The aim of this study was to investigate the effects of the herbal compound YHK on hepatocarcinogenesis induced by diethylntrosamine (DEN) in Sprague Dawley rats. Rats were randomly divided into 3 groups and followed up for 15 weeks. Groups 1 was given standard food and represented the healthy control. Liver preneo-plastic foci were induced using the DEN method in groups 2 and 3 (20 rats each). However, group 3 was concomitantly given 50mg/kg/day of YHK. For quantitative assessment of liver preneoplastic foci, the placental form of glutathione-S-transferase (GST-P) positive foci were measured using immunohistochemical staining and image analysis. Treatment using DEN caused a significant decrease in body weight and increase in liver weight compared to the control group while concomitant supplementation with YHK prevented body weight loss and liver weight increase. As compared to DEN-only treated rats, the group given YHK showed a significant decrease in the number, size and volume of GST-P-positive foci. Moreover, co-administration of YHK significantly reduced the incidence, number, size and volume of hepatocellular carcinoma. Anti-inflammatory, anti-fibrotic as well as antioxidative properties of this compound are mechanisms which are likely to be advocated for to explain its protective effect. It is concluded that herbal compound YHK by preventing hepatocarcinogenesis in DEN-induced liver preneoplastic lesions in rats has the potential to a large clinical application as a functional food.
Nowadays there is a concern over the possible involvement of xenobiotics in carcinogenesis and this has led to a solid research stream in both humans and rodents. Indeed, DNA can be damaged along the whole process of absorption of carcinogens into the body, distribution to most sensitive tissues, metabolism which gives rise to a further form reacting with DNA, detoxification, and excretion. There are many genotoxic carcinogens occurring naturally in our environment, including the large group of heterocyclic amine mutagens.1,2 For instance, 2-amino-3,8-dimethylimidazo[4,5-f] quinoxaline (MelQx), one of food-derived agent, is able to induce in rats DNA adduct formation in the liver3 while an overt hepatocellular carcinomas might develop with treatment at high doses.4 In clinical practice, it has been shown that the human daily intake of MelQx is estimated to be 0.2-2.6 μg/subject5 and this substance can be recovered and quantified in the urine of healthy volunteers after eating cooked meat.6-8 However, most important, MelQx-DNA adducts have been found in kidney and colon tissues in man.9 Besides the primary importance of the detection and removal of such potential carcinogenetic agents, a protective dietary approach would represent an ideal countermeasure. Indeed, against the constant exposure to xenobiotics, several protective natural compounds have been proposed while only few have proven to possess a validated property in experimental tests. We have previously shown either in vitro and in vivo experimental studies that YHK exerts a potent protective effect on hepatocellular damage and on liver microcirculation in an ischemia-reperfusion model10,11 as well as exerting potent in vitro protective effect on metal-induced oxidative stress of hepatocytes.12 Indeed, on the clinical ground, to the contrary of many herbal remedies experimentally tested, the present phytothera-peutic compound has shown to significantly lower within two-three weeks the ALT level in the majority of HCV-related chronic liver disease patients and, moreover, to decrease Maruyama score in an awarded pilot clinical study.13 Quite recently, a separate canadian group has shown its therapeutic effect in patients with non-alcohol steatohepatitis who had been studied either biochemically and histologically.14 The rat liver represents an ideal model to study the whole sequence of cancer initiation and development since within a few days after administration of various genotoxic hepatocarcinogens single hepatocytes express placental glutathione S-transferase [single placental glutathione S-transferase-positive (GST-P) cells].15,16 A separate population of such GST-P single cells develop into GST-P foci, which increase further in number and size on treatment with tumour promoters to a final transformation into GST-P tumours. Therefore, single GST-P cells are considered ‘initiated’ and their number as well as the number and size of GST-P foci can be used as quantitative indicators of subsequent cancer risk15 although not all positive liver foci may necessarily develop tumours. Nonetheless, there is an over 90% correlation between the two events while the assay also correlates with the incidence of hepatocellular carcinomas in parallel long-term studies.17 Given these premises, the aim of the present study was to test the potential protective effect of YHK in the early stage of chemical hepatocarcinogenesis.
Materials & methodsSprague Dawley rats were housed and maintained in 12hour light/dark cycles at 23° with a humidity of 60%/10% in an environmentally-controlled vivarium (temperature, ventilation, humidity and light-dark cycle) and with free access to deionized water and non-nutrient fibers ad libitum. The animals were allowed to acclimatize for 15 weeks.
Experimental protocol Sixty rats were randomly divided into 3 groups of 20 rats each and treated as follows until the end of the experiment: Group 1 was given regular chow pellet and served as healthy control; Group 2, given standard chow pellet and Group 3, given the standard chow pellet containing YHK (panax pseudo-ginseng, Eucommia Ulmoides, polygonati rhizome, glycyrrhiza licorice, panax ginseng, Kyotsu Jigyo, Tokyo, Japan) calculated as to assure a daily intake of 50mg/kg, represented the hepatocarcinogenesis model. Then, they received a single intraperitoneal injection of diethylnitrosamine (DEN) (200 mg/kg/bw in saline), as described by Solt and Farber18 with minor modification. The proper mixture between standard food and powdered YHK was prepared each day and the food trays were checked every day, cleared of debris, weighed and filled.
Histopathological analysis and Glutathione 5-transferase placental form (GST-P) staining and counting At the end of the 15-week study period, rats were sacrificed and macroscopic examination was performed to detect any external pathology. Livers were then excised and weighed. Then, 5 mm-thick slices were cut from each lobe in individual rats and quickly fixed in cold acetone (0-4°C) for 6h. The slices were then embedded in paraffin for subsequent immunohistochemical examination of GST-P. GST-P foci (defined as lesions of the cells of more than 0.01 mm2 in area) were assayed by an immuno-histochemical method using a streptavidin-biotin-peroxidase complex (ABC) as described by Hsu et al.19 Briefly, after being deparaffinized with xylene, quenched with hydrogen peroxide and blocked with normal serum, the liver tissue sections were treated sequentially with normal goat serum, anti-rabbit GST-P antibody (1:2000), biotin-labeled goat anti-rabbit IgG (1:400) and finally with ABC. The diaminobenzidine method20 was used to demonstrate the sites of peroxidase binding. For quantitative assessment of lesions it was considered: the number of GST-positive foci/cm2, the percentage of section area occupied by the foci and diameters of GST-P-positive foci and nodules >0.2 mm, as described elsewhere21,22 by using an image analyzer. Liver lesions were diagnosed according to the criteria described by Squire and Levitt23 and the descriptions given following the guidelines of the Institute of Laboratory Animal Resources.24
Statistical analysisResults are expressed as mean±s.d. Statistical analysis was performed using an SPSS programme for Windows XP. The differences between groups were evaluated using one way analysis of variance, followed by Dunnette’s test for pair-wise comparison and Tukey’s family error rate. In all cases, p<0.05 was considered as the minimum level of statistical significance.
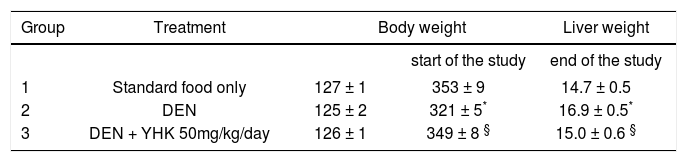
ResultsBody and liver weightAll the rats survived in good condition until the scheduled sacrifices. Treatment with DEN significantly decreased the body weight and increased the liver weight compared to the control group (p<0.05) (Table I). Oral supplementation YHK proved to prevent DEN-induced body weight loss and liver weight increase (p<0.05).
Body and liver weight changes during den-induced hepatocarcinogenesis: Effect of concomitant supplementation with YHK
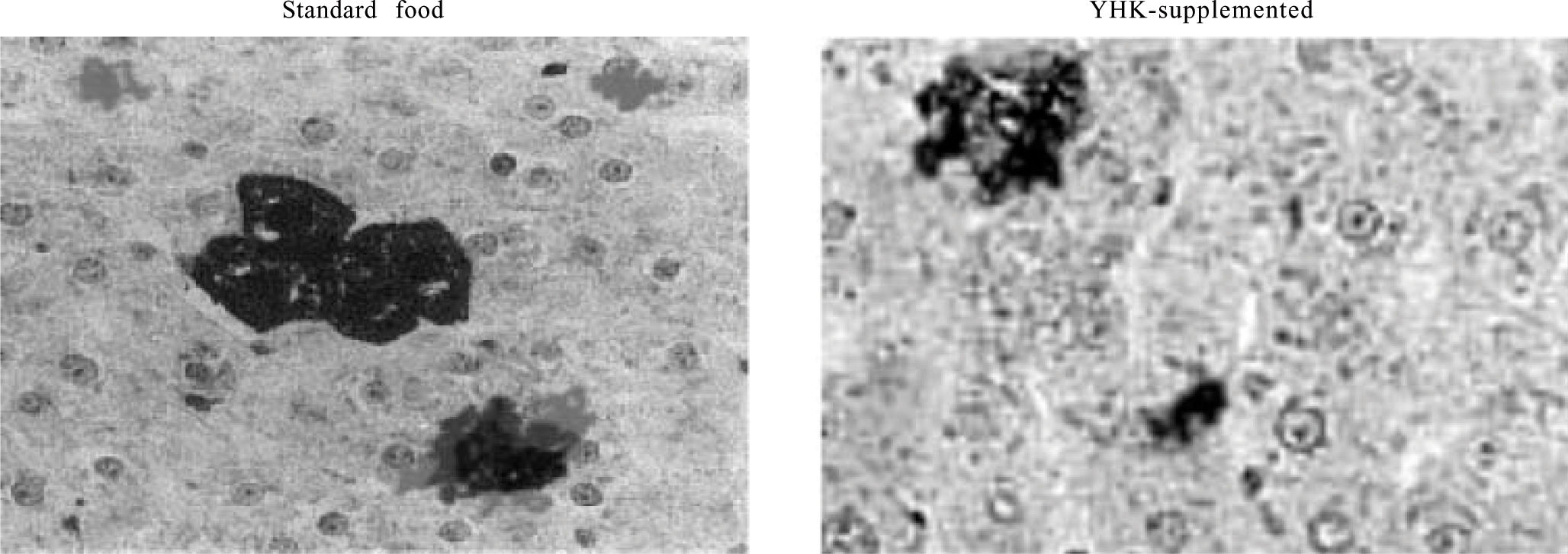
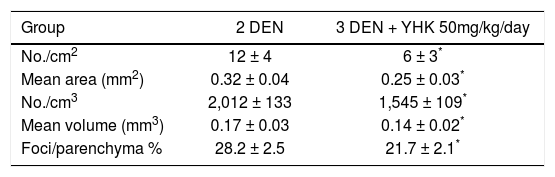
The results of quantitative analysis of the frequency of GST-P-positive foci are summarized in table II. The two-dimensional assessment showed that GST-P-positive lesions were significantly lesser in rats administered YHK (group 3) than in group 2 (Figure 1). The same results appeared when the statistical analysis was applied to volumetric assessment too such as number of lesions per cm3, mean volume and the volume when expressed as a percentage of parenchyma of GST-P-positive lesions.
Number and size of GST-P-positive hepatic lesions in DEN-Induced hepatocarcinogenesis: Effect of concomitant supplementation with YHK
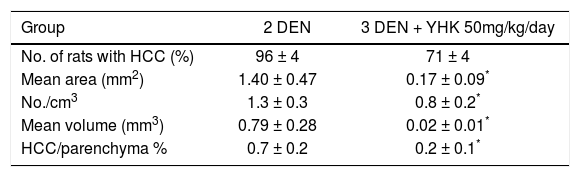
Tumor incidence. No liver tumors was detected in untreated rats while tumor hepatocellular origin were observed in DEN-treated rats (groups 2 and 3). The incidences of carcinoma of group 3 were significantly lower than those of group 2 (p<0.01). The multiplicities of carcinoma and total tumors of groups 3 were significantly smaller than the corresponding values of group 2, when also assessed by volumetric calculation (p<0.05).
Incidence, number, size and volume of DEN-Induced hepatocellular carcinoma: Effect of concomitant supplementation with YHK
Hepatocellular carcinoma (HCC) is a devastating and increasingly common disease and progress in the management of this cancer has been slow while a high rate of recurrence is still a limiting factor in the success of surgical resection.25 While hepatitis C and B and aflatoxin in some areas are the main cause of HCC, there is an increasing concern over the wider involvement of xenobiotics in carcinogenesis. Indeed, there are many genotoxic carcinogens occurring naturally in our environment, such as the large group of heterocyclic amine mutagens.1,2 A number of chemicals agents are currently employed to experimentally mimick such condition since genotoxic carcinogens can induce irreversible DNA damage in primary cells which then proliferate in the presence of promoter substances until they acquire autonomic growth capability. Classically, chemical hepatocarcinogenesis is regarded as a multistep process with at least three stages, i.e. initiation, promotion and progression, and each of these steps involves host biochemical, endocrinological, immunological, and microenvironmental regulatory systems. On a practical ground, DNA can be damaged along the whole process of absorption of carcinogens into the body, distribution to most sensitive tissues, metabolism which gives rise to a further form reacting with DNA, detoxification, and excretion. In this instance, a protective dietary approach would represents an ideal strategy when considering that there is an established evidence that diet plays a major role in the prevention of many diseases, including cancer.26 Thus, nowadays there is an increasing literature supporting the benefit of specific nutrients which, back in the early 1980s, had been termed in Japan as “functional foods”. In the present study we employed YHK, a controlled herbal remedy, which has been shown to exert potent hepatoprotective properties in several experimental models of liver injury.10-12 However, unlike other natural remedies which are either unapplicable to human trials or have shown to occasionally trigger severe side effects,27,28 being regarded as unsafe in long-term treatment,29 this compound can be safely integrated in normal diet and long-term studies have proved to significantly exert a transaminases-lowering effect in HCV-related cirrhotic patients13 and, most recently, in non-alcohol steatohepatitis.14 This study showed that this compound, when orally ingested together with an established chemical hepatotoxin, could counteract the early phase of carcinogenesis, as expressed by the study of GST-P. This represents a stable marker for persistent preneoplastic and neoplastic cells not only at the protein but also at the mRNA level throughout hepatocarcino-genesis in rats.30,31 Overall, the GSTs are a family of dimeric proteins (labelled as Alpha, Mu, Pi, Theta, Sigma, Kappa, and Zeta) that play important roles in both the intracellular transport of hydrophobic molecule and the metabolism of toxic compounds.32 GST-P protein is hardly detectable in normal rat liver but becomes expressed and detectable in hyperplastic nodules and hepatocellular carcinomas, irrespective of the kind of carcinogen used. GST-positive cells are typically characterized by an elevated DNA replication and the growth of GST-P-positive single cells and GST-P-positive liver foci is believed to be results between such replication and the counterbalance determined by death of cells.33,34 However, as stated above, a number of chemical and/or dietary toxins may act as tumor promoters by triggering a progressive cellular damage. In this context, are of worthwhile interest the data of Matsui et al. showing the beneficial effect of a functional food approach in improving the prognosis of postoperative HCC35 which has generated a thoughtful editorial stressing the need of well-planned studies tackling such complex issue.36 In particular, our study showed that the number, size and volume of either GST-P-positive foci and of overt HCC were significantly reduced by co-administration of YHK, the latter event being at an higher significance level. In general, a number of mechanisms underlie the effects of chemopreventive agents, the suppression of lipid peroxidation or DNA adduct formation and the modulation of phase I or II enzymes being among them. More recently, we have shown in that this compound could offer a significant protection to hepatocytes and lysosomal fraction against metal-induced oxidative stress12 as well as modulating phase I and phase II carcinogens-metabolizing enzymes (Marotta et al. personal data, manuscript in preparation). The mechanism by which YHK provides significant protection against hepatocarcinogenesis is not clear as yet. However, taken overall it is conceivable that some YHK components endowed by potent antioxidant/antiinflammatory property such as eucomnia ulmoides and panax pseudo-ginseng37,38 and antiproliferative or pro-apoptotic effect such as gallic acid and glycyrrhiza,39,40 are advocated for to explain its prevention of preneoplastic lesion formation. On the other hand, its safety make it a potential functional food of large clinical application in the quest to achieve a better control of HCC transformation in chronic liver disease.41 This holds of particular interest when considering that a number of “would-be" protective natural compound have failed to do the same42,43 if not even worsen the carcinogenesis process.44,45