Background/objectives. The study evaluates the outcome of patients who performed orthotopic liver transplantation (LT) as treatment for hepatocellular carcinoma (HCC), with percutaneous ethanol injection (PEI) while on the waiting list, verifying the effectiveness of this treatment in producing tumor necrosis and avoiding dropout and identifying treatment-related complications.
Material and methods.Medical records of 97 patients on the waiting list for LT at Hospital Clinic of Barcelona were examined. Sixty-two (56.3%) patients had been treated with PEI (group 1); 35 (31.8%) had not received any anti-tumor therapy before LT (group 2).
Results.Complete necrosis of the tumor was observed in 38/59 (64.3%) patients. The presence of additional nodules in the explant and the diameter of the main tumor of group 1 was significantly lower than in group 2 (p = 0.002). Dropout related to tumor progression occurred in 4.8% and 8.5%, and tumor recurrence in 5% and 6.2% for groups 1 and 2, respectively. Major complications were not evidenced after 421 PEI sessions and there was no tumor implant in the needle traject.
Conclusions. In conclusion, the percutaneous treatment of HCC with PEI is a safe and effective method before the LT.
Hepatocellular carcinoma (HCC) is the fifth most common tumor in the world and the third cause of cancer-related mortality.1 Cirrhosis underlies HCC in more than 80% of affected individuals.2 The only options that can achieve long term control for HCC are surgical resection, liver transplantation (LT) and percutaneous ablation.3
Liver transplantation (LT) is the treatment of choice for patients with early HCC in advanced cirrhosis. However, when it is analyzed in an intention-to-treat manner, the existence of dropouts significantly decreases the long-term outcome of LT.
Dropout for tumor progression or death during the waiting list period varies according to the series analyzed and is around 11% and 38% for the waiting list of six months and one year, respectively.4
Locoregional treatment with Percutaneous Ethanol Injection (PEI) and Radiofrequency Ablation (RFA) can be considered during the waiting time period, since these techniques are able to delay tumor progression and decrease the risk of list exclusion.5 According to the American Association for the Study of the Liver Diseases guidelines,3 PEI is a safe and highly effective treatment for small hepatocellular carcinomas. PEI induces local tumor necrosis as a result of cellular dehydration, protein denaturation, and chemical occlusion of tumor vessels. The major advantages of ethanol ablation are its low cost and low rate of complications. Ethanol injection remains a widely used and effective option for patients waiting for LT. The main limitation of this technique is the presence of fibrous septa inside the lesion, which limits the spreading of ethanol; most of the times, multiple sessions are needed to achieve a complete response.
The advantage of RFA is real in tumors larger than 3 cm. Furthermore, RFA uses larger needles, and some tumors located near to the main biliary tree, abdominal organs or heart represent contraindications, however are not absolute contraindications, for its application.6 Tumor seeding can be detected in approximately 10% of the cases of subcapsular nodules treated with RFA.7 Poor differentiation degree can be other risk factor of neoplastic seeding after RFA.8 In order to decrease the risk of neoplastic seeding the tract ablation must be routinely practiced at completion of tumor RFA. Considering that RFA treatment is much more expensive than PEI, many institutions use PEI as the primary option of percutaneous treatment in patients with HCC. Accessibility to the lesion and the costs may influence the decision of using either RFA or PEI.
Nevertheless, some questions related to treatment with PEI in the pretransplant period remain. For instance: does it reduce the risk of dropout during the waiting list for LT? Despite being a local treatment, does percutaneous ablation increase the risk of intrahepatic seeding? Does it increase the risk of tumor recurrence in the posttransplant period? And, finally, are treatment-related complications significant?
In the literature, there are no randomized controlled trials comparing any treatment option and no treatment at all on waiting lists; thus, there is no strong evidence showing that a given intervention is effective to prevent tumor progression and exclusion from the list.
Some studies9-12 have analyzed the treatment impact in patients on the waiting list for LT; however, the samples in these studies were not homogeneous because they used different types of treatment, such as PEI, RFA and Transarterial Chemoembolization (TAE), which makes it difficult to evaluate the real benefits of these individual interventions.
We conducted a retrospective study in order to analyze the outcome of a group of patients treated with PEI before LT in relation to complications of the percutaneous treatment and efficacy of PEI in the production of tumor necrosis. We compared this group to another group who did not receive any antitumor treatment during the pre-LT period. The following end points were compared: recurrence, dropout and survival.
Material and MethodsThe medical records of 97 patients with HCC who were on the waiting list for LT between August 2001 and January 2005 were analyzed; all patients were visited at the Ultrasound Unit of Hospital Clinic of Barcelona. Sixty-two (56.3%) patients performed HCC percutaneous treatment guided by ultrasound with PEI (group 1) and 35 (31.8%) did not receive any treatment before LT (group 2). Mean follow-up period after LT was 23.5 months (1-46 months) in group 1 and 36.5 months (1-46 months) in group 2. Patients with neoplastic nodules who received RFA, TACE or multiple different modalities of treatment were excluded from the study.
Patients’ characteristics, etiology and severity of liver disease are shown in table 1.
Patient characteristics.
| Patients | Group 1 (%) n = 62 | Group 2 (%) n = 35 |
|---|---|---|
| Gender | ||
| Male | 39 (62.9) | 28 (80.0) |
| Female | 23 (37.1) | 7(20.0) |
| Mean age (variation) | 58.8 (36-69) years | 57.4 (36-67) years |
| Etiology of chronic hepathopathy | ||
| Hepatitis C with or without alcohol | 47 (75.8) | 26 (74.2) |
| Hepatitis B | 3 (4.8) | 4 (11.4) |
| Hepatitis B and C | 3 (4.8) | 1 (2.8) |
| Alcohol | 7(11.2) | 4 (11.4) |
| Primary biliary cirrhosis | 1 (1.6) | 0 |
| Cryptogenic | 1 (1.6) | 0 |
| Child-Pugh | ||
| A | 22 (35.4) | 18 (51.4) |
| B | 34 (54.8) | 13 (37.1) |
| C | 6 (9.6) | 4 (11.4) |
All patients met the Milan criteria, i.e., they either had a single tumor of 5 cm or less or three tumors of 3 cm or less, without any evidence of vascular invasion or extrahepatic spread. In total, 79 HCC nodules were treated with PEI.
The HCC diagnosis was confirmed with biopsy in 55/62 (88.7%) in group 1 and in 21/35 (60%) in group 2. In 21 (21.6%) other cases (seven patients in group 1 and 14 in group 2) the HCC diagnosis was based on noninvasive methods according to the European Association for the Study of the Liver2 and defined as intense arterial uptake with contrast washout in the venous/delayed phase in two available dynamic modalities (contrast ultrasound, computed tomography, and magnetic resonance imaging). Hepatocellular carcinomas characteristics are shown in table 2.
Hepatocellular carcinomas characteristics.
| HCC | Group 1 n = 62 | Group 2 n = 35 |
|---|---|---|
| Number of nodules | ||
| 1 | 45 (72.5%) | 21 (60.0%) |
| 2 | 15 (24.1%) | 10 (28.5%) |
| 3 | 2 (3.2%) | 4 (11.4%) |
| Tumour size | 25.8 mm | 26.1 mm |
| (rate: 13-50 mm) | (rate: 13-50 mm) | |
| Degree differentiation | ||
| Well | 27 (43.5%) | 8 (22.8%) |
| Moderate | 18 (29.0%) | 8 (22.8%) |
| Poor | 1 (1.6%) | 0 |
| Not available | 16 (25.8%) | 19 (54.2%) |
| AFP (ng/mL) | ||
| 0-10 | 30 (48.3%) | 13 (37.1%) |
| 10-100 | 24 (38.7%) | 17 (48.5%) |
| 100-1000 | 8 (12.9%) | 3 (8.5%) |
| > 1000 | 0 | 2 (5.7%) |
All the proceedings were performed in inpatients. All tumors were treated by board-certified abdominal interventional radiologists (R.V, L.B). In all cases, PEI was performed with local anesthesia with lidocaine 2%, using a 22-gauge needle, a 5-mL syringe and absolute ethanol, with real-time ultrasound (US) guidance using a 4.0 MHz sector-probe (Acuson Sequoia 512). Gray-scale imaging was used for continuous monitoring at the local area of ablation during the ethanol injection. Sedation with fentanyl IV was reserved to cases in which the nodule was superficial and it was performed under the control of an anesthesiologist.
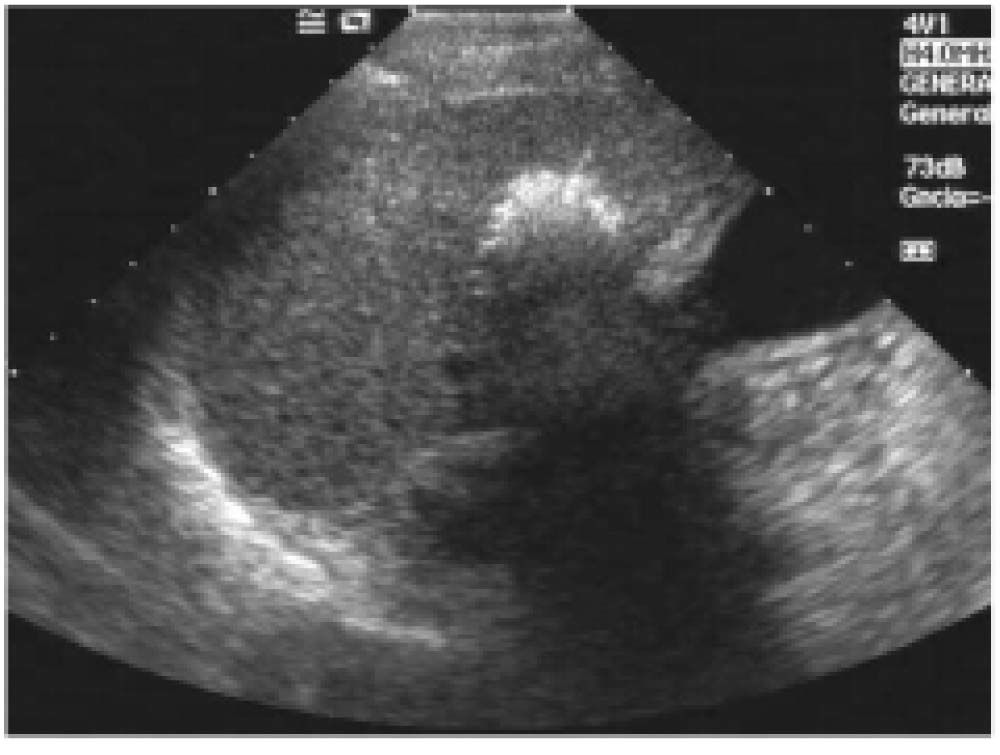
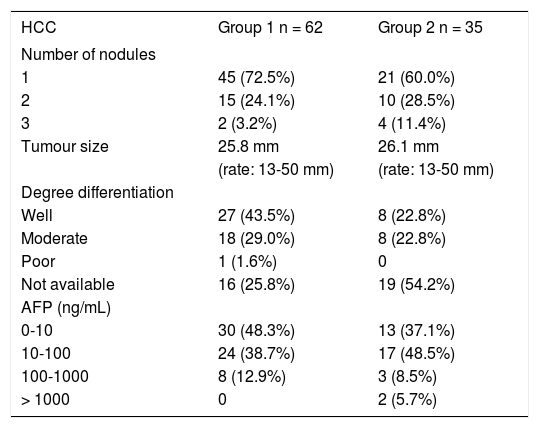
Injections were repeated in different tumor areas until they appeared completely hyperechoic (Figure 1). The amount of ethanol used varied according to the size of the lesion and the compliance of the patient (mean 2-3 mL per session). The procedure was repeated for up to four or five sessions per week completing one treatment cycle. A mean of 6.79 sessions (range: 1-21 sessions) was performed in each patient.
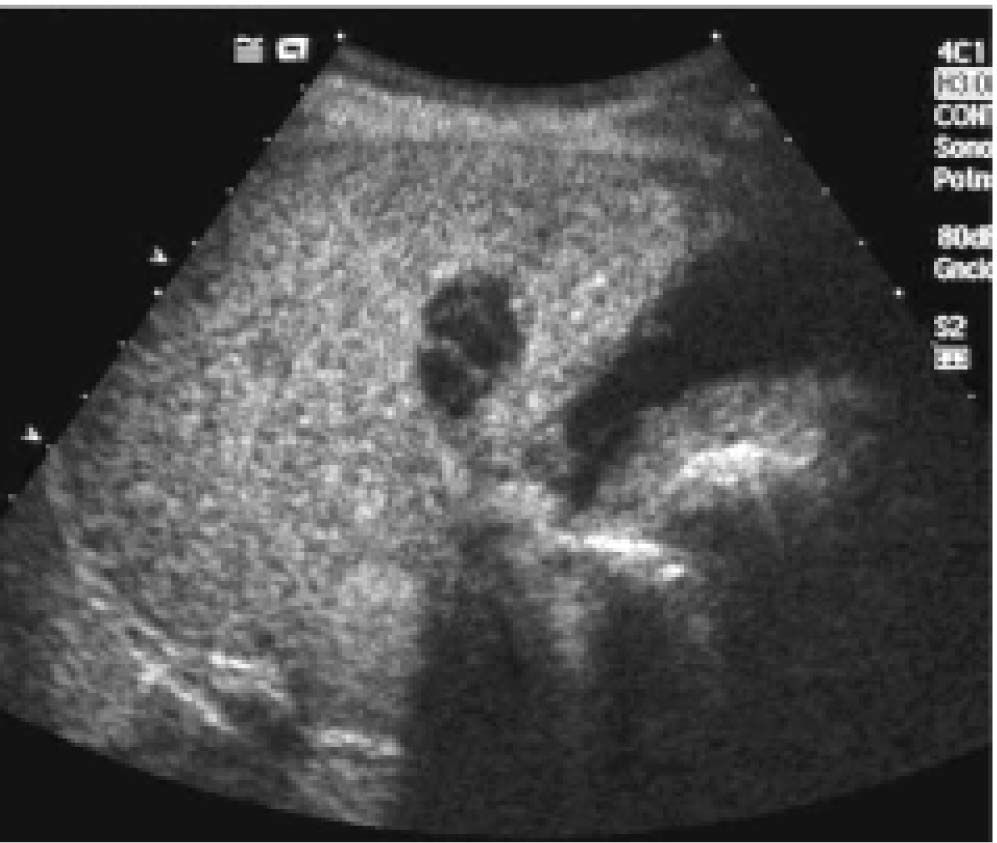
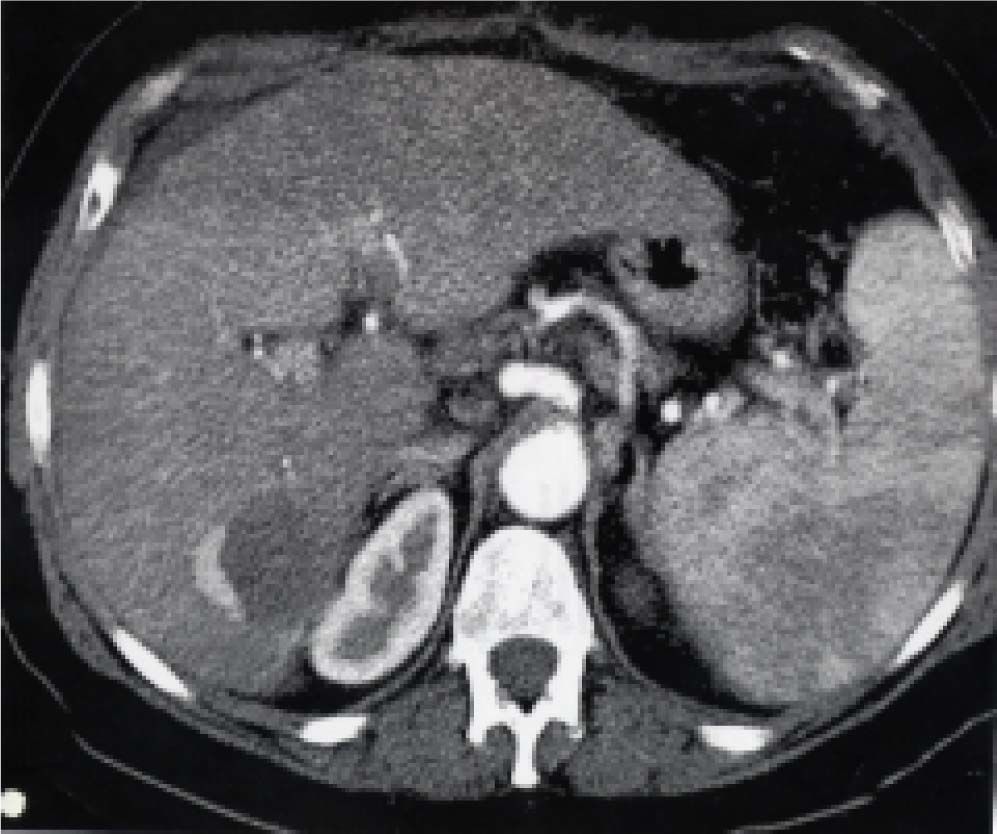
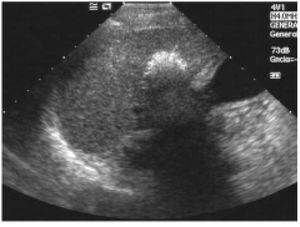
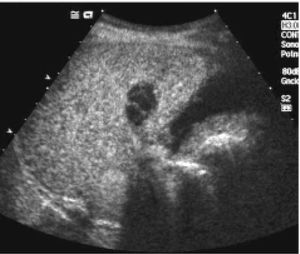
Posttreatment imaging control was repeated with contrast-enhanced US (Sonovue, Bracco, Italy) and contrast-enhanced dual-phase computed tomography (CT) or gadolinium-enhanced magnetic resonance imaging (MRI) at the end of the first month and every three months after ablation until the LT. Complete response was defined as the absence of enhanced tumoral areas showing complete tissue necrosis (Figure 2). Any focus of abnormal enhancing tissue, within or along the margin of the ethanol injection zone, was considered a residual tumor, and then a new cycle of treatment was performed (Figure 3).
Explanted liversAll explanted liver specimens were fixed in formalin and processed using routine protocol, consisting of 0.5-1 cm sections on transverse planes. The liver graft was investigated for HCC nodules, the presence of satellite nodules (i.e., small tumors localized < 1 cm from the main HCC), and microvascular invasion. Complete histological response was defined as the absence of tumor cells in the treated zone.
A written informed consent was obtained from all patients before PEI.
Statistical methodsStatistical analysis was performed using SPSS statistical software 13.0. Continuous variables were analyzed using either Student’s t-test or ANOVA. Categorical variables were analyzed using Fisher’s test. Survival was analyzed using the log-rank test. Statistical significance was considered when p < 0.05 and CI = 95%.
ResultsSeventy-nine HCC nodules in 62 liver transplantation candidates were treated with PEI.
Mean time on the waiting list was eight months (range: 1-15 months). Exclusion from the list during the waiting period for LT happened in 3/62 (4.8%) patients from group 1 and in 3/35 (8.5%) patients from group 2, all due to tumor progression which exceeded the limits established by the Milan criteria. Proportionally there were more dropouts in group 2; however, the difference was not statistically significant (p = 0.46).
Of the 59 transplanted patients in group 1, the mean maximal diameter of treated nodules was 24.1 mm (range: 5-55 mm) at the latest available imaging follow-up (mean: 2.8 months) before transplantation.
Complications after PEI treatmentIn a total of 421 PEI sessions, no major complications were detected. Minor complications occurred in 10 (2.37%) cases. Segmentary or partial portal thrombosis happened in seven cases; self-limited hemoperitoneum occurred in one case, and transaminases increased (more than 10-fold the normal level) with no clinical relevance in other two cases.
No deaths or dropouts occurred as a result of the treatment. No evidence of tumor seeding in the needle track was reported during laparotomy or follow-up related to biopsy or PEI.
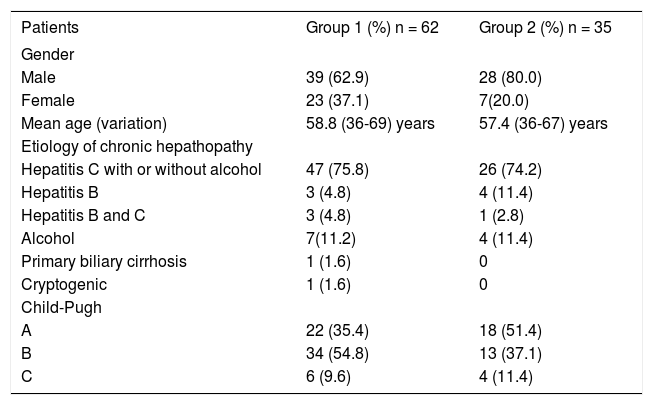
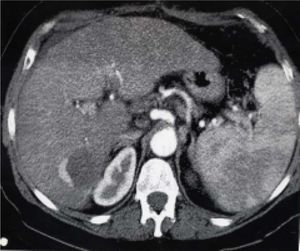
Characteristics of tumor on explanted liversFifty-nine explanted livers were analyzed in group 1 and thirty-two in group 2. In group 1, complete tumor necrosis without histological evidence of viable carcinoma or just a minimum quantity of cells was observed in 38/59 (64%) patients (Figure 4). In 14 patients (23%), there were less than 50% of viable cells, considering the total diameter of the lesion, and in seven patients (12%) there was more than 50% of viable tumor.
The mean maximal diameter of the tumor in explanted liver was 21.6 mm in group 1 and 32.2 mm in group 2 (p = 0.03).
Microvascular invasion or satellite nodules were present in 23/59 in group 1 patients (38.9%) and in 19/32 in group 2 patients (59.3%). The presence of additional HCC nodules in the explant of group 1 patients was significantly lower when compared to group 2 patients (p = 0.002).
When the number of tumors presents in the explanted livers was compared to the number radiologically verified before LT, it was shown that the patients were understaged in 19/59 (32.2%) cases in group 1 and in 21/32 (65.5%) cases in group 2. There was not any case of upstaging.
HCC recurrence after LTThree patients from group 1 (5.1%) and two from group 2 (6.2%) showed tumor recurrence after LT (p = 0.81).
In group 1, the mean time of follow-up was 23.5 months, with a minimum of one month for a patient who died 30 days after LT for neoplastic dissemination during surgery (tumor adhesion to the diaphragm). Recurrence was intra-hepatic in the other two patients, 16 and 29 months after LT. Recurrences were all confirmed with nodule biopsy. The longest follow-up in group 1 was 46 months.
In the first case, two nodules were treated with 12 sessions of PEI; in the second case, one nodule was treated with five sessions; and in the last case, two nodules were treated with nine sessions. Two patients died, and one was alive until the end of the follow up.
In all recurrence cases, explants showed at least four nodules; the largest tumor in each case had 65 mm of diameter in the first case, 35 mm of diameter in the second case, and 80 mm of diameter in the third case. All cases had microvascular invasion and satellite nodules in their explant. Then, it is correct to state that all the recurrence cases had been understaged.
In group 2, recurrence was diagnosed in two cases (6.2%) one and 19 months after LT. In the first case, the explant showed nine nodules; the largest had 50 mm, and death took place one month after LT due to disseminated HCC. In the second case, five tumors were seen in the explant, the largest had 22 mm; this patient was alive until the end of the follow-up. Both cases presented microvascular invasion and satellite nodules in their explants.
Survival rate after liver transplantationIn the follow-up period, 11/59 (18.6%) patients from group 1 died. Five of these patients died of postoperative complications. One died of lung cancer; one of lymphoma; one of lung Aspergillus infection; and one of hepatorenal syndrome after HCV recurrence; deaths occurred 36, 7, 18 and 36 months after transplantation, respectively. The two other died from HCC recurrence one and 29 months after LT. Intention to treat analysis from the time of entering in the waiting list showed that 14/62 (22.5%) patients died; of these, 5/62 (8%) died from HCC progression. In group 2 transplanted patients, 7/32 (21.8%) died until the end of follow-up. Four died of infectious diseases at 26, 7, 13 and three months after LT; two died of complications related to the surgical procedure, and one died of tumor progression. The intention to treat analysis showed death in 10/35 (28.5%) patients and tumor progression in 4/35 (11.4%). No statistically significance was found in survival between the two groups (p = 0.79). After LT, estimated 3-year survival of group 1 and 2 was 67.7% and 77.4%, respectively (p = 0.29), and there was a decrease to 64.4% and 70.7% in the 3-year survival in groups 1 and 2, respectively, when dropout cases were considered (p = 0.48).
DiscussionFor patients with early HCC in the setting of advanced cirrhosis, the best treatment option is liver transplantation. This treatment provides excellent outcomes if its indication is restricted to patients with early stage disease as defined by the Milan criteria.13 However, the main problem is the lack of organs due to the scarce number of donations. Even more, transplant lists are filled with severely sick patients and the velocity of donations does not follow patients’ inclusion; therefore, in many centers, the average time on the waiting list for LT is over one year. During this time, tumors of patients with HCC may progress, and any tumor growth increases the risk of microvascular invasion and satellite nodules, impeding LT. Hence, intention-to-treat survival is significantly reduced when the waiting time is too long (more than six months); as a result, most groups treat HCC before LT.14
Percutaneous ablation with radiofrequency and ethanol injection therapy have been used for local control of HCC and provide an effective bridge to transplantation over a prolonged waiting period.10,14,15
The radiofrequency ablation has emerged as the most effective method for local tumor destruction, but the results of the studies do not provide significant improvement in survival favoring RFA over PEI.6,16-18 The present study has special importance for centers that perform PEI as the primary option of percutaneous treatment, considering the high cost of radiofrequency, sometimes unfeasible for many institutions.
PEI is a highly effective treatment for HCC smaller than 3 cm and provides an initial complete response in more than 80% of cases.19,20 The rate of initial complete responses is an independent predictor of survival in HCC patients treated with PEI, and tumor size has been considered the main factor to determine the efficacy of this treatment.21
The effectiveness of percutaneous treatments for HCC depends on the induction of necrosis of the tumor after the procedure and absence of local recurrence. However, the main objective of PEI for patients on the waiting list for LT is to control tumor growth, thus avoiding complications and preventing exclusion from the waiting list.
The rate of dropout of the waiting list due to advanced disease is around 25%.4 The evaluation of the dropout rate must consider the kind of selection of patients for LT used. Therefore, Castroagudin, et al.22 analyzed the explant of 19 patients with HCC < 5 cm in diameter treated with PEI before LT, and in two cases (10.5%) dropouts occurred due to tumoral progression. Using the TNM classification, including patients with advanced tumors (> 5 cm), dropout rate was 5/41 (12%) on a nine-month waiting list in another study using multimodality treatments,11 on the other hand, there was no dropout considering only patients classified as T1. We described 4.8% of dropout in our series of treated patients following the Milan criteria, with a mean time of eight months on the waiting list. Although there was no significant difference between groups, lower dropout rate was seen in the treated group.
Severe complications of PEI can be detected in 2.2% of cases,23 as well as tumor seeding.24 In the present study, no tumor seeding and no major complications were observed after 421 sessions of PEI. It is important to make it clear that this study included only BCLC stage A cirrhotic patients, once BCLC stages B, C and D patients are not candidates for curative therapies, such as LT. Despite having early stage tumors, more than 50% of the patients of the present study were Child-Pugh class B and almost 10% of the sample was Child-Pugh class C, which denotes an important liver dysfunction.
Moreover, in the present study, we demonstrated not only the safety of percutaneous treatment with PEI, but also the efficacy of this therapeutic method in the control of hepatic neoplastic disease, since significantly more nodules were seen in the explant of the non-treated group, and the diameter of the main nodule was smaller in the treated group. This finding could be related to a possible beneficial effect of the treatment in the control of tumor progression, slowing down the appearance of satellite nodules.
Tumor recurrence after LT is not uncommon; mean recurrence rates are 10-20% in five years. In this study, 3/59 patients in group 1 (5%) and 2/32 patients in group 2 (6%) presented HCC recurrences in the posttransplant follow-up. The presence of microvascular invasion and satellite nodules in the explant of all recurrence cases confirms that these findings are the most important predictors of recurrence as previously reported.25 Besides, the liver explant of the five patients who presented with tumor recurrence after LT demonstrated that their neoplastic disease exceeded Milan criteria; therefore, these patients should have been excluded from the waiting list if their radiological studies had detected the actual number and size of the tumors.
Studies of explant pieces have demonstrated that image methods performed in the pre-LT period not only fail to diagnose small tumors (> 20 mm), but also underestimate the diameter of the main nodule.10,22,26 In the present study, 32% of patients in group 1 and 65% of patients in group 2 had more HCC nodules in their explant than previously diagnosed by CT. This should be considered when one discusses the expansion of Milan criteria to include patients with more and larger tumors, since this criteria consider both the dimensions and the number of HCCs based on image methods and not on explant findings.27-29
We verified that the survival rate after LT was lower when dropout cases due to tumor progression during the waiting period were included in the analysis. In the present study, mean survival based on intention-to-treat analysis was 37 months for group 1 and 48 months for group 2; no statistical difference was found. When we considered the analysis based on intention-to-treat, we found that 5/62 (8%) patients treated with PEI died from HCC progression. In the Markov model5 applied to the analysis of patients on a waiting list to LT, the percutaneous treatment with PEI was cost-effective and increased the seven-year survival in all waiting times before LT. Yao, et al.10 have show that preoperative loco-regional therapy, utilizing a multimodality treatment regimen, may confer a survival benefit after LT in patients with T2 and T3 HCC, when compared to a group without treatment (5-year recurrence-free survival 93.8% vs. 80.6%; p = 0.04).
We believe that, since this was a retrospective, case-controlled study, some of our finding could be the result of inherent flaws that could have caused a selection bias, such as a shorter time of follow-up for group 1 in relation to group 2 (23.5 months x 36.5 months) and a larger number of patients from group 2 who had their tumors understaged, when comparing their diagnosis before the LT and the explant. Even so, the presented data may help to guide the decision of using PEI in patients with HCC before LT.
In conclusion, although no difference was found between the survival rates of the two groups, we can conclude that percutaneous treatment of HCC with PEI is a safe and effective method in a BCLC stage A cirrhotic population, who met Milan criteria, since a significantly smaller number of additional nodules in the explant of the treated group and a smaller diameter of the main nodule were detected. PEI was confirmed as a possible bridge to liver transplantation, since the rate of dropout and tumor recurrence in explanted liver was low. Furthermore, no tumor seeding and no major complications were observed during pretransplant period. Finally, further studies are needed to evaluate the influence of tumor necrosis after PEI on dropout rates and on overall survival of these patients.
Abbreviations- •
AASLD.American Association for the Study of Liver Disease.
- •
AFP. Alfafetoprotein.
- •
BCLC.Barcelona Clinic Liver Cancer.
- •
HCC. Hepatocellular carcinoma.
- •
EASL.European Association for the Study of the Liver.
- •
PEI. Percutaneous ethanol injection.
- •
TAE. Transarterial Chemoembolization.
- •
RF. Radiofrequency.
- •
MRI. Magnetic Resonance Imaging.
- •
CT. Computed Tomography.
- •
LT. Liver transplantation.
- •
US. Ultrasound.
- •
HCV. Hepatitis C virus.