD, or hypertension (Table 1).
The national prevalence of NAFLD in US adults was 26.7% (95% CI: 25.3%-28.1%) using a USFLI cut-off of ≥ 30. As shown in Fig. 1, the prevalence of NAFLD was the highest among Mexican Americans; males had higher prevalence compared with females; those aged 18–44 years had higher prevalence compared with those aged ≥ 45 years. We also observed a strong association between BMI categories and the prevalence of NAFLD, with the highest prevalence observed among those with BMI ≥ 35 kg/m2 (class 2 and 3 obesity). Weighted estimates of NAFLD prevalence according to comorbidities are also shown in Fig. 1, with the highest NAFLD prevalence observed among those with T2D [58.8% (95%CI: 54.7–63.0%)].
Projected national prevalence (95% CI) of NAFLD among US adults, overall and in subgroups, NHANES 2011-2018. Abbreviations: BMI, body mass index; CI, confidence interval; FLI, fatty liver index; NAFLD, Non-alcoholic fatty liver disease; NHANES, National Health and Nutrition Examination Survey; SE, standard error; T2D, type 2 diabetes; USFLI, United States fatty liver index ; US, United States.
Of those meeting the USFLI criterion for NAFLD, 18.1% and 3.7% were determined as having a high probability of advanced fibrosis based on NFS ≥ 0.676 and FIB-4 ≥ 2.67 cut-offs, respectively. The proportion of subjects estimated to have a low probability of having advanced fibrosis also varied, based on NFS and FIB-4 (33.9% with NFS< −1.445 and 63.6% with FIB-4 < 1.30) (Table 2).
Fibrosis status based on NFS or FIB-4 cut-offs among those meeting the USFLI criterion for NAFLD in the NHANES 2011-2018.
| Probability of advanced (Stage 3/4) fibrosis | NFS | FIB-4 | ||
|---|---|---|---|---|
| Cut-off scores | n (%) | Cut-off scores | n (%) | |
| High probability | ≥0.676 | 369 (18.1%) | ≥2.67 | 75 (3.7%) |
| Intermediate probability | –1.445 – <0.676 | 977 (48.0%) | 1.30 – <2.67 | 668 (32.7%) |
| Low probability | <-1.445 | 690 (33.9%) | <1.30 | 1,298 (63.6%) |
| Total | 2,036 | 2,041 | ||
NFS, Non-Alcoholic Fatty Liver Disease Fibrosis Score; FIB-4, Fibrosis Index-4; USFLI, The United States Fatty Liver Index; NAFLD, Non-Alcoholic Fatty Liver Disease; NHANES, National Health and Nutrition Examination Survey.
In this study, the NAFLD prevalence in US adults was 26.7% based on data from NHANES 2011-2018. This is similar to prior NHANES analyses where a prevalence of approximately 30% was reported recently [13–16]. The study presents a stratified analysis of the prevalence of NAFLD in subgroups based on age, sex, race/ethnicity, BMI, and various comorbidities, and the findings support an increased prevalence of NAFLD in specific subpopulations [14,15,39,40]. The study presents a stratified analysis of the prevalence of NAFLD The study has demonstrated that the prevalence of NAFLD is higher in males and increases with age. Consistent with prior analyses [14,16,40], our data also suggest that Mexican American adults had the highest prevalence compared to other racial/ethnic groups. This may be related to genetic predisposition. Chincilla-Lopez et al have reported that polymorphism of the patatin-like phospholipase domain-containing protein 3 (PNPLA3) gene might be more common in native Mexicans, and this association places these individuals at higher odds for NAFLD (odds ratio, 1.711; 95% CI, 1.014-2.886) [41]. Another study based on the NHANES III data found that some single nucleotide polymorphisms were associated with particular races or ethnicities, such as the high prevalence of the SNP G allele of rs738409 of PNPLA3 in Mexican Americans [42]. Fleischman et al conducted a multi-ethnic study and reported that Hispanics of Mexican origin had a significantly higher prevalence of NAFLD, compared to Hispanics of Dominican and Hispanics of Puerto Rican origins, after adjustment for confounders [43]. The authors mentioned that PNPLA3 likely contributed to the observed differences in the subgroups of the Hispanic population. A number of risk factors predispose individuals to develop NAFLD, including obesity, T2D, hypertension, and hyperlipidemia [5,6,44]. Our analysis confirmed the high prevalence of NAFLD among those individuals, and the results are in line with previous cross-sectional studies [45–47].
Another important finding is the likelihood for having advanced fibrosis based on NFS and FIB-4 cut-offs in the NAFLD population. NFS and FIB-4 are recommended to rule-out advanced fibrosis in clinical practice [24–28,48]. In this study, we found that among those meeting the USFLI criterion for NAFLD in the NHANES 2011–2018, the proportions of having a high likelihood of advanced fibrosis were 18.1% based on NFS cut-off (NFS ≥ 0.676), and 3.7% based on FIB-4 cut-off (FIB-4 ≥ 2.67), respectively. Proportion of having intermedia/low likelihood of advanced fibrosis also varies based NFS and FIB-4. The observed difference in our study may be related to the difference in sensitivity and specificity of the two scoring systems based on the choice of cut-off values [49,50]. In addition to the cut-off values, patients’ age, liver function, and comorbidity conditions (e.g., diabetes, obesity) may also have an impact on the performance of the two scoring systems; cut-off values that work good for patients without certain conditions (e.g., non-diabetic patients) may not work as good for those with the conditions (e.g., diabetic patients) [51–55]. For those with diabetes or obesity, FIB-4 might be more useful to rule out advanced fibrosis [56]. Others reported that NFS and FIB-4 had similar capability to predict outcomes, yet FIB-4 may perform slightly better with higher sensitivity, based on individual participant data meta-analysis [56,57].
A study based on NHANES 2017-2018 data used results from transient elastography (FibroScan®), another non-invasive test, to predict NAFLD prevalence and fibrosis stage. It was reported that the prevalence of NAFLD and advanced fibrosis was 25.3% and 2.9%, respectively [58]. This was similar to the NAFLD prevalence estimate based on USFLI and advanced fibrosis prevalence estimate based on FIB-4 cut-off (FIB-4 ≥ 2.67) in our study using data that included subjects over a longer time frame (NHANES 2011-2018). However, as compared to USFLI, FIB-4 and NFS, transient elastography is less readily available.
Several limitations of our study should be noted. Noninvasive fibrosis indices (NFS and FIB-4) were used to assess the likelihood of having advanced fibrosis in NAFLD patients. We were not able to evaluate the predicative capability of the two scoring systems because of the lack of liver biopsy data. Furthermore, self-reported alcohol consumption was captured in the NHANES as the number of drinks per day, not the actual alcohol intake in grams. This could result in an underestimation of the true NAFLD prevalence, as people who had actual alcohol intake between 15-20 g/day could have reported as having 2 drinks per day and in turn, were not eligible for the NAFLD definition of this study. Besides, the NAFLD patients were identified based on USFLI calculated with anthropometric measurements and lab results collected during the NHANES, rather than formal diagnosis based on imaging or histologic findings, and therefore misclassification of disease status may exist. Lastly, as NHANES only sampled noninstitutionalized adults, the findings may not be generalizable to institutional settings such as nursing homes.
5ConclusionsIn conclusion, our findings extend previous national estimates of the prevalence of NAFLD and NAFLD with advanced fibrosis in US adults and support an increased prevalence of NAFLD in specific subpopulations. Male, Mexican Americans, and people with T2D and obesity were the most affected groups assessed in this study. The observed difference in the prevalence of advanced fibrosis as estimated by NFS and FIB-4 may be due to the cut-off values of choice. Future studies are warranted to validate the cut-off values of the two scoring systems for advanced fibrosis diagnosis in NAFLD patients with various comorbidity conditions (e.g., diabetes, obesity).
FundingThis analysis and article processing charges were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author ContributionsTongtong Wang, Yuzhi Xi, Annaswamy Raji, Michael Crutchlow, Gail Fernandes, Samuel S Engel, and Xiao Zhang are responsible for the work described in this paper. Tongtong Wang and Yuzhi Xi conceived, designed, and/or planned the study. Yuzhi Xi analyzed the data. Tongtong Wang, Yuzhi Xi, Annaswamy Raji, Michael Crutchlow, Gail Fernandes, Samuel S Engel, and Xiao Zhang interpreted the results. Tongtong Wang drafted the manuscript. Tongtong Wang, Yuzhi Xi, Annaswamy Raji, Michael Crutchlow, Gail Fernandes, Samuel S Engel, and Xiao Zhang critically reviewed and/or revised the manuscript for important intellectual content. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The authors acknowledge Dr. Ira Gantz for comments and suggestions in the earlier versions of this manuscript.