Observations of hepatitis C virus (HCV) infection in adults with hemochromatosis are limited.
Materials and methodsWe determined associations of serum ferritin (SF) with anti-HCV in non-Hispanic white North American adults in a post-screening examination. Cases included p.C282Y homozygotes (regardless of screening transferrin saturation (TS) and SF) and participants (regardless of HFE genotype) with high screening TS/SF. Controls included participants without p.C282Y or p.H63D who had normal screening TS/SF. Participants with elevated alanine aminotransferase underwent anti-HCV testing. We determined prevalence of chronic HCV infection in consecutive Alabama and Ontario referred adults with HFE p.C282Y homozygosity.
ResultsIn post-screening participants, anti-HCV prevalence was 0.3% [95% CI: 0.02, 2.2] in 294 p.C282Y homozygotes, 9.5% [7.2, 12.3] in 560 Cases without p.C282Y homozygosity, and 0.7% [0.2, 2.3] in 403 Controls. Anti-HCV was detected in 7.2% of 745 participants with and 0.8% of 512 participants without elevated SF (odds ratio 9.9 [3.6, 27.6]; p<0.0001). Chronic HCV infection prevalence in 961 referred patients was 1.0% (10/961) [95% confidence interval (CI): 0.5, 2.0]. Ten patients with chronic HCV infection had median age 45y (range 29–67) and median SF 1163μg/L (range 303–2001). Five of eight (62.5%) patients had biopsy-proven cirrhosis.
ConclusionsOdds ratio of anti-HCV was increased in post-screening participants with elevated SF. Prevalence of anti-HCV in post-screening participants with HFE p.C282Y homozygosity and chronic HCV infection in referred adults with HFE p.C282Y homozygosity in North America is similar to that of Control participants with HFE wt/wt and normal screening TS/SF.
Hemochromatosis due to HFE p.C282Y homozygosity in North America occurs predominantly in non-Hispanic whites of European descent [1]. Among persons in North America with hemochromatosis phenotypes, ∼90% have HFE p.C282Y homozygosity [3]. In a screening study of 99,711 adults in North America, the estimated prevalence of p.C282Y homozygosity in non-Hispanic whites was 0.44% (∼1 in 227) [1]. The prevalence of p.C282Y homozygosity is highest in Ireland (∼1.0%), Denmark, United Kingdom, Iceland, and Norway [4,5]. p.C282Y homozygosity is also common in Australia, New Zealand, and South Africa due to migration of Europeans to these areas [6]. In large countries of Central and South America, patients with hemochromatosis phenotypes are uncommon and the proportions of them who also have p.C282Y homozygosity are low [7–9]. Among rural-dwelling southern Africans with a high probability of having the putative African iron overload gene, none had p.C282Y [10]. In Asia, hemochromatosis is rare. Novel cases are due to deleterious mutations in genes that encode hemojuvelin (HJV), hepcidin (HAMP), and ferroportin (SLC40A1), but not HFE (HFE) [11]. HFE p.C282Y/H63D compound heterozygosity occurs in 2.0% of non-Hispanic whites in North America [1] but is infrequently associated with morbidity due to iron overload [12,13].
The prevalence of hepatitis C virus (HCV) infection in p.C282Y homozygotes in North America is unknown. We determined the prevalence of anti-HCV antibody and HFE genotype and serum ferritin (SF) associations in non-Hispanic white participants in the Hemochromatosis and Iron Overload Screening (HEIRS) Study who attended a post-screening examination wherein participants with elevated alanine aminotransferase (ALT) levels underwent reflex testing for anti-HCV. We also sought to compare the prevalence and presenting characteristics of chronic HCV infection in two cohorts of referred patients with hemochromatosis and HFE p.C282Y homozygosity in Alabama and Ontario. We compare our prevalence estimates of anti-HCV positivity and chronic HCV infection in p.C282Y homozygotes with those in population cohorts from North America, review observations of the prevalence of p.C282Y homozygosity among patients with chronic HCV infection, and discuss SF levels in patients with HCV infection.
2Methods2.1Selection of subjectsThis study was conducted in accordance with the principles of the Declaration of Helsinki [14]. HEIRS Study participants, recruited from Field Centers in the US and Canada, were least 25y of age, gave informed consent, and at screening underwent measurement of serum transferrin saturation (TS) and SF and HFE genotyping to detect HFE alleles p.C282Y and p.H63D, as described in detail elsewhere [1]. The HEIRS Study component of this study describes observations in self-identified non-Hispanic white participants who attended a post-screening examination [15].
Observations of consecutive referred patients with hemochromatosis and HFE p.C282Y homozygosity diagnosed between 1996 and 2018 were compiled in retrospective reviews of medical care records at practices located in Birmingham, Alabama (Ja.C.B.) and London, Ontario (P.C.A.). The present study design did not include reviewing medical records of potential control subjects from general clinic populations in Alabama and Ontario.
2.2Evaluation of subjectsHEIRS Study post-screening examination participants designated as Cases included: all HFE p.C282Y homozygotes regardless of screening TS and SF; and participants with other genotypes who had high TS and SF at screening, in accordance with the HEIRS Study design [15]. Elevated SF levels were defined as >300μg/L (men) and >200μg/L (women) [1]. Participant Controls had HFE wt/wt (defined as the absence of HFE p.C282Y and p.H63D) and screening TS and SF levels between the 25th and 75th percentiles of sex-specific distributions [15]. At the examination, HFE genotypes were confirmed and anti-HCV was measured if serum ALT level was elevated (>40IU/L men, >31IU/L women) [16]. Anti-HCV test results were interpreted as positive or negative [16]. The median interval between screening and the post-screening examination was 8 months [15].
2.3Laboratory testingReflex testing for HCV antibody was performed in post-screening participants with elevated ALT levels using Vitros Eci Immunodiagnostic System (Ortho-Clinical Diagnostics Incorporated, Linden, NJ, USA).
Referred patients underwent evaluations and laboratory testing at Alabama and Ontario clinics. Serum TS, SF, and ALT levels and HFE genotypes were determined by affiliated clinical laboratories. At presentation, patients with ALT levels greater than the upper reference limit and most patients who had elevated SF were evaluated for anti-HCV, HCV RNA quantification, HCV genotype, and liver histology, as indicated. HCV antibody in referred patients was measured using an enzyme immunoassay (Laboratory Corporation of America, Burlington, North Carolina, USA). In referred patients, HCV RNA was quantified using Roche COBAS® Ampliprep (COBAS® Taqman® 96, Roche Diagnostics USA, Indianapolis, Indiana, USA). Hepatitis C genotyping was determined using Abbott m2000® RealTime HCV Genotype ll (Abbott Laboratories, Lake Bluff, Illinois, USA).
2.4Definition of chronic hepatitis C virus infectionWe defined chronic HCV infection in accordance with the Council of State and Territorial Epidemiologists position statement [17]. Clinical criteria included evidence of chronic liver disease consistent with HCV infection. Laboratory criteria included a positive test for anti-HCV and HCV RNA (qualitative, quantitative, or genotype testing).
2.5Histologic scoringPathologists affiliated with Alabama and Ontario clinics interpreted liver biopsy specimens using the METAVIR system to grade (inflammation) and stage (fibrosis) hepatic disease caused by chronic HCV infection [18]. Stage 4 fibrosis was defined as cirrhosis.
2.6StatisticsThe datasets for analyses included observations on: 1257 HEIRS Study post-screening examination participants (854 Cases (294 p.C282Y homozygotes and 560 participants without HFE p.C282Y homozygosity who had high screening TS and SF values) and 403 Controls with HFE wt/wt and normal screening TS and SF values); 307 unrelated non-screening p.C282Y homozygotes from Alabama; and 654 p.C282Y homozygotes from Ontario. Descriptive data are displayed as enumerations, percentages, mean±1 SD, mean [95% confidence interval (CI)], or proportions [95% CI] with continuity corrections. Proportions were compared using Fisher's exact test (two-tailed) or odds ratio [95% CI], as appropriate. Respective TS and SF values in participant subgroups positive and negative for anti-HCV were compared using Mann–Whitney U test (two-tailed). Analyses were performed with SAS v. 9.1 (SAS Institute Inc., Cary, NC), GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA), USA Excel 2000® (Microsoft Corp., Redmond, WA), and GB-Stat® (v. 10.0, Dynamic Microsystems, Inc., Silver Spring, MD). We defined values of p<0.05 to be significant.
3Results3.11257 post-screening examination participantsParticipants included 854 Cases (294 p.C282Y homozygotes and 560 participants without HFE p.C282Y homozygosity who had high screening TS and SF values) and 403 Controls with HFE wt/wt and normal screening TS and SF values. There were 612 men (48.7%) and 645 women (51.3%).
3.2Anti-HCV in 1257 post-screening examination participantsElevated ALT levels were detected in 271 of 1271 participants (21.6%). Thus, reflex testing for anti-HCV was performed only in these 271 participants. Anti-HCV was detected in 58 of 271 participants who had elevated ALT levels (21.4%). The aggregate prevalence of combined elevated ALT and anti-HCV in all 1257 participants was 4.6% [95% CI: 3.6, 6.0]. The proportions of men and women with combined elevated ALT and anti-HCV were 5.9% (36/612) and 3.4% (22/645), respectively (p=0.0433).
3.3Anti-HCV in post-screening examination participant subgroupsAnti-HCV was detected in 0.3% (1/294) [95% CI: 0.02, 2.2] of HFE p.C282Y homozygotes. The single p.C282Y homozygote with anti-HCV was a 50 year-old man with TS 98% and SF 2710μg/L. Anti-HCV was detected in 9.5% (53/560) [95% CI: 7.2, 12.3] of Cases without p.C282Y homozygosity. Anti-HCV was detected in 1.0% (4/403) [95% CI: 0.3, 2.7] of Controls with HFE wt/wt (p=0.4036). The odds ratio of anti-HCV positivity in 294 p.C282Y homozygotes vs. 403 control participants with HFE wt/wt was 0.3 ([95% CI: 0.04, 3.1]; p=0.3363).
3.4Anti-HCV, transferrin saturation, and serum ferritin in 271 post-screening examination participantsIn 58 participants positive for combined elevated ALT and anti-HCV, median screening TS and SF were 55% and 447μg/L, respectively. In 213 participants with elevated ALT who were negative for anti-HCV, median screening TS and SF were 47% and 419μg/L, respectively. Although respective median screening TS and SF values in participants positive for combined elevated ALT and anti-HCV were numerically greater than those of participants with elevated ALT who were negative for anti-HCV, neither comparison achieved statistical significance (p=∼1.0, respectively).
3.5Anti-HCV and serum ferritin in post-screening examination participantsAnti-HCV was detected in 7.2% (54/745) of participants who had elevated screening SF levels. Anti-HCV was detected in 0.8% (4/512) of participants whose screening SF levels were not elevated. Odds ratio of anti-HCV in participants with elevated screening SF levels was 9.9 ([95% CI: 3.6, 27.6]; p<0.0001).
3.6961 referred HFE p.C282Y homozygotesThere were 307 referred probands from Alabama (171 men, 136 women). There were 654 referred patients from Ontario (375 men, 279 women; 249 probands, 406 non-probands). The percentages of men from Alabama and Ontario were not significantly different (55.7% vs. 57.3%, respectively; p=0.6324).
3.7Chronic hepatitis C virus infection in referred HFE p.C282Y homozygotesThe prevalence of chronic HCV infection was 2.0% (6/307) [95% CI: 0.8, 4.4] in Alabama patients and 0.6% (4/654) [95% CI: 0.2, 1.7] in Ontario patients (p=0.0831). The odds ratio of chronic HCV infection in Alabama vs. Ontario patients was 3.2 [95% CI: 0.9, 11.4] (p=0.0735). The aggregate prevalence of chronic HCV infection in 961 referred p.C282Y homozygotes was 1.0% (10/961) [95% CI: 0.5, 2.0].
Proportions of Alabama men and women with chronic HCV infection were 2.9% and 0.7%, respectively (p=0.1698). Proportions of Ontario men and women with chronic HCV infection were 0.8% and 0.4%, respectively (p=0.6399). In aggregate, the prevalence of chronic HCV infection in 961 referred patients was 1.5% in men and 0.4% in women (p=0.1096).
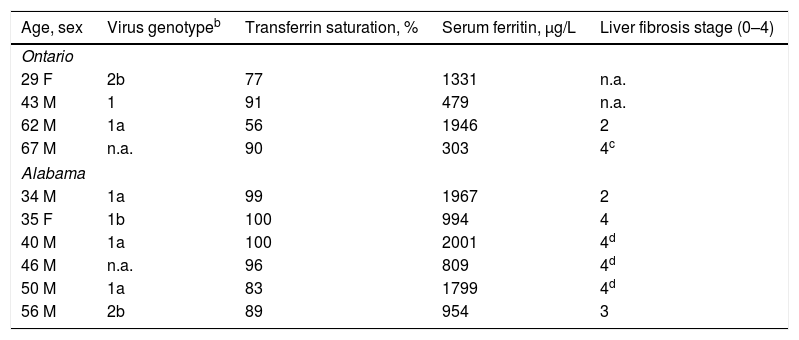
Ten referred patients had chronic HCV infection (Table 1). Median age at diagnosis of hemochromatosis was 45y (range 29–67). Eight of ten patients (80.0%) were men. Median TS and median SF at diagnosis were 91% (range 56–100) and 1163μg/L (range 303–2001), respectively. Eight of the ten patients underwent HCV genotyping. Six of the eight patients had HCV genotype 1.
Chronic hepatitis C virus infection in 10 referred HFE p.C282Y homozygotes.a
| Age, sex | Virus genotypeb | Transferrin saturation, % | Serum ferritin, μg/L | Liver fibrosis stage (0–4) |
|---|---|---|---|---|
| Ontario | ||||
| 29 F | 2b | 77 | 1331 | n.a. |
| 43 M | 1 | 91 | 479 | n.a. |
| 62 M | 1a | 56 | 1946 | 2 |
| 67 M | n.a. | 90 | 303 | 4c |
| Alabama | ||||
| 34 M | 1a | 99 | 1967 | 2 |
| 35 F | 1b | 100 | 994 | 4 |
| 40 M | 1a | 100 | 2001 | 4d |
| 46 M | n.a. | 96 | 809 | 4d |
| 50 M | 1a | 83 | 1799 | 4d |
| 56 M | 2b | 89 | 954 | 3 |
Observations at diagnosis of hemochromatosis. n.a., not available. All patients were positive for anti-HCV and a HCV RNA or genotype test. A 51 year-old man evaluated in Ontario had p.C282Y homozygosity, transferrin saturation 90%, serum ferritin 2134μg/L, and anti-HCV positivity (data not tabulated).
Hepatitis C virus RNA quantification using PCR technique yielded values of 43,000–3,800,000IU/mL. Nine of the 10 patients were treated with pegylated interferon and ribavirin. Three of the 9 patients were re-treated with ledipasvir/sofosbuvir. One of the three patients had persistent viremia after re-treatment. A tenth patient underwent liver transplantation before anti-viral therapy and did not survive.
Eight patients underwent liver biopsy. Five of the eight patients (62.5%) had cirrhosis. Three of the five patients with cirrhosis reported heavy alcohol consumption. Another patient with cirrhosis had hepatocellular carcinoma (Table 1). No patient was diagnosed to have porphyria cutanea tarda.
4DiscussionThe prevalence of combined elevated ALT and anti-HCV in 403 HEIRS Study Control participants with HFE wt/wt and normal screening TS and SF values was 1.0% [95% CI: 0.3, 2.7]. The prevalence of chronic HCV infection in 961 referred non-Hispanic white patients with hemochromatosis and HFE p.C282Y homozygosity in this study was 1.0% [95% CI: 0.5, 2.0]. The prevalence of combined elevated ALT and anti-HCV in 294 HEIRS Study participants with p.C282Y homozygosity was 0.3% [95% CI: 0.02, 2.2]. In a study of 368 p.C282Y homozygotes from US, Canada, and Australia who underwent liver biopsy, one participant (0.3%; [95% CI: 0.01, 2.7]) had HCV infection [19]. These four estimates are consistent with the proportion of 9931 civilian non-institutionalized adult non-Hispanic white participants aged ≥20y in the 2003–2010 National Health and Nutrition Examination Survey who were positive for HCV RNA (1.1% [95% CI: 1.0, 1.4]) [20] and with estimates of HCV infection prevalence in Canada (0.6–0.7%) [21].
The prevalence of chronic HCV infection in Italian patients with iron overload and HFE p.C282Y homozygosity (n=269) or p.C282Y/p.H63D compound heterozygosity (n=69) reported from Milan was 5.9% [22]. The overall prevalence of chronic HCV infection in European countries is highest in Italy (3.0–4.4%) [23]. In central and southern Italy and the major islands of Italy, the prevalence of HCV infection is 12.6–26% [23]. It is likely that the greater prevalence of HCV infection reported in Italian patients with HFE-related hemochromatosis than in US and Canada HFE p.C282Y homozygotes is due in part to the higher general population prevalence of HCV infection in Italy.
The present results suggest that susceptibility to anti-HCV positivity or HCV infection or in non-Hispanic white adults with HFE p.C282Y homozygosity in North America is no greater than that of non-Hispanic white adults from US and Canada populations unselected for HFE genotypes or with HFE wt/wt. The prevalence of p.C282Y homozygosity in 100 Massachusetts Caucasian patients with chronic HCV infection did not differ significantly from that of 73 local controls [24] or 2016 non-Hispanic white participants from the Third National Health and Nutrition Examination Survey 1992–1994 [24,25]. In a study of 246 German patients with chronic HCV infection and 200 control subjects, no participant had p.C282Y homozygosity [26]. In a study of 184 Austrian patients with chronic HCV infection, five patients (2.7% [95% CI: 1.0, 6.6]) were discovered to have HFE p.C282Y homozygosity [27], whereas the prevalence of chronic HCV infection in Austria is <0.5% [28]. Because the numbers of patients in this Austrian hepatitis cohort were relatively small, it is not possible to discern whether the prevalence of chronic hepatitis C in Austrian p.C282Y homozygotes differs significantly from that in the general Austrian population. In a meta-analysis of subjects from diverse geographic areas (mainly case-control studies), the odds ratio for HCV infection in p.C282Y homozygotes vs. subjects without p.C282Y or p.H63D was 4.1 (1.2–14) [29]. Taken together, these observations do not resolve whether p.C282Y homozygotes are more susceptible to developing chronic HCV virus infection than persons with other HFE genotypes, although p.C282Y homozygotes may be more likely to develop hyperferritinemia if they also have chronic HCV infection.
In the present referred patients with hemochromatosis and HFE p.C282Y homozygosity, eight of the ten patients with HCV infection were men. In another US cohort of ten patients with HFE p.C282Y homozygosity, chronic HCV infection, and stage 3 or 4 fibrosis, all were men [30]. In the present post-screening examination participants, the prevalence of anti-HCV was significantly greater in men than women. In National Health and Nutrition Examination Survey 2003–2010, the prevalence of chronic hepatitis C infection was significantly greater in men than women [20].
In this study, the odds ratio of anti-HCV in post-screening examination participants with elevated screening SF levels was more than nine-fold higher than that of participants whose screening SF levels were not elevated. Case participants without HFE p.C282Y homozygosity were invited to the post-screening examination solely because they had elevated screening TS and SF. These selection criteria would account in part for the strong positive association of anti-HCV and elevated screening SF levels we observed. These results are also consistent with previous observations of SF and liver disease in HEIRS Study participants [31] and SF levels in non-Hispanic white participants with chronic HCV infection who participated in the 1988–1994 National Health and Nutrition Examination Survey [32].
In a study performed in Italy before HFE was discovered, Piperno et al. reported that most patients with hemochromatosis and chronic HCV infection also had cirrhosis, and that their mean SF levels and quantities of iron removed by phlebotomy to achieve iron depletion were significantly lower than those of hemochromatosis patients without chronic HCV infection and fibrosis/cirrhosis [33]. In three of the present referred patients with HFE p.C282Y homozygosity, chronic HCV infection, and stage 4 fibrosis/cirrhosis, SF was 303–994μg/L. In contrast, stage 4 fibrosis/cirrhosis in a large series of Alabama and Ontario p.C282Y homozygotes was significantly associated with SF >1000μg/L, after adjustment for other variables [34]. In another study from the US, ten p.C282Y homozygotes with chronic HCV infection were diagnosed to have stage 3 or 4 fibrosis at a lower mean age and had a significantly lower mean hepatic iron concentration than 13 p.C282Y homozygotes with stage 3 or 4 fibrosis who did not have chronic HCV infection [30]. These findings support the postulate that the combination of hemochromatosis-associated iron overload and chronic HCV infection are synergistic in causing hepatic fibrogenesis. In contrast, each of five Austrian patients with p.C282Y homozygosity and chronic HCV infection had elevated SF levels, but only one of the five patients had hepatic iron index >1.9mmolFe/g dry weight/y and histologic abnormalities of liver typical of hemochromatosis [27]. This report is consistent with observations in patients with chronic HCV infection that hepatic iron overload is uncommon and hepatic iron concentration is unassociated with inflammation grade and fibrosis stage [35].
The proportion of patients with chronic HCV infection who have elevated hepatic iron levels measured by atomic absorption spectrometry is 5–14% [35,36]. Elevated hepatic iron levels in patients with chronic HCV infection are incompletely explained by p.C282Y and p.H63D alleles [27,37]. Serum hepcidin levels are lower in patients with chronic HCV infection than control subjects [38,39]. Hepcidin expression is suppressed in persons with chronic HCV infection via multiple control pathways [40] and HCV-induced oxidative stress [41]. Decreased hepcidin expression may increase iron absorption via up-regulation of ferroportin in patients with chronic HCV infection [42]. The expression of many iron-related genes is increased in chronic HCV infection and could lead to hepatocyte iron deposition and retention [43]. Hepatic iron levels were higher in patients with chronic HCV infection due to genotype 1b than those with genotypes 2a or 2b [44], indicating that HCV genotype influences liver iron deposition and retention. Low concentrations of free or transferrin-bound iron reduced interferon-gamma signaling in the human myelomonocytic cell line THP-1 [45], suggesting that iron overload could reduce in vivo macrophage antiviral activity. Other “immunologic” mechanisms by which iron may decrease resistance to HCV infection have been reviewed [46].
Heavy alcohol consumption, reported by three of five referred patients with chronic HCV infection and cirrhosis, is a risk factor for cirrhosis in p.C282Y homozygotes [19,47] and adults with chronic HCV infection [48]. Although none of the present referred patients were diagnosed to have porphyria cutanea tarda, the proportion of HFE p.C282Y homozygotes in US case series of porphyria cutanea tarda is greater than the proportion of p.C282Y homozygotes in the general US population [49,50]. Among p.C282Y homozygotes with porphyria cutanea tarda, liver injury is greatest in those who also have chronic HCV infection and heavy alcohol consumption [50].
One of the present referred patients also had hepatocellular carcinoma, possibly due to interaction of iron overload [34] and chronic HCV infection [51]. The present results and those of a previous report [19] suggest that chronic HCV infection contributes less than iron overload and heavy alcohol consumption to overall cirrhosis risk in p.C282Y homozygotes in North America because the prevalence of HCV infection in this region is low.
Limitations of the present study include lack of hepatitis C RNA analyses and liver biopsies in post-screening participants with anti-HCV because obtaining these studies was beyond the scope of the HEIRS Study. Liver biopsy specimens were unavailable in two referred HFE p.C282Y homozygotes with chronic HCV infection. It is possible that some HEIRS Study participants and referred patients had occult HCV infection [52], chronic HCV infection with normal ALT levels [53], no HCV infection associated with low levels of anti-HCV [54], or developed chronic HCV infection after diagnosis of hemochromatosis. HEIRS Study participants and some referred patients without elevated ALT levels were not tested for anti-HCV. Among US subjects with HCV infection who were unselected for hemochromatosis, ∼30% have normal ALT levels [55]. This suggests that our estimates of the prevalence of anti-HCV and chronic HCV infection are conservative. Compiling and evaluating other risk factors for chronic HCV infection in adults, including blood transfusion before 1992, lifetime drug use, and lifetime sexual partners [20], were not feasible for referred patients or HEIRS Study post-screening participants.
We conclude that the odds ratio of anti-HCV was increased in post-screening participants with elevated SF. Prevalence of anti-HCV in post-screening participants with HFE p.C282Y homozygosity and chronic HCV infection in referred adults with HFE p.C282Y homozygosity in North America is similar to that of Control participants with HFE wt/wt and normal screening TS/SF.AbbreviationsALT alanine aminotransferase confidence interval hepatitis C virus Hemochromatosis and Iron Overload Screening Study serum ferritin transferrin saturation
The HEIRS Study (January 2000–January 2006) was supported by the National Heart, Lung, and Blood Institute, in conjunction with the National Human Genome Research Institute. Individual investigators, grants, and contracts have been acknowledged in detail previously [1,2]. The present work was supported in part by Southern Iron Disorders Center.
Author contributionsJa.C.B. conceived the work, evaluated patients, compiled data, performed statistics, and drafted the manuscript. J.Cl.B. compiled data and performed statistics. P.C.A. evaluated patients and compiled data. All authors contributed and agreed to the final form of the manuscript.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors recognize and appreciate the contributions of all HEIRS Study investigators and participants.