Terms of use: This graphical abstract and icons were created by Yun-I, chou solely.
HCC is one of the most common malignancies and the second leading cause of cancer-related deaths worldwide [1]. The incidence of HCC is increasing globally, particularly in developing countries [2]. HCC is characterized by an insidious onset, rapid progression, poor prognosis, and frequent relapses [3]. The leading risk factors for HCC are chronic hepatitis B virus infection in Africa and East Asia, and chronic hepatitis C virus infections in developed Western countries and Japan [4–6].
HCC can be diagnosed using non-invasive criteria in patients with risk factors, or with evidence of pathology but without known risk factors [7]. Treatments are implemented after establishing a diagnosis based on the Barcelona Clinic Liver Cancer (BCLC) staging system [4]. For patients with a diagnosis of advanced HCC, novel agents, including chemotherapy, targeted therapy, and immune checkpoint inhibitors, have shown benefits in terms of progression-free survival and local recurrence (LR) rate [8,9]. For those with early-stage disease, i.e., those in the curative-intent category, resection remains the gold standard, and liver transplantation remains the first-line treatment for patients who fulfill the Milan criteria. Nevertheless, most patients are not eligible for such surgical interventions, and impaired liver function after the procedure is usually anticipated. The current scarcity of liver donors and high tumor recurrence rates make RFA and TACE acceptable alternative therapeutic management options.
RFA, first described by Rossi et al. in 1993 [10], induces coagulation necrosis through ionic agitation and subsequent heating of tissue. In the evolution of the ablation technique and machinery, RFA has shown results comparable to those of surgery in the early BCLC stage, especially when operation time, intraoperative bleeding, and disease-free survival is considered [11,12]. Although it has some limitations [13–15], RFA is still considered to be the curative therapy for HCC tumors sized <3 cm in diameter [16,17]. Another ablative technique, PEI, can diminish the heat-sink effect by inducing coagulation and obliterating small intratumoral vessels [18]. Although PEI showed less efficacy than RFA, irrespective of the HCC tumor size [19,20], PEI conducted prior to RFA treatment may theoretically eliminate the heat-sink effect and probably yield a larger ablation zone. This could improve treatment outcomes, especially if performed on areas of the liver which are difficult to reach with the RFA needle. The mechanisms of RFA-PEI are as follows: (1) RFA can enhance the ablative effect of ethanol due to its low boiling point (78.3°C), (2) an ethanol injection can embolize small vessels to reduce the heat-sink effect, (3) ethanol can be distributed to RFA-unablated areas (or difficult-to-treat areas), (4) ethanol can diffuse beyond the RFA ablation zone to establish a safety margin, and (5) an ethanol injection can make the tissue around the electrode less prone to carbonization and further thermal conduction [18,21,22]. The combination of local regional therapies has recently been established with the aim of magnifying HCC treatment outcomes.
Zhu et al.'s [23] 2016 meta-analysis (with three RCTs and five cohort studies that included a total of 969 patients) and Zheng et al.'s [24] 2017 meta-analysis (with two RCTs for synthesis) both demonstrated that RFA-PEI significantly improved the OS and significantly reduced the LR rate. However, the research team also noted a higher incidence of fever in the RFA-PEI than in the RFA group. Although significant outcomes were found in Zhu et al.’s and Zheng et al.’s work, both had either a small number of included studies, or a lack of further subgroup analyses according to tumor location, study type, or needle type. Hence, we performed a meta-analysis to find more comprehensive and updated evidence, and to not only compare the therapeutic efficacy between RFA-PEI and RFA, but also to conduct subgroup analyses such as needle type according to specific conditions.
2Materials and methods2.1Selection criteria2.1.1Searching strategy and eligibility criteriaRandomized clinical trials (RCTs) which assessed the efficacy of RFA monotherapy, or a combination of RFA and PEI, were eligible for inclusion in this systematic review. The following inclusion criteria were applied: (1) patients with HCC diagnosed through histological examination or clinical diagnostic criteria, regardless of Child-Pugh class, tumor size, or previous treatment for liver tumors; and (2) outcome information, including OS, LRF, complete tumor necrosis (CTN), treated tumor size, and complications had to be provided. After reading the publications, abstracts, and full texts, we initially excluded article types such as editorials, case-control studies, case reports, reviews, cohort studies, duplicates, and conference abstracts. Other exclusion criteria for this study were (1) simultaneous serious liver dysfunction or extrahepatic metastasis and (2) ablative treatment (RFA or RFA-PEI) combined with transhepatic artery chemoembolism (TACE).
2.1.2Intervention and comparisonCombined therapy (RFA-PEI) vs. monotherapy (RFA).
2.1.3OutcomesPrimary outcomes were OS, LRF proportion, and CTN rates. The secondary outcomes included the mean number of treatment sessions, treated tumor sizes, and treatment-related complications.
2.1.4Protocol registrationThis systematic review and meta-analysis was registered with PROSPERO (registration no. CRD42018097443).
2.2Search strategyFollowing the guidelines of the PRISMA statement, we performed a systematic search for eligible studies from inception to January 1, 2022, in electronic databases including PubMed, EMBASE, Scopus, and CNKI. We used search terms including "hepatocellular carcinoma[Mesh]" OR "liver neoplasm" AND "radiofrequency" OR "thermal ablation" AND "alcohol" OR "ethanol injection,” "combination therapy" OR "multiple ablation therapy." No language restrictions were imposed.
2.3Study screening and data extractionStudy screening and data extraction based on the selection criteria were independently performed by two investigators (SW Cheng and DE Lu). Discrepancies were resolved through discussion with a senior investigator (KH Chen). All data were extracted from published articles and included study authors, publication year, patient characteristics, tumor size, number of tumors, treatment methods, and follow-up duration. We contacted the authors whenever important information was unavailable in the published work.
A high-risk location was defined as a tumor located near the gallbladder, inferior vena cava, portal vein, kidney, or immediately under the diaphragm. The distance between the edge of the nodule and the large vessels or an extrahepatic organ was considered “high-risk” whenever it was under 5 mm [25,26]. CTN was defined as complete ablation in the case of low attenuation, non-enhancing ablated lesions, which correspond in shape and size to previously identified HCC on computed tomography, in both arterial and porto-venous phases, without newly developed lesions elsewhere in the liver. The time point for the assessment was four weeks after ablation. LRF was defined as the length of time from primary HCC treatment to the confirmation of disease relapse with imaging studies. Adverse effects were subdivided into major complications(portal vein thrombosis, single-organ failure, intracapsular hemorrhage) and minor complications(fever and pain).
2.4Quality assessmentWe used the risk of bias tool from the Cochrane Handbook for Systematic Reviews of Interventions (ver. 5.1.0) [27] to assess the quality of the included RCTs. The assessment included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective outcome reporting, and other types of bias.
2.5Statistical methodsThe meta-analysis was performed using the Review Manager (RevMan) vers. 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Mean and standard deviation values were extracted for continuous data, and the numbers of events and people in each group were extracted to analyze categorical data. Furthermore, a pooled estimate was computed using the DerSimonian and Laird random-effects model [28]. The Cochrane Q and I2 tests were used to assess heterogeneity. When the Q value showed a significant difference (p < 0.1), we considered that heterogeneity is present in the study samples. The I2 test was used to determine the level of heterogeneity among study samples. The final heterogeneity results were collectively portrayed in a forest plot to exhibit the effect size and 95% confidence interval (CI). Heterogeneity was considered significant when I2 was ≥50%. For the heterogeneous studies, subgroup, random-effects model, and sensitivity analyses were used to pool the estimates.
2.6Subgroup and sensitivity analysisSubgroup analyses were performed for OS (divided by tumor size, high-risk and non-high-risk locations, and Cool-tip™ or non-Cool-tip™), LRF (divided into high-risk or non-high-risk), complications (divided into major complications, fever, and pain), and the CTN rate (divided into high-risk or non-high-risk, and Cool-tip™ or non-Cool-tip™). Tumor response was measured using the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [29]. A sensitivity test was conducted if the included trials did not use exactly the same intervention methods.
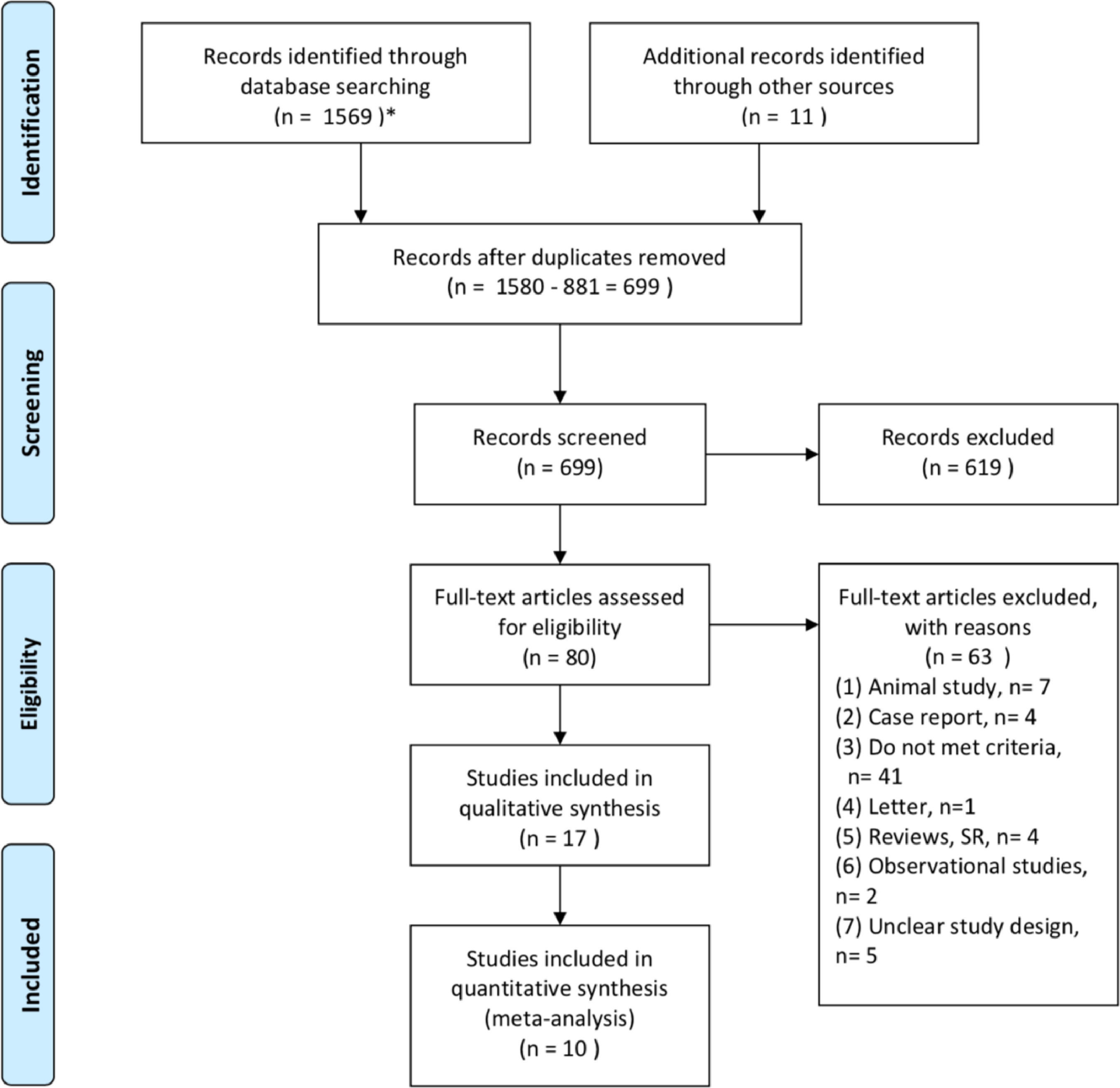
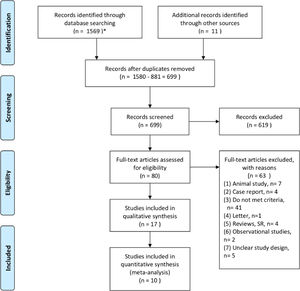
3Results3.1Study selectionA total of 1580 studies were identified after searching the databases and other sources. After excluding duplicates, 699 records were screened. Additionally, 619 records were excluded based on the title or the abstract. Eighty full-text articles were reviewed to determine their eligibility based on the inclusion criteria, and 63 were excluded for various reasons (Fig. 1). As a result, 17 studies were included in the qualitative synthesis [22,25,30–44]. However, Shi et al.’s study [36] was removed due to duplication. Takata et al. [37] only provided data in an abstract from the American Association for the Study of Liver Diseases in 2015, and their study only included high-risk location tumors. Liu et al. [22] and Kai et al.[33] conducted retrospective studies; the former [22] included patients who received RFA-PEI for HCC only in the caudate lobe. Luo et al. [35] reported imbalanced tumor sizes between their combination therapy group and their monotherapy group. The monotherapy group contained tumors sized >3 cm (Group 2) and <3 cm (Group 3a), whereas the combination group only contained tumors sized >3 cm (Group 3b). In addition, Luo et al. [35] did not analyze the data separately for the combination group, meaning that the results were mixed (containing RFA-PEI and TACE-PEI). Xu et al. [38] included patients with recurrent HCCs who underwent hepatectomy before receiving RFA or RFA-PEI, meaning that the therapeutic effect might have been affected by decompensated livers. Zhang et al.[44] used a microwave ablative technique that produces higher intertumoral temperatures and is less susceptible to the heat-sink effect. Considering the mechanism of the beneficial effect expected from RFA-PEI compared with RFA alone, this study was excluded. Finally, 10 RCT studies [25,30–32,34,39–43], with a total of 854 patients were included in the quantitative synthesis (Fig. 1).
3.2Characteristics of included studiesAll 10 studies were published between 2002 and 2020 [25,30–32,34,39–43]; the number of patients in each study ranged from 16 to 76, with a total of 854 participants combined. All the studies included patients with HCC who received either combination therapy (RFA-PEI), or RFA monotherapy. The patients’ baseline liver function was normal, and the Child-Pugh class was either A or B. A variety of RFA needles were used in the studies, including Cool-tip™, RITA®, LeVeen™, WHK-411®, and blade®, with the majority of ablation methods using Cool-tip™ [25,30,34,42] (Table 1).
Baseline characteristics of the included studies.
| Study | Treatment arms (patients, n) | Tumors (n) | No. of tumors (1/≥2) (%) | Age, years (mean±SD) | Gender (M/F) | Child A/B/C | Main HCC size (cm) (mean±SD) |
|---|---|---|---|---|---|---|---|
| Azab et al. 2011 (Egypt) | RFAa(30) | 33 | N/A | N/A | N/A | N/A | N/A |
| PEI(30) | 32 | N/A | N/A | N/A | N/A | N/A | |
| RFAa-PEI(30) | 33 | N/A | N/A | N/A | N/A | N/A | |
| Chen et al. 2005 (China) | RFAb(41) | N/A | 41/0(100/0) | 50 | 37/4 | 28/11/2 | N/A |
| RFAb-PEI (45) | N/A | 45/0(100/0) | 48 | 36/9 | 34/11/0 | N/A | |
| Du et al. 2011 (China) | RFAc (37) | N/A | 52/23(69/31) | 59.5 | N/A | 6/10/21 | 5.3±1.4 |
| RFAc-PEI (38) | N/A | 69.5 | N/A | 7/12/19 | 5.8±1.7 | ||
| Kalra et al. 2017 (India) | RFAa (25) | N/A | 20/5(80/20) | 61.56±9.2 | 24/1 | 17/8/- | N/A |
| RFAa-PEI (25) | N/A | 16/9(64/36) | 58.36±10.1 | 22/3 | 18/7/- | N/A | |
| Kurokohchi et al. 2002 (Japan) | RFAc (20) | N/A | N/A | 68 | 13/7 | 14/6/0 | 1.9 |
| RFAc-PEI (19) | N/A | N/A | 66 | 14/5 | 14/5/0 | 2.6 | |
| RFAa-PEI (34) | N/A | N/A | 69 | 25/9 | 20/13/1 | 3 | |
| Liu et al. 2014 (China) | RFAe (30) | N/A | N/A | N/A | N/A | N/A | N/A |
| RFAe-PEI (30) | N/A | N/A | N/A | N/A | N/A | N/A | |
| Sun et al. 2012 (China) | RFAa (16) | 34 | 6/10(37.5/62.5) | N/A | 11/5 | N/A | 3.5±1.1 |
| RFAa+ PEI (27) | 51 | 11/16(40.1/59) | N/A | 19/8 | N/A | 3.6±1.2 | |
| Sung et al. 2012 (China) | RFAf (47) | N/A | N/A | N/A | N/A | N/A | N/A |
| RFAf-PEI (47) | N/A | N/A | N/A | N/A | N/A | N/A | |
| Zhang et al. 2005 (China) | RFAd (74) | 85 | N/A | N/A | 49/25 | 61/11/2 | 5.2±1.8 |
| RFAd+ PEI (76) | 83 | N/A | N/A | 50/26 | 65/9/2 | 5.5±2.4 | |
| Zhang et al. 2007 (China) | RFAb (67) | 210 | 43/24(64/36) | 52.2±10.3 | 58/9 | 37/24/6 | N/A |
| RFAb-PEI (66) | 40/26(61/39) | 53.3±11.3 | 57/9 | 42/20/4 | N/A |
RFA, radiofrequency ablation; PEI, percutaneous ethanol injection; Child, Child-Pugh score; HCC, hepatocellular carcinoma; TACE, transarterial chemo-embolization; N/A, not available;
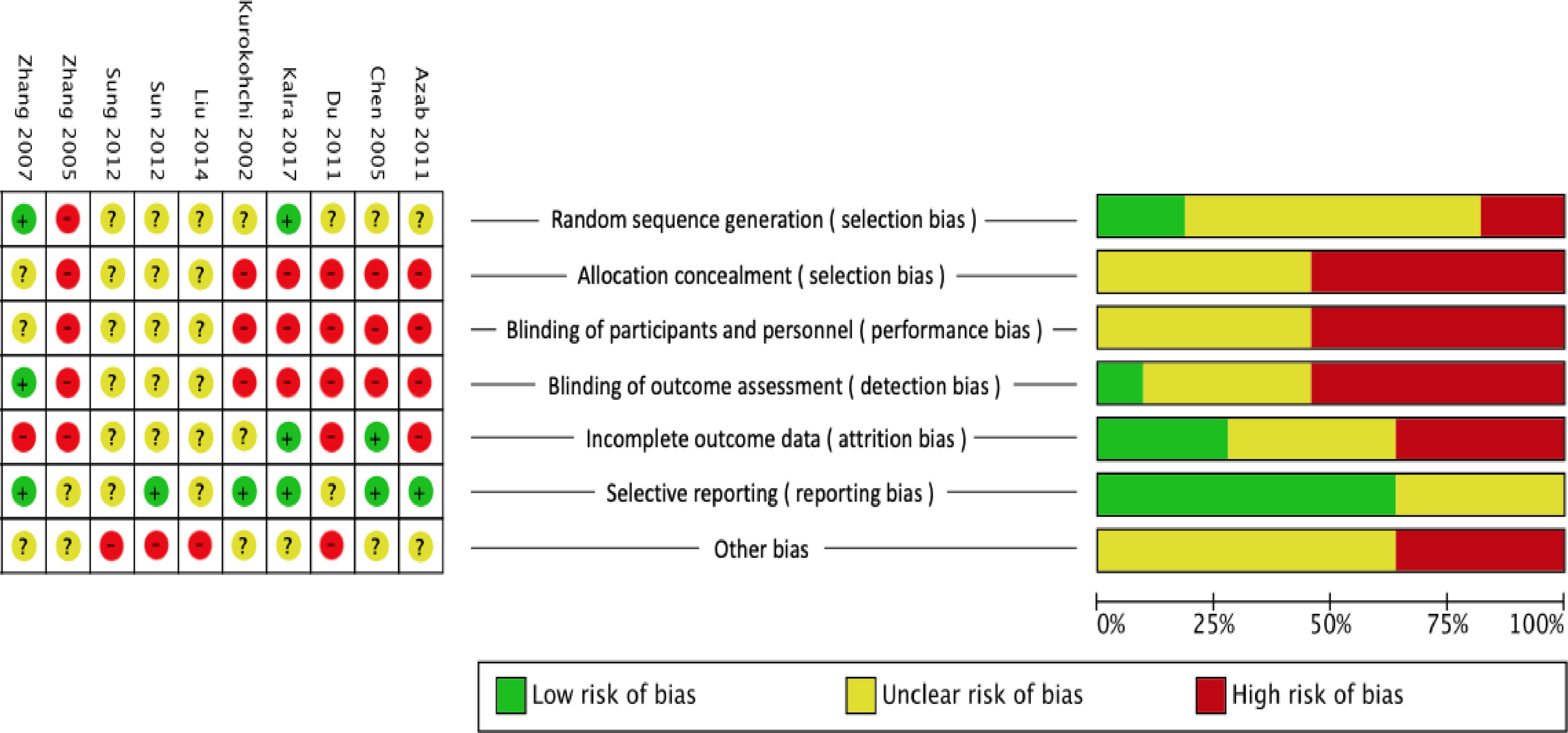
A quality analysis of the included studies is shown in Fig. 2. Two studies [34,40] had low risk due to random computer sequence generation, one [39] randomly assigned odd/even days, while the rest had unclear random sequence reporting. For the domain of allocation concealment, the other studies were unclear; for the domains of blinding participants, personnel, and outcome assessment, only one study [40] had low risk. For the domain of incomplete outcome data, two studies [31,34] had low risk with an acceptable attrition rate; for the domain of selective reporting, six studies had low risk [25,30,31,34,40,42]. The domains of other biases were mainly unclear.
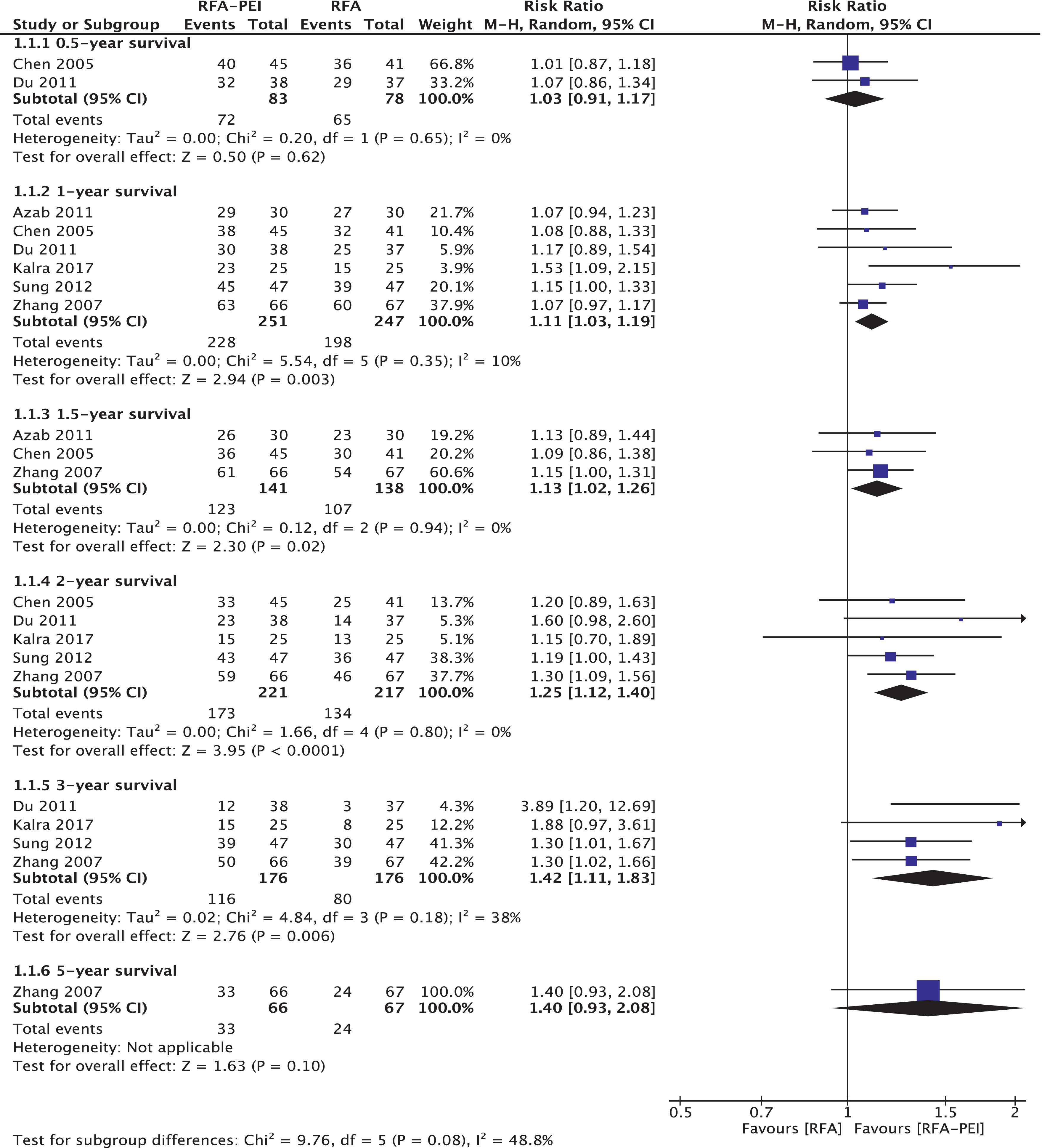
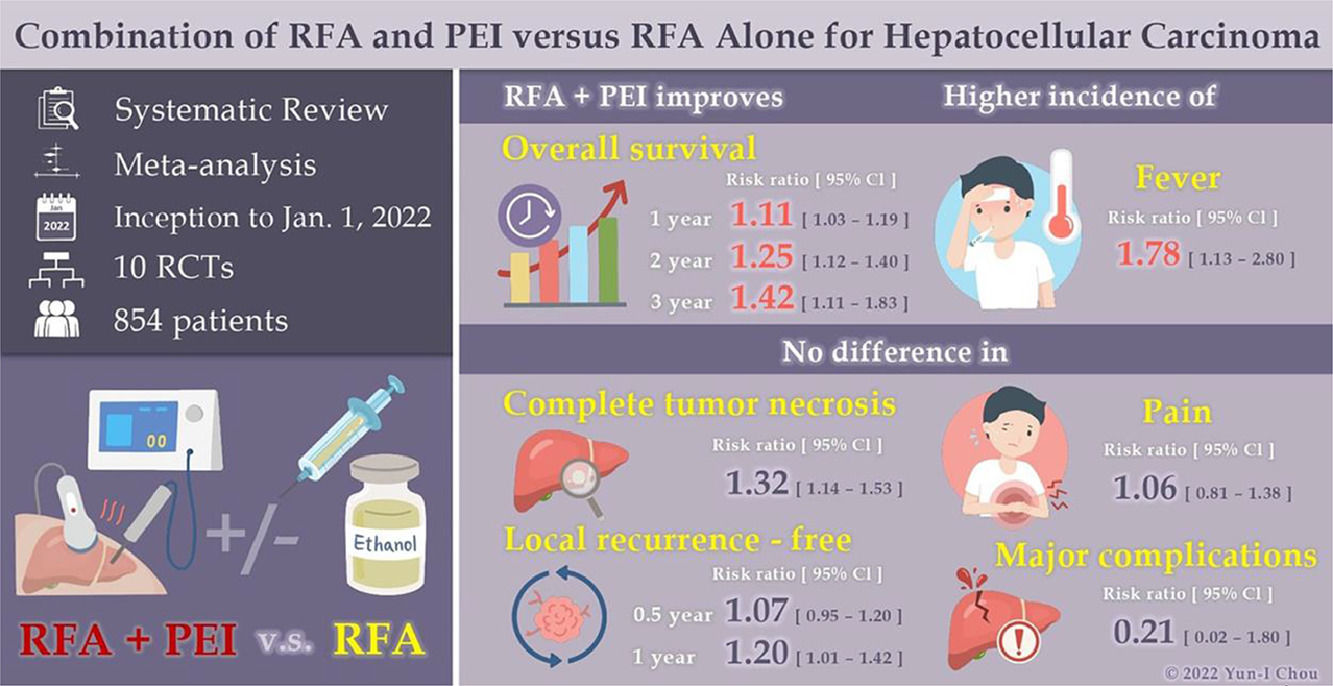
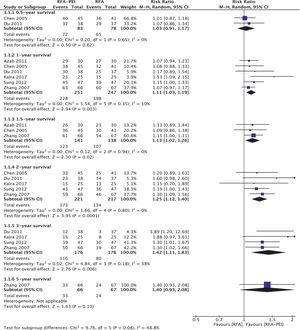
3.4Outcome measurements3.4.1Overall survival (OS)Six RCTs with 528 patients [30–32,34,40,42] analyzed OS between RFA and RFA-PEI. Compared with RFA monotherapy, the pooled results demonstrated a significantly higher OS in the RFA-PEI group at 1 year (RR: 1.11; 95% CI: 1.03, 1.19, I2 = 10%), 1.5 years (RR: 1.13; 95% CI: 1.02, 1.26, I2 = 0%), 2 years (RR: 1.25, 95% CI: 1.12, 1.40, I2 = 0%), and 3 years (RR: 1.42, 95% CI: 1.11, 1.83, I2 = 38%) (Fig. 3).
The tumor sizes in the RFA-PEI group before treatment were slightly larger than those in the RFA group. Therefore, we conducted a subgroup analysis of tumor location (tumor sizes <3 cm vs. 3–5 cm, supplementary Figs. 3 and 4) and high-risk vs. non-high-risk location (supplementary Figs. 5 and 6). In the 3–5-cm tumor size subgroup, the 2-year OS was slightly higher in the RFA-PEI than in the RFA group (RR: 1.35, 95% CI: 1.02, 1.79) [31,40]. Additionally, we noted that the OS rates at 1 year (RR: 1.09; 95% CI: 1.02, 1.17), 1.5 years (RR: 1.14; 95% CI: 1.01, 1.29), 2 years (RR: 1.25; 95% CI: 1.10,1.42), and 3 years (RR: 1.30; 95% CI: 1.09, 1.55) in the RFA-PEI group were significantly higher in the non-high-risk location group. In the high-risk location group, improvement in OS was seen at 3 years (RR, 3.89; 95% CI: 1.20, 12.69) (Table 2).
Subgroup analysis of overall survival (OS), local tumor recurrence free proportion (LRF), the complete tumor necrosis rate, and complications between the radiofrequency ablation (RFA) and percutaneous ethanol injection (PEI)+RFA groups (Supplementary Figs. 1–6)
| Outcome | No. of included studies | RFA-PEI | RFA | Risk ratio | I2 | p value | ||
|---|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | |||||
| OS | ||||||||
| Cool tip™ | ||||||||
| 1 year survival | 2 | 52 | 55 | 42 | 55 | 1.25 | 82% | 0.30 |
| 2 year survival | 1 | 15 | 25 | 13 | 25 | 1.15 | ** | 0.57 |
| 3 year survival | 1 | 15 | 25 | 8 | 25 | 1.88 | ** | 0.06 |
| Non-cool tip™ | ||||||||
| 1 year survival | 3 | 131 | 149 | 117 | 145 | 1.08 | 0% | 0.08 |
| 2 year survival | 3 | 115 | 149 | 85 | 145 | 1.30 | 0% | 0.0005 |
| 3 year survival | 2 | 62 | 104 | 42 | 104 | 1.97 | 73% | 0.24 |
| Tumor <3 cm | ||||||||
| 1 year OS | 2 | 49 | 53 | 48 | 54 | 1.04 | 0% | 0.49 |
| 2 year OS | 2 | 46 | 53 | 40 | 54 | 1.17 | 0% | 0.08 |
| Tumor 3∼5 cm | ||||||||
| 1 year OS | 2 | 41 | 46 | 33 | 40 | 1.07 | 0% | 0.38 |
| 2 year OS | 2 | 37 | 46 | 24 | 40 | 1.35 | 0% | 0.04 |
| 3 year OS | 1 | 19 | 25 | 11 | 22 | 1.52 | 45.1% | 0.08 |
| High-risk location | ||||||||
| 0.5 year OS | 1 | 32 | 38 | 29 | 37 | 1.07 | ** | 0.52 |
| 1 year OS | 1 | 30 | 38 | 25 | 37 | 1.17 | ** | 0.27 |
| 2 year OS | 1 | 23 | 38 | 14 | 37 | 1.60 | ** | 0.06 |
| 3 year OS | 1 | 12 | 38 | 3 | 37 | 3.89 | ** | 0.02 |
| Non-high-risk location | ||||||||
| 1 year OS | 3 | 137 | 143 | 126 | 144 | 1.09 | 0% | 0.64 |
| 1.5 year OS | 2 | 87 | 96 | 77 | 97 | 1.14 | 0% | 0.03 |
| 2 year OS | 2 | 102 | 113 | 82 | 114 | 1.25 | 0.0% | 0.0007 |
| 3 year OS | 2 | 89 | 113 | 69 | 114 | 1.30 | 10.0% | 0.99 |
| Local recurrence-free proportion (LRF) | ||||||||
| High-risk location | ||||||||
| 0.5 year LRF | 1 | 35 | 38 | 29 | 37 | 1.18 | ** | 0.10 |
| 1 year LRF | 1 | 33 | 38 | 22 | 37 | 1.46 | ** | 0.01 |
| Non-high-risk location | ||||||||
| 0.5 year LRF | 2 | 84 | 92 | 76 | 88 | 1.03 | 22% | 0.56 |
| 1 year LRF | 3 | 109 | 125 | 91 | 121 | 1.14 | 51% | 0.11 |
| 2 years LRF | 2 | 59 | 83 | 37 | 78 | 1.47 | 23% | 0.01 |
| Complete tumor necrosis | ||||||||
| Cool tip™ | 2 | 76 | 84 | 43 | 67 | 1.38 | 38% | 0.01 |
| Non-cool tip™ | 4 | 113 | 181 | 83 | 181 | 1.45 | 63% | 0.03 |
| High-risk location | 3 | 100 | 119 | 54 | 101 | 1.52 | 56% | 0.007 |
| Non-high-risk location | 3 | 128 | 150 | 91 | 134 | 1.26 | 47% | 0.15 |
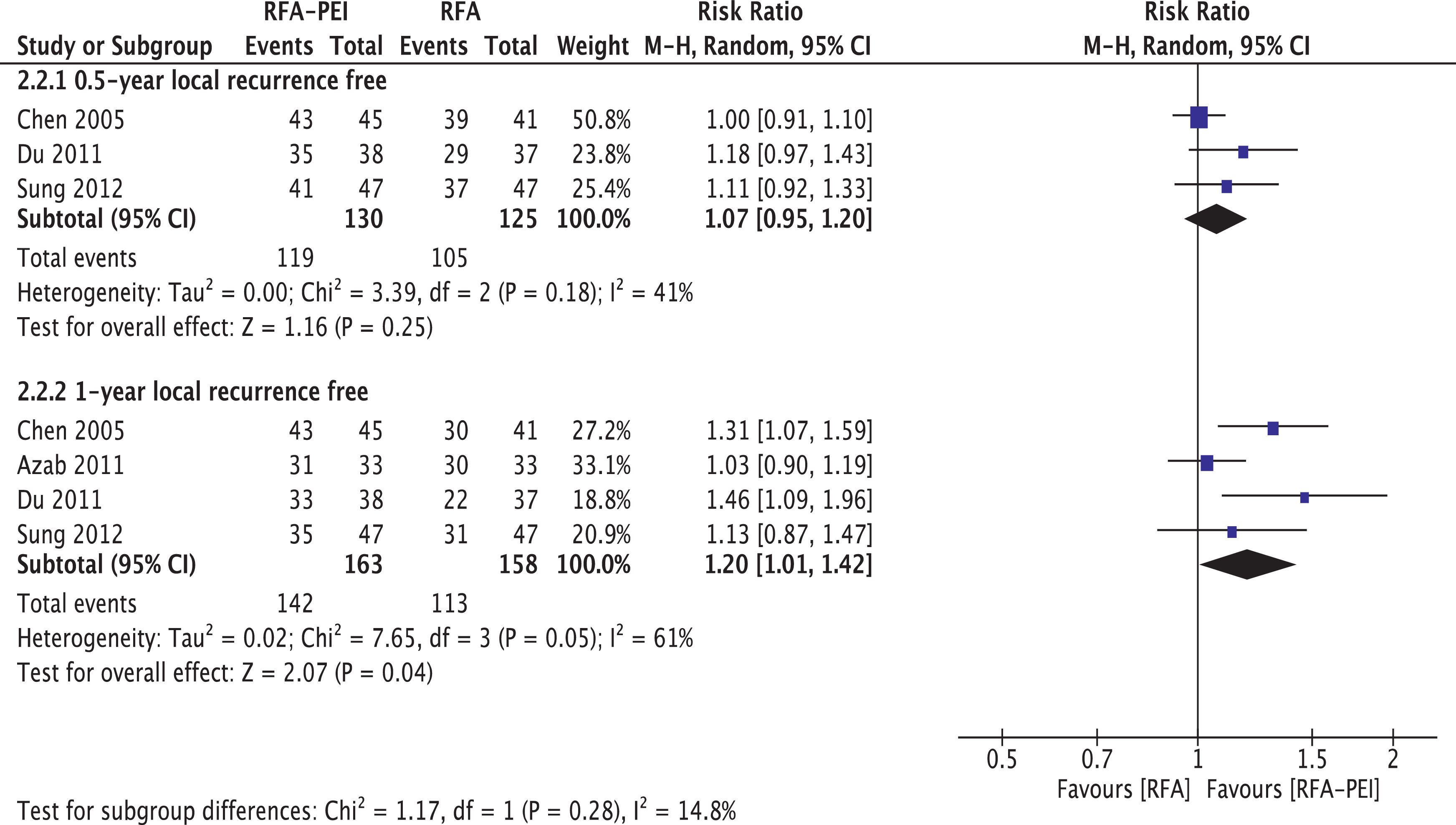
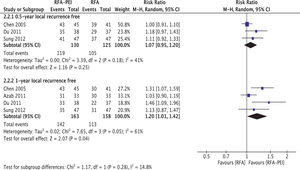
Four studies provided LRF data [30–32,43]. Pooled results and subgroup analyses are shown in Fig. 4 and Supplementary Figs. 7 and 8. Generally, LRF proportion at 0.5 years (three studies, 255 patients) did not differ with statistical significance between the RFA and RFA-PEI groups (RR: 1.07; 95% CI: 0.95, 1.20, I2 = 41%). Compared with RFA monotherapy, LRF proportion at 1 year (four studies, 321 patients) was significantly higher in the RFA-PEI group, despite the high heterogeneity found in this analysis (RR: 1.20, 95% CI: 1.01, 1.42, I2 = 61%).
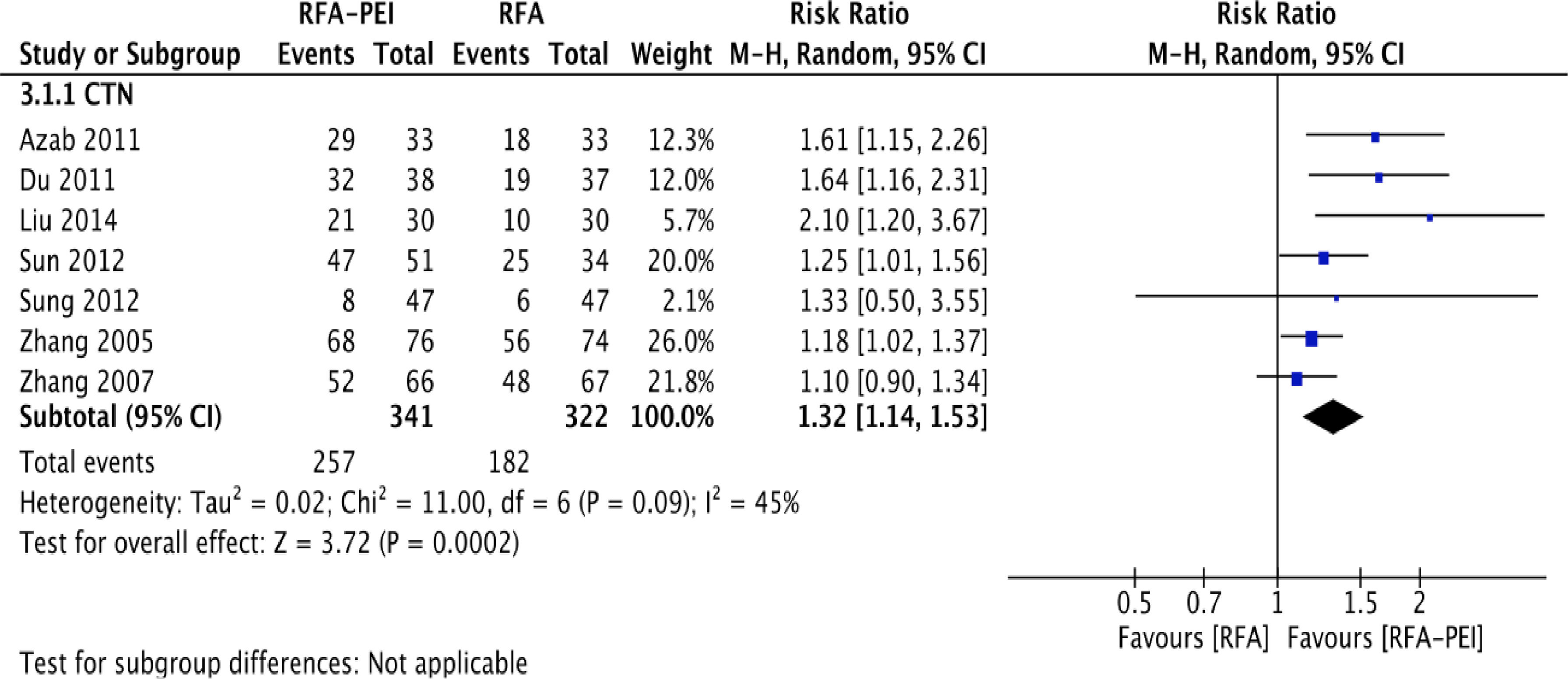
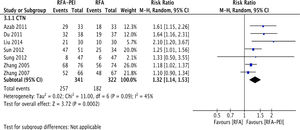
3.4.3CTNIn total, seven studies [30,32,39–43] with 663 patients demonstrated CTN data for the RFA and RFA-PEI groups. Compared with RFA monotherapy, pooled results demonstrated a significantly higher CTN rate in the RFA-PEI and in the high-risk location groups (RR: 1.32; 95% CI: 1.14, 1.53; I2 = 45%, RR: 1.52; 95% CI: 1.12, 2.06, I2 = 56%, respectively). Nevertheless, high heterogeneity was still detected (Fig. 5, Supplementary Figs. 9 and 10).
3.4.4ComplicationsIn order to assess safety concerns, we compared the major complications in the RFA and RFA-PEI groups. Six studies addressed non-specific major complications [25,30,31,34,39,40], with three major complications in the RFA group being portal vein thrombosis [30], single-organ failure, and intracapsular hemorrhage [34]; there were no adverse events reported in the RFA-PEI group. Two studies [30,40] assessed fever occurrence, and found that pooled results indicated a higher incidence of fever in the RFA-PEI group (RR: 1.78; 95% CI: 1.13, 2.80, I2 = 0%); fever occurred in 38.5% (37 of 96) patients in the RFA-PEI group and 21.6% (21 of 97) patients in the RFA group (p = 0.01). The meta-analysis did not show any statistically significant differences in pain intensity (Supplementary Figs. 11 and 12).
4DiscussionIn this meta-analysis, the results of the primary outcomes were consistent with the findings of Zhu et al. [23] and Zheng et al. [24], showing significant clinical improvements in the combination group in terms of the 1-, 1.5-, 2-, 3-, and 5-year OS. Furthermore, we performed subgroup analyses based on tumor size, tumor location, and needle type. We found that the combination group exhibited significant improvements in (1) 2- and 3-year OS in the 3-5-cm tumor size group; (2) 2- and 3-year OS in the high-risk group; (3) 1-, 2-, and 3-year OS in the non-high-risk group; (4) 2-year OS in the non-Cool-tip™ group; and (5) CTN rates in the non-high-risk group. In contrast, post-procedural major complications and pain did not significantly differ between the RFA and RFA-PEI groups.
The combination of locoregional therapies is a better way to achieve successful treatment outcomes than monotherapy [20,23]. However, a relatively small number of studies have addressed the efficacy of RFA-PEI compared with RFA alone. Ethanol is not the only substance that can be injected to enhance ablation outcomes; some studies have suggested that saline injections can also achieve favorable results. Lavraghi et al. [45] used a continuous saline injection (1 ml/min) during ablation therapy to make the tissue electrically uniform, and thereby improve the uniformity of heat deposition and avoid or limit tissue charring. Fifteen patients with 25 tumors were treated, with a mean tumor size of 3.1 (range: 1.2–4.5) cm. CTN was achieved in 13 tumors (52%), whereas 12 tumors had a partial response (48%). All tumors that had a complete response were <4 cm in size. Honda et al. [46] suggested a heat-induced coagulation necrosis method using boiling physiological saline, and devised the percutaneous hot-saline-injection therapy. They method was used on 20 patients with tumors sized <3 (1.8 ± 0.7) cm, with 2.5 (2.5 ± 0.5) mean treatment sessions, and achieved LRF. However, this was a one-arm study, and the tumors were <3 cm in size. Kurokohchi et al. [25] reported that RFA-PEI yields an excellent coagulation effect. Their case study included five patients with HCC at a high-risk location. The patients received a 99.5% ethanol injection prior to RFA. All tumors were found to be completely coagulated, and no major complications were detected. From their findings, it can be concluded that RFA-PEI may be effective for treating HCC located in regions that are difficult to treat with RFA alone, as well as for treating large-sized HCCs. However, there was no comparison group in their study, and most patients had compensated liver function.
Regarding the probability of ethanol toxicity, Livraghi et al. [45] suggested that ethanol injection decreases the patients’ immune function and leads to severe impairment of liver function due to the damage to peripheral liver tissues caused by ethanol diffusion. In patients who present with liver cirrhosis before treatment, the curative dose of ethanol may cause severe complications, such as hemorrhage in the congestive ducts. In our included studies, most patients [25,31,32,34,39] had conditions with compensated liver function; evidence on RFA-PEI with decompensated liver function remains controversial and inconsistent. Therefore, ethanol should be used with great caution in patients with decompensated liver disease.
Based on this synthesis, we found that combination therapy resulted in significant improvement in 2- and 3-year OS with medium-sized tumors (3–5 cm). These results were obtained from two previous studies [31,40]. In addition, Azab et al. [30] performed a subgroup analysis of complete ablation based on tumor size. After one treatment session, RFA-PEI had a better complete ablation rate than RFA alone (Z = 2.96, p = 0.003). The difference can be attributed to the subgroup with tumor sizes of 3–5 cm (Z = 2.74, p = 0.018), rather than that with sizes <3 cm (Z = 1.43, p = 0.38). This indicates that the combined therapy was beneficial for medium-sized tumors, and the efficacy of RFA alone might be sufficient for small-sized tumors (<3 cm).
Evidence of the RFA-PEI effect on intrahepatic or extrahepatic recurrence is limited. Zhang et al. [40] reported that RFA-PEI could only improve LRF, but not intrahepatic or extrahepatic recurrence. In addition, we cannot implement LRF related to OS, since multiple comorbidities contribute to OS. Therefore, treatments which aim to control risk factors for HCC should be considered to avoid intrahepatic or extrahepatic recurrence, such as antiviral agents for chronic hepatitis B or C, and alcohol abstinence.
No previous meta-analyses of tumors in high-risk locations have been conducted. We included one study which contained such tumors. Nevertheless, RFA-PEI did not seem to significantly improve outcomes in the high-risk group in terms of OS, LRF, or CTN rates. In fact, OS in the non-high-risk group was more favorable. This was probably due to the relatively small number of available studies.
Lastly, we found that the combination group had larger tumor sizes than the monotherapy groups [25,32,39], despite there being no statistical difference prior to treatment. One study also assessed other types of tumors, not only pure HCC [32], which could have affected the quality of selection and probably influenced the outcomes. The included studies contained significant heterogeneity with some reporting bias. Numerous inconsistencies and the inability to adequately access the clinical data preclude the superiority of RFA-PEI in the treatment of HCC.
4.1Study limitationsThis meta-analysis and the included RCTs had several limitations. First, all the included studies used monopolar electrodes, so we cannot apply our conclusions on bipolar electrode settings. Second, most of the included studies were from China, as were the analytical results for the main outcomes. Therefore, poor generalizability may be a factor. Third, none of the locoregional therapies had pathological analyses (to evaluate satellite lesions or micro-metastasis-like perineural invasion and lymphovascular invasion), except for biopsies prior to treatment; Thus, it was difficult to clarify the safety margins. Lastly, the underlying risk factors for HCC were different, which may have affected the treatment outcomes.
5ConclusionsBased on this meta-analysis, combined therapy with RFA-PEI appears to be suitable for HCC patients with a compensated liver condition with respect to OS. Nevertheless, the results of LR and CTN were not solid due to the heterogeneity of existing evidence. The high risk of bias and lack of sufficient evidence made it difficult to create a definite assessment. Additionally, the current evidence supports the hypothesis that combination therapy is associated with a higher risk of fever than monotherapy.
We hope that more high-quality RCTs will be conducted in the future, with lower risks of systemic errors and bias. In addition, it would be beneficial if further studies analyzed tumor size, tumor location, compared different equipment, and assessed the correlation between LRF and OS.
FundingThis research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author's contributionLDE, CSW, LYS: conception or design of the work, data collection, data analysis and interpretation, drafting the article and critical revision of the article; TMW, LCH: conception or design of the work, data collection , data analysis and interpretation; CCF: conception or design of the work and critical revision of the article; CKH: data analysis and interpretation and critical revision of the article. All the authors gave their final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Declaration of interestNone