Background. African Americans are disproportionately affected by hepatitis C (HCV) and are less likely to undergo HCV treatment. Underserved populations are especially at risk for experiencing health disparity. Aim. To identify reasons for HCV non-treatment among underserved African Americans in a large safety-net system.
Material and methods. Medical records of HCV-infected African Americans evaluated at San Francisco General Hospital liver specialty clinic from 2006-2011 who did not receive HCV treatment were reviewed. Treatment eligibility and reasons for non-treatment were assessed. Factors associated with treatment ineligibility were assessed using logistic regression modeling.
Results. Among 118 patients, 42% were treatment ineligible, 18% treatment eligible, and 40% were undergoing work-up to determine eligibility. Reasons for treatment ineligibility were medical (54%), non-medical (14%), psychiatric (4%), or combined (28%). When controlling for age and sex, active/recent substance abuse (OR 6.65, p = 0.001) and having two or more medical comorbidities (OR 3.39, p = 0.005) predicted treatment ineligibility. Excluding those ineligible for treatment, 72% of all other patients were lost to follow-up; they were older (55 vs. 48 years, p = 0.01) and more likely to be undergoing work up to determine treatment eligibility (86 vs. 21%, p < 0.0001) than those not lost to follow-up.
Conclusions. Medical comorbidities and substance abuse predicted HCV treatment ineligibility in underserved African Americans. Importantly, the majority of those undergoing work-up to determine HCV treatment eligibility were lost to follow-up. While newer anti-HCV agents may increase treatment eligibility, culturally appropriate interventions to increase compliance with evaluation and care remain critical to HCV management in underserved African Americans.
The prevalence of hepatitis C virus (HCV) infection is disproportionately higher in African Americans, with 3% being affected compared to 1.5% of the non-Hispanic White population in United States.1 Although HCV-infected African Americans have a lower prevalence of cirrhosis, they are at higher risk for complications of end-stage liver disease and the rate of hepatocellular carcinoma (HCC) is two times higher in this population compared to Whites.2–4 Despite the higher prevalence of HCV and related complications, African Americans are less likely to receive HCV treatment compared to other racial and ethnic groups.5–8 Considering the changing era of HCV treatment with new and potent direct acting antiviral agents, understanding reasons for the lower uptake of HCV therapy in this population is critical to reduction of HCV disparity in this at risk group.9
Treatment ineligibility and non-receipt of treatment in eligible patients have been identified as reasons for HCV non-treatment in prior studies of multiethnic populations.5,7,8,10–13 Data on reasons for HCV non-treatment in African Americans is Limited and none have focused on the underserved populations. Some have shown that African Americans are more likely to be ineligible for HCV treatment, and to defer treatment when eligible, compared to non-African Americans.5–7 African Americans also suffer from higher rates of medical comorbidities that may be contraindications to interferon and ribavirin based treatment regimens.14 Additionally, they are less likely to respond to interferon-based HCV therapies even with use of protease inhibitors.7,15 While these could contribute to higher rates of treatment ineligibility and deferral, further studies are needed to more comprehensively assess reasons for HCV non-treatment among African Americans.
Safety net populations suffer disproportionately from health disparities.16 African American patients in the safety net have lower baseline knowledge of HCV infection, though the rate of increase in knowledge following HCV education is similar to that of other racial and ethnic groups.17 In keeping with the lower rates of HCV treatment reported elsewhere, in the San Francisco safety net liver specialty clinic only 8% of African American patients with chronic HCV received treatment compared to 26% of non-African Americans over a 4-year period.18 The aim of this study was to evaluate reasons for HCV non-treatment, as well as factors associated with treatment ineligibility, in the underserved population of African Americans within the San Francisco safety net healthcare system.
Material and MethodsPatient population and study designThis study is a retrospective review of the electronic medical records of patients who were evaluated at the San Francisco General Hospital (SFGH) liver specialty clinic between January 1, 2006 and June 30, 2011. Patients were referred to the liver specialty clinic from primary care clinics within the San Francisco safety net healthcare system, which provides services to over 150,000 patients annually including most of the county’s uninsured and underinsured population.19 This system is comprised of 11 non-profit primary care clinics affiliated with the San Francisco Community Clinic Consortium, as well as the San Francisco Department of Public Health’s Community Health Network, which includes 15 community-based primary care clinics and SFGH, an acute care and referral facility with on-site primary care, and specialty clinics.
All adult (18 years and older) patients who self-identified as African American race with chronic HCV (evidence of HCV antibody positivity ≥ 6 months and detectable HCV viral load) and who had completed at least one liver specialty clinic visit but did not undergo HCV treatment during the study period were included in the study. This study was approved by the Committee on Human Research of the University of California San Francisco.
Data extractionData was extracted from the electronic medical records with respect to demographics, medical history, laboratory data, clinical data, and referring primary care clinic location (community clinics vs. San Francisco General Hospital clinics). Hepatitis C treatment eligibility, potential barriers to treatment, and reasons for treatment ineligibility cited in the clinic notes were captured and categorized into: medical conditions, uncontrolled or poorly controlled psychiatric disease, and non-medical barriers (either active substance abuse or unstable housing or unstable social situation such lack of support with inability to independently attend clinic appointments or take medications). Patient’s interest in receipt of HCV therapy and reasons for declining HCV therapy were recorded. Income data was not collected in the database as income in the medical record reflected current income status which may not have been representative of income during the study period. However, the safety net population represents those with low income status with the payer source distribution as described above.
In November 2007, the liver specialty clinic at SFGH instituted a mandatory formal HCV education class offered by this specialty service for patients referred from the San Francisco safety net healthcare system. Providers who wished to refer patients to liver specialty clinic scheduled them in this formal HCV education class prior to evaluation in the liver specialty clinic. The class provides information on HCV transmission, diagnosis, symptoms, natural history, severity of liver disease, appropriate candidacy for treatment, response rates of antiviral therapy and side effects of treatment.18 Formal HCV education class attendance was recorded for all patients in this study.
Determining treatment eligibility status and loss to follow-upTreatment eligibility status was determined based on data from the last attended liver specialty clinic visit provider report. Treatment eligibility was categorized as: treatment-eligible, treatment ineligible, or potentially eligible for therapy (patients who were in the process of determining treatment eligibility at their last liver specialty clinic visit). Patients were also classified as lost to follow up if the patient was given a follow-up appointment in the liver specialty clinic but did not attend any subsequent clinic visits.
Statistical analysisDescriptive statistics of the patient characteristics were generated using mean ± SD and median (range) for continuous variables and frequency (%) for qualitative variables. Patient characteristics were compared between the three treatment eligibility categories using the Chi-square test (Fisher’s exact test when appropriate) for categorical variables and Kruskal-Wallis test for continuous variables. Similarly, patient characteristics were compared between those lost to follow-up versus not lost to follow-up using Chi-square (Fisher’s exact when appropriate) for categorical and MannWhitney test for continuous variables. The difference in the proportion of categorical variables among those lost to follow-up versus not lost to follow-up was further assessed using the Z-test.
Univariate analysis was used to evaluate the factors associated with treatment ineligibility (compared to treatment eligible or potentially eligible patients). Multivariate stepwise forward selection logistic regression modeling was then performed from an a priori compiled list and controlled for age and sex in all models. Statistical significance was assessed at a p-value of < 0.05 (2-sided). All analyses were performed using Stata version 12 statistical software, Stata Corp LP, College Station, TX.
ResultsPatient characteristicsDuring the study period, of the 132 African American patients evaluated in liver specialty clinic, 121 did not receive HCV treatment. Of these, 3 patients did not have active HCV infection and 118 met inclusion criteria for this study. Overall, 22 patients were considered eligible for treatment, 50 were determined as ineligible for treatment, and 46 were in the process of further work-up to determine treatment eligibility, defined as potentially eligible.
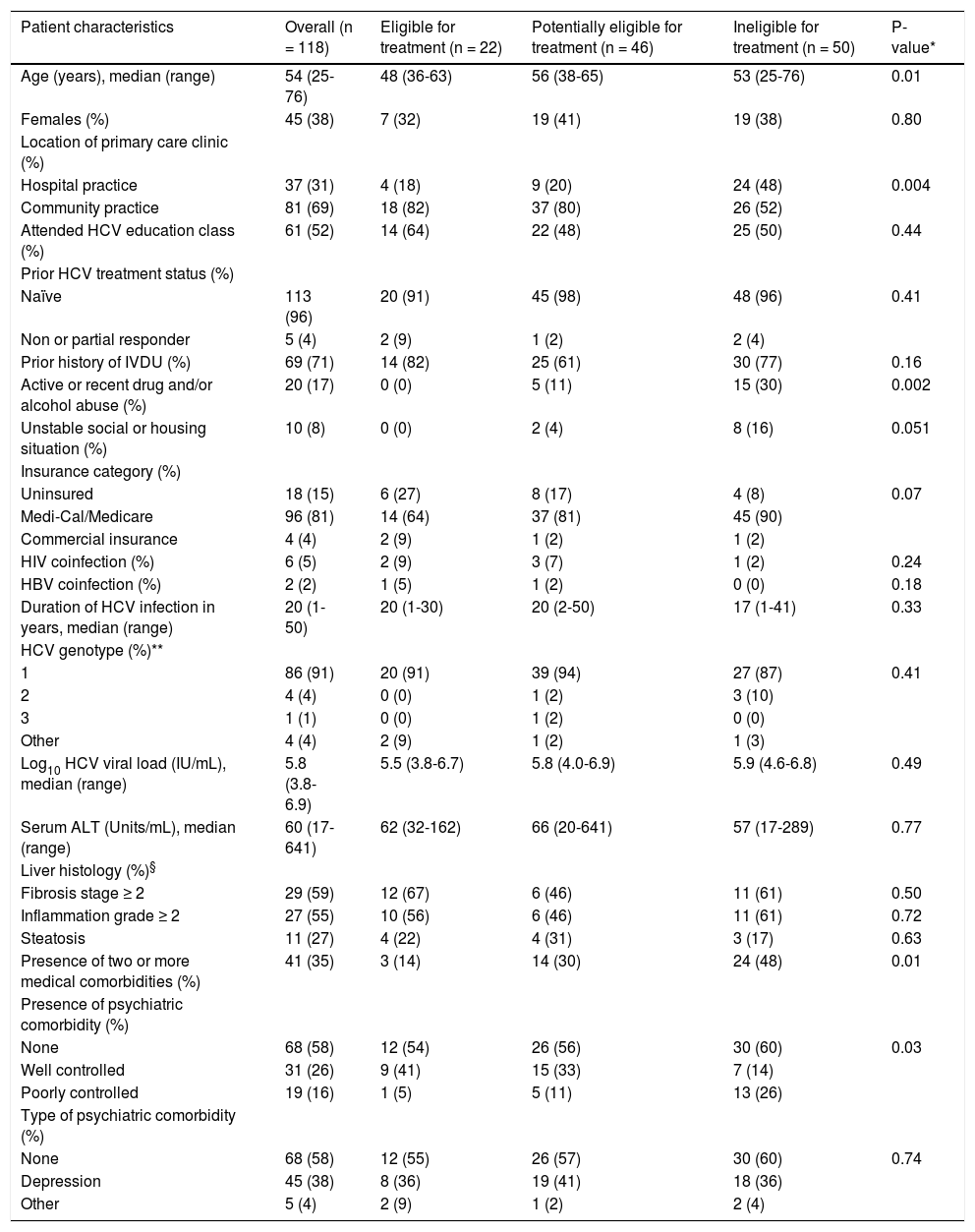
Table 1 summarizes patient characteristics overall and by treatment eligibility subgroup. Overall, the majority of patients were middle aged and 45% were female. Nearly all patients were infected with genotype 1 (91%) and were treatment naïve (96%). Among those who underwent a liver biopsy, 59% had moderate to severe liver disease. History of intravenous drug use was the most common mode of transmission of HCV and about 70% were referred from a community primary care practice. One third of patients had at least two medical comorbidities, and approximately 42% had concomitant psychiatric disease. Approximately half of all patients had attended formal HCV education by the liver specialty clinic. There were statistically significant differences among treatment eligible categories with respect to patient age, location of the primary care clinic, active or recent (within the past 6 months) drug and/or alcohol abuse, number of medical comorbidities, and control of psychiatric disease. On pairwise comparison, patients who were treatment eligible were younger than those who were potentially eligible (48 vs. 56 years old, p = 0.004). Patients who were ineligible for treatment were more likely to have active or recent drug and/or alcohol abuse (30 vs. 0%, p = 0.003 and 30 vs. 11%, p = 0.03, respectively) and poorly controlled psychiatric disease (26 vs. 5%, p = 0.02 and 26 vs. 11%, p = 0.04, respectively) compared to treatment eligible and potentially eligible patients. In addition, treatment ineligible patients were less likely to be referred from community-based primary care clinics (52 vs. 82%, p = 0.02 and 52 vs. 80%, p = 0.005, respectively). Moreover, treatment ineligible patients were more likely to have two or more medical comorbidities (48 vs. 14%, p = 0.008) than those who were treatment eligible.
Patient characteristics of based on treatment eligibility category.
| Patient characteristics | Overall (n = 118) | Eligible for treatment (n = 22) | Potentially eligible for treatment (n = 46) | Ineligible for treatment (n = 50) | P-value* |
|---|---|---|---|---|---|
| Age (years), median (range) | 54 (25-76) | 48 (36-63) | 56 (38-65) | 53 (25-76) | 0.01 |
| Females (%) | 45 (38) | 7 (32) | 19 (41) | 19 (38) | 0.80 |
| Location of primary care clinic (%) | |||||
| Hospital practice | 37 (31) | 4 (18) | 9 (20) | 24 (48) | 0.004 |
| Community practice | 81 (69) | 18 (82) | 37 (80) | 26 (52) | |
| Attended HCV education class (%) | 61 (52) | 14 (64) | 22 (48) | 25 (50) | 0.44 |
| Prior HCV treatment status (%) | |||||
| Naïve | 113 (96) | 20 (91) | 45 (98) | 48 (96) | 0.41 |
| Non or partial responder | 5 (4) | 2 (9) | 1 (2) | 2 (4) | |
| Prior history of IVDU (%) | 69 (71) | 14 (82) | 25 (61) | 30 (77) | 0.16 |
| Active or recent drug and/or alcohol abuse (%) | 20 (17) | 0 (0) | 5 (11) | 15 (30) | 0.002 |
| Unstable social or housing situation (%) | 10 (8) | 0 (0) | 2 (4) | 8 (16) | 0.051 |
| Insurance category (%) | |||||
| Uninsured | 18 (15) | 6 (27) | 8 (17) | 4 (8) | 0.07 |
| Medi-Cal/Medicare | 96 (81) | 14 (64) | 37 (81) | 45 (90) | |
| Commercial insurance | 4 (4) | 2 (9) | 1 (2) | 1 (2) | |
| HIV coinfection (%) | 6 (5) | 2 (9) | 3 (7) | 1 (2) | 0.24 |
| HBV coinfection (%) | 2 (2) | 1 (5) | 1 (2) | 0 (0) | 0.18 |
| Duration of HCV infection in years, median (range) | 20 (1-50) | 20 (1-30) | 20 (2-50) | 17 (1-41) | 0.33 |
| HCV genotype (%)** | |||||
| 1 | 86 (91) | 20 (91) | 39 (94) | 27 (87) | 0.41 |
| 2 | 4 (4) | 0 (0) | 1 (2) | 3 (10) | |
| 3 | 1 (1) | 0 (0) | 1 (2) | 0 (0) | |
| Other | 4 (4) | 2 (9) | 1 (2) | 1 (3) | |
| Log10 HCV viral load (IU/mL), median (range) | 5.8 (3.8-6.9) | 5.5 (3.8-6.7) | 5.8 (4.0-6.9) | 5.9 (4.6-6.8) | 0.49 |
| Serum ALT (Units/mL), median (range) | 60 (17-641) | 62 (32-162) | 66 (20-641) | 57 (17-289) | 0.77 |
| Liver histology (%)§ | |||||
| Fibrosis stage ≥ 2 | 29 (59) | 12 (67) | 6 (46) | 11 (61) | 0.50 |
| Inflammation grade ≥ 2 | 27 (55) | 10 (56) | 6 (46) | 11 (61) | 0.72 |
| Steatosis | 11 (27) | 4 (22) | 4 (31) | 3 (17) | 0.63 |
| Presence of two or more medical comorbidities (%) | 41 (35) | 3 (14) | 14 (30) | 24 (48) | 0.01 |
| Presence of psychiatric comorbidity (%) | |||||
| None | 68 (58) | 12 (54) | 26 (56) | 30 (60) | 0.03 |
| Well controlled | 31 (26) | 9 (41) | 15 (33) | 7 (14) | |
| Poorly controlled | 19 (16) | 1 (5) | 5 (11) | 13 (26) | |
| Type of psychiatric comorbidity (%) | |||||
| None | 68 (58) | 12 (55) | 26 (57) | 30 (60) | 0.74 |
| Depression | 45 (38) | 8 (36) | 19 (41) | 18 (36) | |
| Other | 5 (4) | 2 (9) | 1 (2) | 2 (4) |
IVDU: intravenous drug use. HCV: hepatitis C virus. HIV: human immunodeficiency virus. HBV: hepatitis B virus.
Among all patients, 42% were ineligible for treatment. Reasons for treatment ineligibility were predominantly medical (54%), followed by non-medical (14%), and psychiatric (4%), with the remaining 28% having a combination of these factors. The most commonly cited medical barriers to treatment were decompensated liver disease or presence of hepatocellular carcinoma, followed by low blood counts (leukopenia and/or anemia). The most commonly cited non-medical barriers to treatment were active drug and alcohol abuse followed by unstable social or housing situation. All cases of psychiatric barriers to treatment were due to poorly controlled psychiatric disease.
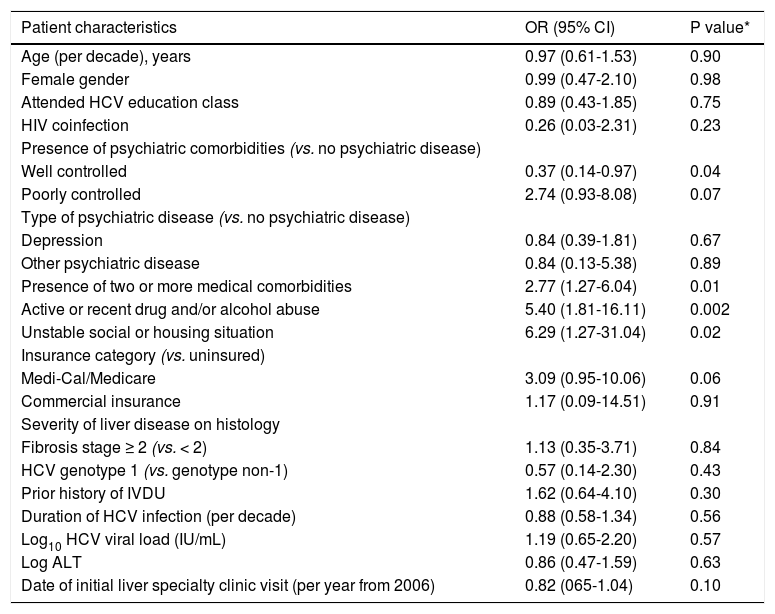
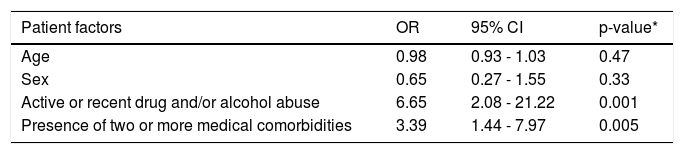
On univariate analysis (Table 2), factors associated with treatment ineligibility included having two or more medical comorbidities (OR 2.77, p = 0.01), active or recent drug and/or alcohol abuse (OR 5.4, p = 0.002), and unstable social or housing situation (6.29, p = 0.02). Well-controlled psychiatric disease was negatively associated with treatment ineligibility (OR 0.37, p = 0.04). On multivariate analysis, when controlling for age and sex, active or recent drug and/or alcohol abuse (OR 6.65, p = 0.001) and having two or more medical comorbidities (OR 3.39, p = 0.005) were the only independent predictors of treatment ineligibility (Table 3).
Univariate analysis of factors associated with HCV treatment ineligibility.
| Patient characteristics | OR (95% CI) | P value* |
|---|---|---|
| Age (per decade), years | 0.97 (0.61-1.53) | 0.90 |
| Female gender | 0.99 (0.47-2.10) | 0.98 |
| Attended HCV education class | 0.89 (0.43-1.85) | 0.75 |
| HIV coinfection | 0.26 (0.03-2.31) | 0.23 |
| Presence of psychiatric comorbidities (vs. no psychiatric disease) | ||
| Well controlled | 0.37 (0.14-0.97) | 0.04 |
| Poorly controlled | 2.74 (0.93-8.08) | 0.07 |
| Type of psychiatric disease (vs. no psychiatric disease) | ||
| Depression | 0.84 (0.39-1.81) | 0.67 |
| Other psychiatric disease | 0.84 (0.13-5.38) | 0.89 |
| Presence of two or more medical comorbidities | 2.77 (1.27-6.04) | 0.01 |
| Active or recent drug and/or alcohol abuse | 5.40 (1.81-16.11) | 0.002 |
| Unstable social or housing situation | 6.29 (1.27-31.04) | 0.02 |
| Insurance category (vs. uninsured) | ||
| Medi-Cal/Medicare | 3.09 (0.95-10.06) | 0.06 |
| Commercial insurance | 1.17 (0.09-14.51) | 0.91 |
| Severity of liver disease on histology | ||
| Fibrosis stage ≥ 2 (vs. < 2) | 1.13 (0.35-3.71) | 0.84 |
| HCV genotype 1 (vs. genotype non-1) | 0.57 (0.14-2.30) | 0.43 |
| Prior history of IVDU | 1.62 (0.64-4.10) | 0.30 |
| Duration of HCV infection (per decade) | 0.88 (0.58-1.34) | 0.56 |
| Log10 HCV viral load (IU/mL) | 1.19 (0.65-2.20) | 0.57 |
| Log ALT | 0.86 (0.47-1.59) | 0.63 |
| Date of initial liver specialty clinic visit (per year from 2006) | 0.82 (065-1.04) | 0.10 |
IVDU: intravenous drug use. OR: odds ratio. CI: confidence interval. HCV: hepatitis C virus.
Multivariate analysis of factors associated with HCV treatment ineligibility.
| Patient factors | OR | 95% CI | p-value* |
|---|---|---|---|
| Age | 0.98 | 0.93 - 1.03 | 0.47 |
| Sex | 0.65 | 0.27 - 1.55 | 0.33 |
| Active or recent drug and/or alcohol abuse | 6.65 | 2.08 - 21.22 | 0.001 |
| Presence of two or more medical comorbidities | 3.39 | 1.44 - 7.97 | 0.005 |
OR: odds ratio. CI: confidence interval.
A total of 68 patients were either eligible for treatment or were in the process of determination of treatment eligibility (defined as potentially eligible). Among the eligible patients (n = 22), one patient declined treatment, 18 decided to defer treatment to a later time of whom 4 were subsequently lost to follow-up, and 3 were agreeable to receive treatment but all were subsequently lost to follow-up. Reasons for deferring treatment included wanting to further discuss HCV treatment with family members, specific life events like a starting a new job, desire to conceive within the near future, and awaiting approval of direct acting HCV anti-viral medications.
Among potentially eligible patients, 91% were lost to follow-up while in the process of undergoing workup to determine treatment eligibility. One patient declined further work-up and the remaining three continued to follow in liver specialty clinic during the study period.
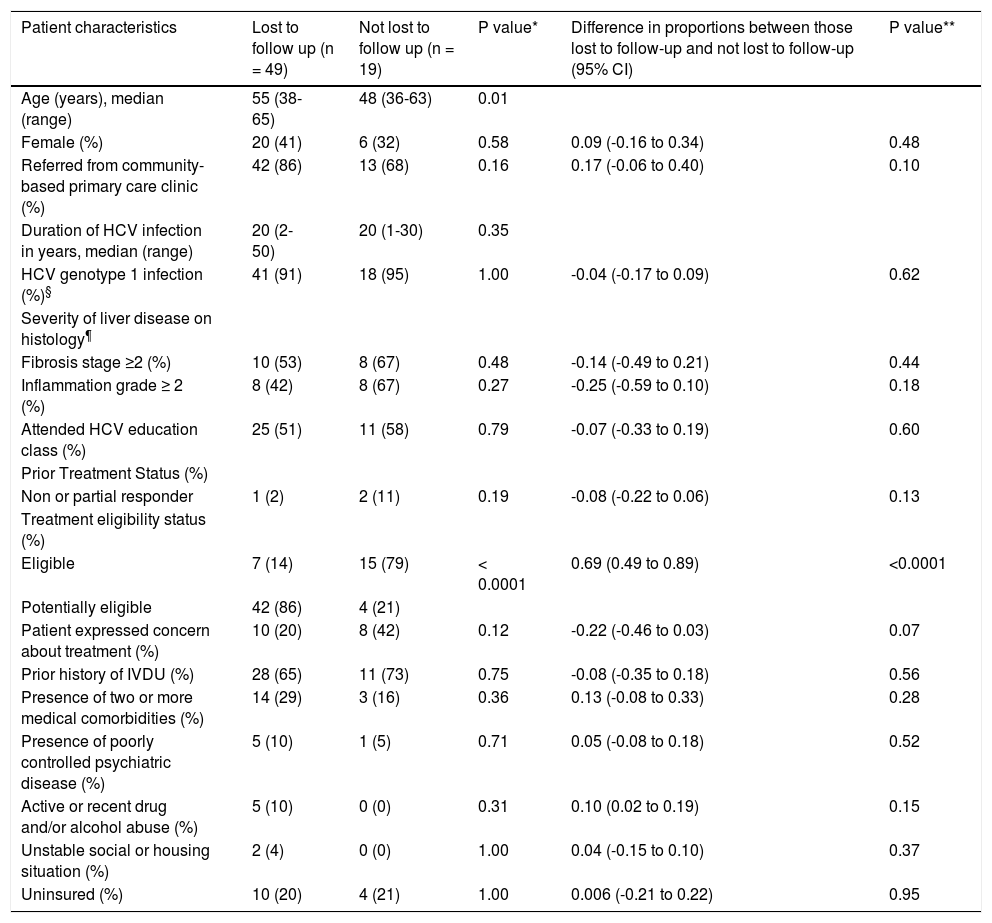
Characteristics of patients who were or were not lost to follow-up are listed in table 4. As anticipated, those lost to follow-up were more likely to be potentially eligible (in the process of determining treatment eligibility) compared to those not lost to follow-up (difference of 69%, p ≤ 0.0001). Patients lost to follow-up were also older (55 vs. 48 years, p = 0.01). Although statistically not significant, patients lost to follow-up were more likely to be referred from the community-based primary care clinics (difference of 17%) and to have active or recent drug and/or alcohol abuse (difference of 10%). They were however less likely to have received prior HCV treatment (difference of 8%), have severe liver disease on histology (difference of 25% for inflammation grade and 14% for fibrosis stage), or to have expressed concerns about HCV treatment (difference of 22%).
Characteristics of patients who were and were not lost to follow up among HCV treatment eligible and potentially eligible groups.
| Patient characteristics | Lost to follow up (n = 49) | Not lost to follow up (n = 19) | P value* | Difference in proportions between those lost to follow-up and not lost to follow-up (95% CI) | P value** |
|---|---|---|---|---|---|
| Age (years), median (range) | 55 (38-65) | 48 (36-63) | 0.01 | ||
| Female (%) | 20 (41) | 6 (32) | 0.58 | 0.09 (-0.16 to 0.34) | 0.48 |
| Referred from community-based primary care clinic (%) | 42 (86) | 13 (68) | 0.16 | 0.17 (-0.06 to 0.40) | 0.10 |
| Duration of HCV infection in years, median (range) | 20 (2-50) | 20 (1-30) | 0.35 | ||
| HCV genotype 1 infection (%)§ | 41 (91) | 18 (95) | 1.00 | -0.04 (-0.17 to 0.09) | 0.62 |
| Severity of liver disease on histology¶ | |||||
| Fibrosis stage ≥2 (%) | 10 (53) | 8 (67) | 0.48 | -0.14 (-0.49 to 0.21) | 0.44 |
| Inflammation grade ≥ 2 (%) | 8 (42) | 8 (67) | 0.27 | -0.25 (-0.59 to 0.10) | 0.18 |
| Attended HCV education class (%) | 25 (51) | 11 (58) | 0.79 | -0.07 (-0.33 to 0.19) | 0.60 |
| Prior Treatment Status (%) | |||||
| Non or partial responder | 1 (2) | 2 (11) | 0.19 | -0.08 (-0.22 to 0.06) | 0.13 |
| Treatment eligibility status (%) | |||||
| Eligible | 7 (14) | 15 (79) | < 0.0001 | 0.69 (0.49 to 0.89) | <0.0001 |
| Potentially eligible | 42 (86) | 4 (21) | |||
| Patient expressed concern about treatment (%) | 10 (20) | 8 (42) | 0.12 | -0.22 (-0.46 to 0.03) | 0.07 |
| Prior history of IVDU (%) | 28 (65) | 11 (73) | 0.75 | -0.08 (-0.35 to 0.18) | 0.56 |
| Presence of two or more medical comorbidities (%) | 14 (29) | 3 (16) | 0.36 | 0.13 (-0.08 to 0.33) | 0.28 |
| Presence of poorly controlled psychiatric disease (%) | 5 (10) | 1 (5) | 0.71 | 0.05 (-0.08 to 0.18) | 0.52 |
| Active or recent drug and/or alcohol abuse (%) | 5 (10) | 0 (0) | 0.31 | 0.10 (0.02 to 0.19) | 0.15 |
| Unstable social or housing situation (%) | 2 (4) | 0 (0) | 1.00 | 0.04 (-0.15 to 0.10) | 0.37 |
| Uninsured (%) | 10 (20) | 4 (21) | 1.00 | 0.006 (-0.21 to 0.22) | 0.95 |
IVDU: intravenous drug use. CI: confidence interval. HCV: hepatitis C virus.
To our knowledge, this is the first study to investigate reasons for HCV non-treatment among African Americans in a safety net healthcare system. Approximately 40% of HCV-infected African American patients who did not receive treatment were deemed treatment ineligible with contraindications to interferon-based HCV treatment. Presence of more than one medical comorbidity or active substance abuse predicted treatment ineligibility. Most strikingly, over 90% of patients in the process of evaluation of HCV treatment eligibility were lost to follow-up. In addition, about a third of patients who were eligible to receive therapy were also lost to follow-up.
The African American population within the safety net healthcare system warrants dedicated consideration. In addition to the disparate HCV disease burden nationally, African Americans within this setting may experience additional health disparities due to social, economic, or environmental disadvantage.16 Reduction of health disparities in viral hepatitis is a priority shared by the Institute of Medicine, Centers for Disease Control (CDC), and Department of Health and Human Services (DHHS).20–22 These organizations have underscored the need to better understand determinants of health, apart from access to care, which may impact a populations’ uptake of hepatitis treatment. Issues of health literacy and education, HCV awareness, cultural perception of disease, and psychosocial support encountered in the safety net healthcare system may further impact rates of HCV treatment uptake among African Americans.
To date, data on HCV non-treatment in African Americans is insufficient and none of the studies have focused on the underserved African American populations. While some have reported that African Americans are more likely to be ineligible for treatment or decline therapy when eligible, few have detailed the reasons for HCV non-treatment in this population.5–8 In the multi-ethnic HCV studies, the most commonly cited reasons for treatment ineligibility are normal liver enzymes, uncontrolled medical disease, uncontrolled psychiatric disease, and recent drug or alcohol use.7,13 Reasons for declining or deferring HCV treatment include asymptomatic or mild disease status and concerns over medication side effects.5,6,11,12 In this study, the most common reason for HCV non-treatment was treatment ineligibility which consistent with prior studies included decompensated liver disease, poorly controlled medical or psychiatric disease, and active drug or alcohol use. In addition, social and housing instability also contributed to reasons for treatment ineligibility in this underserved population. As expected, the San Francisco safetynet population represents patients who are predominantly uninsured or insured through government programs including Medi-Cal and Medicare. In 2012-2013, the proportion of various payer sources among patients attending the outpatient clinics at SFGH were: 10% were uninsured, 27% were enrolled in the Healthy San Francisco (a program operated by the San Francisco Department of Public Health that makes the healthcare services accessible and affordable to uninsured residents of San Francisco), 52% had Medi-Cal/Medicare, 2% commercial insurance, and 9% other sources.23 Although a higher number of HCV-infected safetynet patients attending the liver specialty clinic in this study had Medi-Cal/Medicare (81%) than the general SFGH population, insurance status was not associated with treatment eligibility or loss to follow-up. In this study, active substance abuse and higher number of medical comorbidities were the only independent predictors of treatment ineligibility when controlling for age and sex. Though the high cost of newer potent and better tolerated direct acting anti-HCV agents may prohibit access, they will likely increase rates of treatment eligibility and be more appealing to patients who have had prior concerns regarding treatment efficacy and adverse effects. Indeed, one of the reasons for deferring therapy in this study was interest in receipt of upcoming newer anti-HCV agents. Nevertheless, a proportion of the patients awaiting newer therapies were subsequently lost to follow-up highlighting the importance of timing of HCV therapy while engaged with healthcare in the at-risk underserved and disadvantaged populations.
While access to care remains an important consideration in the resource limited safety net healthcare setting, maintaining patient engagement in viral hepatitis care once accessed is critical to reducing HCV-related health disparity in this population. With the enactment of the Affordable Care Act to assist patients in accessing care, understanding other barriers to treatment in the most vulnerable groups becomes even more urgent. The most critical finding of our study was that despite access to liver specialty care, a high number (over 90%) of African American patients, especially older patients, were lost to follow-up while awaiting further workup to determine HCV treatment eligibility. Although not as extreme, the rate of loss to follow-up was also high at approximately 30% among those who were deemed eligible to initiate treatment. This finding was higher than expected based on available data to date from other healthcare settings. Reports from the IDEAL study and another from the VA Pittsburg Healthcare system suggest a loss to follow-up rates of approximately 25% among both eligible patients and those undergoing evaluation to assess HCV treatment eligibility.7,13 However, results from clinical trials may not be representative as patients who agree to participate in clinical trials may differ from the general population. In addition, other healthcare settings may have a higher level of care coordination compared to the safety net setting where resources may be limited. While health system limitations may contribute to patient retention, it is likely that other factors also play a role in adherence to HCV care. While provider attitudes play a significant role in accessing specialty services for HCV care, patient-related factors including patient preference, interest in treatment, health literacy, and social perception of disease may also affect their decision to engage in ongoing HCV management.16,18,24 Better understanding of healthcare system, provider, and patient factors associated with enhanced engagement with healthcare specifically among the underserved African American HCV-infected population, will likely result in improved adherence to HCV care and requires further investigation.
The main limitation of this study is a retrospective study design whereas the main strength is a comprehensive assessment of HCV non-treatment among the underserved African American population. While these findings are representative of the safety net population, a large proportion of the HCV-infected individuals in this country are from “health disparity populations” such as those with low income, low education, and poor health literacy and who are uninsured or underinsured.16,24 Evaluating reasons for HCV non-treatment in this population is therefore important especially in light of the enactment of the Affordable Care Act (ACA), whereby private and public health systems alike will have an influx of previously uninsured patients with chronic HCV similar to those described in this study.
Reducing health disparities in chronic hepatitis C requires a multimodal approach, and is a public health priority.16,20–22 We have shown that while medical comorbidities and active substance abuse are significant reasons for HCV non-treatment, disengaging from HCV care is also highly prevalent in the underserved African American population. While effective new direct acting antiviral agents improve treatment eligibility rates in those previously deemed ineligible, prospective studies aimed at better understanding why patients disengage from care, identifying gaps in outreach and education, and improving care coordination within health systems are needed in order to inform further interventions to reduce hepatitis C disparities in African Americans.
Abbreviations- •
ACA: Affordable care act.
- •
CDC: Centers for Disease Control.
- •
DHHS: Department of Health and Human Services.
- •
HCC: Hepatocellular carcinoma.
- •
HCV: Hepatitis C.
- •
SFGH: San Francisco General Hospital.
This work was supported by National Institutes of Health (NIH) K24AA022523 (to M.K.), and P30DK026743 (UCSF Liver Center).
Disclosure of Financial ArrangementsThere are no conflicts of interest.