Background and aims. Heterogeneous data has been reported regarding liver transplantation (LT) for hepatocellular carcinoma (HCC) in Latin America. We aimed to describe treatment during waiting list, survival and recurrence of HCC after LT in a multicenter study from Latin America.
Material and methods. Patients with HCC diagnosed prior to transplant (cHCC) and incidentally found in the explanted liver (iHCC) were included. Imaging-explanted features were compared in cHCC (non-discordant if pre and post-LT were within Milan, discordant if pre-LT was within and post-LT exceeding Milan).
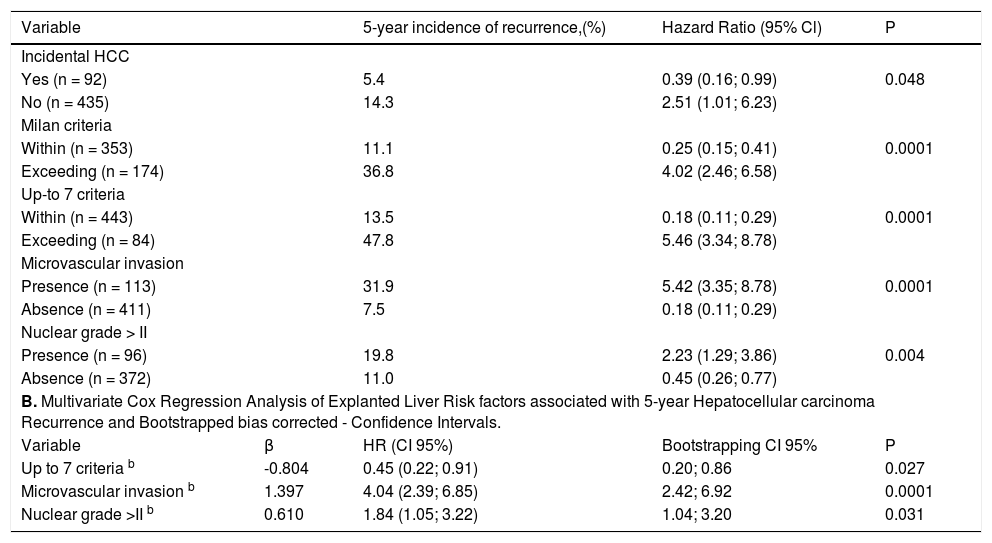
Results. Overall, 435 patients with cHCC and 92 with iHCC were included. At listing, 81% and 91% of cHCC patients were within Milan and San Francisco criteria (UCSF), respectively. Five-year survival and recurrence rates for cHCC within Milan, exceeding Milan/within UCSF and beyond UCSF were 71% and 16%; 66% and 26%; 46% and 55%, respectively. Locoregional treatment prior to LT was performed in 39% of cHCC within Milan, in 53% beyond Milan/within UCSF and in 83% exceeding UCSF (p < 0.0001). This treatment difference was not observed according to AFP values (<100, 44%; 101-1,000, 39%, and > 1,000 ng/mL 64%; p = 0.12). Discordant imaging-explanted data was observed in 29% of cHCC, showing lower survival HR 2.02 (CI 1.29; 3.15) and higher recurrence rates HR 2.34 when compared to AFP <100 ng/mL. Serum AFP > 1,000 ng/mL at listing was independently associated with a higher 5-year recurrence rate and a HR of 3.24 when compared to AFP <100 ng/mL.
Conclusion. Although overall results are comparable to other regions worldwide, preLT treatment not only considering imaging data but also AFP values should be contemplated during the next years.
Recent data from liver transplant centers in Europe and the United States have shown that approximately 20-30% of all liver transplants (LT) are performed for hepatocellular carcinoma (HCC).1,2 The Milan criteria were published 20 years ago and have been established as standard selection criteria for LT.3 However, other extended criteria have challenged the Milan criteria over the past years, including imaging data4 or combination of imaging and molecular markers.5,6
Currently, there are actually 13 countries in Latin America with Liver Transplant programs.7 More than 2,500 liver transplants are performed each year in this region, with a deceased donation rate of 8.3 per million population.7 Heterogeneous data has been reported to date related to LT for HCC in Latin America. Single or multicenter center experience have been published from different countries.8–17
With these isolated data, reporting a regional global measure for results of survival and recurrence at 5 years is still difficult. That is why we set this study at a regional level with the aim of describing selection criteria, treatment during waiting list and results in terms of survival and recurrence of hepatocellular carcinoma after liver transplantation in a multicenter study from Latin America.
Material and MethodsStudy design, setting and participating centersThis study was conducted including a multicenter Latin American cohort of consecutive adult patients (> 17 years of age) who underwent a first LT between June 1 2005 and December 31 2011 in 17 different LT centers and were prospectively followed after transplantation. Participating centers appointed a study coordinator responsible for data collection. In cases of conflicting or missing data, central revision and resubmission were requested.
Eligibility criteria and study variablesCriteria for inclusion required patients to be adult cirrhotic or non-cirrhotic recipients with confirmed HCC in the explanted liver. Patients were excluded if other tumors than HCC were confirmed in the explanted liver.
Recipient characteristics, pre-transplant tumor characteristics and serum α-fetoprotein (AFP) levels were obtained at listing. Subjects with HCC diagnosis prior to transplant (cHCC) based on imaging criteria18 were classified according to Milan (MC)3 and University of California San Francisco criteria (UCSF),4 depending on size and number of lesions detected on pre-LT computerized tomography (CT) or magnetic resonance images (MRI). Pre-transplant AFP levels were registered in parallel with imaging data at listing and the following cut-offs were considered: ≤ 100 ng/mL, 101-1,000 ng/mL, and > 1,000 ng/ mL.5 Standard patient selection in all centers was limited to patients with tumors meeting MC. Transplantation for patients exceeding MC was discussed at each transplant center on a case-by-case basis. Site-specific organ allocation policies were also registered.
Tumor treatment before transplantation was recorded including trans-arterial chemoembolization (TACE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI) and liver resection. Among patients who received any local/regional tumor treatment prior to transplant, both last serum AFP and imaging results for restaging were also considered. In patients exceeding MC, downstaging was defined as reducing the tumor size specifically to meet MC.19
Pre-LT images (CT or MRI) were compared with explanted liver findings, including: macroscopic and microscopic evaluation of each nodule, number and diameter (cm) of each, presence of microvascular invasion (mvi), and degree of tumor differentiation according to Edmonson-Steiner grading system.20 Finally, Milan and Up-to seven criteria21 were also applied to the explanted liver specimen. Patients with tumors discovered on final pathology without a preceding diagnosis were catalogued as incidental HCC (iHCC). If a patient had previously known HCC and more nodules were found in the explanted liver, these nodules were not categorized as iHCC.
Discordant imaging-explanted features were assessed and defined as non-discordant if a patient met imaging pre-LT Milan criteria at listing and remained within Milan criteria in the explanted liver; whereas discordant was define as patients meeting Milan at listing and exceeding this criteria after explanted liver analysis. This latter analysis was done excluding iHCC.
Study end-pointsPrimary end-points analyzed were 5-year patient survival and HCC recurrence. All patients were followed-up until death or last outpatient visit. Post-transplant followup for HCC recurrence consisted of one CT or MRI, bone scintigraphy and serum AFP assay every 6 months, as recommended.22 Recurrence was determined on the basis of imaging criteria plus serum AFP or by biopsy. Time to recurrence (TTR) was considered a robust clinical outcome measure and calculated as the time in months elapsed between transplantation and diagnosis of recurrence.
All procedures followed were in accordance with STROBE guidelines.23 This study was approved by the Austral University Hospital and School of Medicine and by each center ethics committee. It complied with the ethical standards (institutional and national) and with Helsinki Declaration of 1975, as revised in 2008. Patient free term consent was obtained from all subjects included.
Statistical analysisCategorical data were compared using Fisher’s exact test or Chi-Square (χ2) test (2-tailed). Continuous variables were compared with Student’s T test or Wilcoxon ranksum test according to their distribution, respectively. A multivariate Cox regression analysis, with hazard ratios (HR) and 95% confidence intervals (95% CI) for identifying baseline pre and post-transplant risk variables for 5-year mortality and recurrence was carried out evaluating potential confounding variables. Clinical effect modifiers related to HCC recurrence were also evaluated including type of immunosuppression after LT. Dummies for ordinal or categorical variables were assessed. Variables with a P value < 0.05 after the univariate analysis were included in the multivariate model, generated by stepwise forward elimination evaluating P values (Wald test) and considering adjusted HR with confounding variables (> 20% of change in crude HR). Adjustment of each final model was evaluated with proportional hazards through graphic and statistical evaluation (Schoenfeld residual test). Calibration was assessed by comparison of observed and predicted curves and evaluation of the goodness of fit of the model by Harrell’s c-statistic index. A competing risk analysis with death and recurrence was done with calculation of subhazard ratios (SHR) and 95% CI. Kaplan Meier survival curves were compared using the log-rank test (Mantel-Cox). Collected data were analyzed with STATA 10.0.
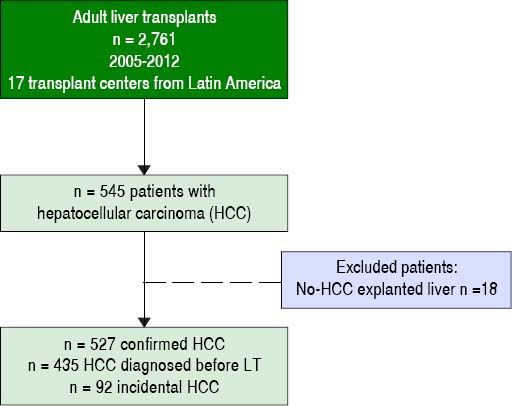
ResultsParticipating centers and patients characteristicsFrom a total of 2,761 consecutive adult LT patients in 17 different centers during the study period previously described, 435 patients with cHCC and 92 with iHCC were included (Figure 1). Percentage of patients contributed by participating LT centers per country was as follows: 2 from Brazil (n = 221, 41.9%), 5 transplant programs from Argentina (n = 136, 25.8%), 2 from Colombia (n = 77, 14.6%), 4 from Chile (n = 55, 10.4%), 2 from Mexico (n = 13, 2.5%), and 1 from Peru (n = 14, 2.7%) and Uruguay (n = 11, 2.1%). Patients within MC from Argentina, Brazil, Uruguay, Peru and Chile could receive additional MELD points while on the waiting list. Table 1 shows a description of the overall cohort. Subsequent analysis was considered excluding incidental HCC.
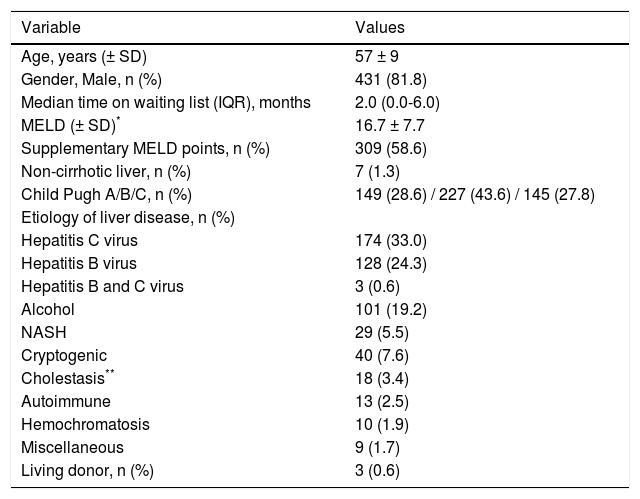
Patients’ Baseline Characteristics.
| Variable | Values |
|---|---|
| Age, years (± SD) | 57 ± 9 |
| Gender, Male, n (%) | 431 (81.8) |
| Median time on waiting list (IQR), months | 2.0 (0.0-6.0) |
| MELD (± SD)* | 16.7 ± 7.7 |
| Supplementary MELD points, n (%) | 309 (58.6) |
| Non-cirrhotic liver, n (%) | 7 (1.3) |
| Child Pugh A/B/C, n (%) | 149 (28.6) / 227 (43.6) / 145 (27.8) |
| Etiology of liver disease, n (%) | |
| Hepatitis C virus | 174 (33.0) |
| Hepatitis B virus | 128 (24.3) |
| Hepatitis B and C virus | 3 (0.6) |
| Alcohol | 101 (19.2) |
| NASH | 29 (5.5) |
| Cryptogenic | 40 (7.6) |
| Cholestasis** | 18 (3.4) |
| Autoimmune | 13 (2.5) |
| Hemochromatosis | 10 (1.9) |
| Miscellaneous | 9 (1.7) |
| Living donor, n (%) | 3 (0.6) |
MELD: Model for End Stage Liver Disease. NASH: Non-Alcoholic Steatohepatitis.
Among patients with cHCC, median time on the waitlist was 3 (IQR 1-9 months); 58.6% (n = 309) were granted with supplementary MELD points. At listing, 81% (n = 354) and 91% (n = 393) of the patients were within MC and UCSF criteria, respectively. Considering extended criteria, 9.2% of the patients were beyond MC/ within UCSF (n = 40) and 9.4% (n = 41) were beyond UCSF criteria. Four patients had missing AFP values.
With respect to specific pre-LT images, among cHCC patients a CT alone was done in 58% of the cohort (n = 251), MRI alone in 27% (n = 117) and both CT/MRI in 15% (n = 66). A tumor biopsy for HCC diagnosis was performed in 8 patients (1.8%). In 315 cHCC patients, a last additional image evaluation was done with a CT alone in 63% (n = 199), an MRI alone in 35% (n = 111) and with both methods in 5 patients. Median time from last imaging evaluation to transplantation was 3 months (IQR 1-5 months).
Bridging therapies prior to transplantation were performed in 44.4% of patients with cHCC (n = 193). Of these, 71.5% (n = 138), 10.9% (n = 21) and 17.6% (n = 34) were within Milan, beyond Milan/within UCSF, and beyond UCSF at listing, respectively. Median time from last treatment to LT was 4.0 months (IQR 2.0-9.0 months) (Table 2). Among patients receiving local/regional treatments, 143 patients were restaged after bridging therapies. At last evaluation 82% of the patients were within Milan, 8% were beyond Milan/within UCSF and 10% were beyond UCSF.
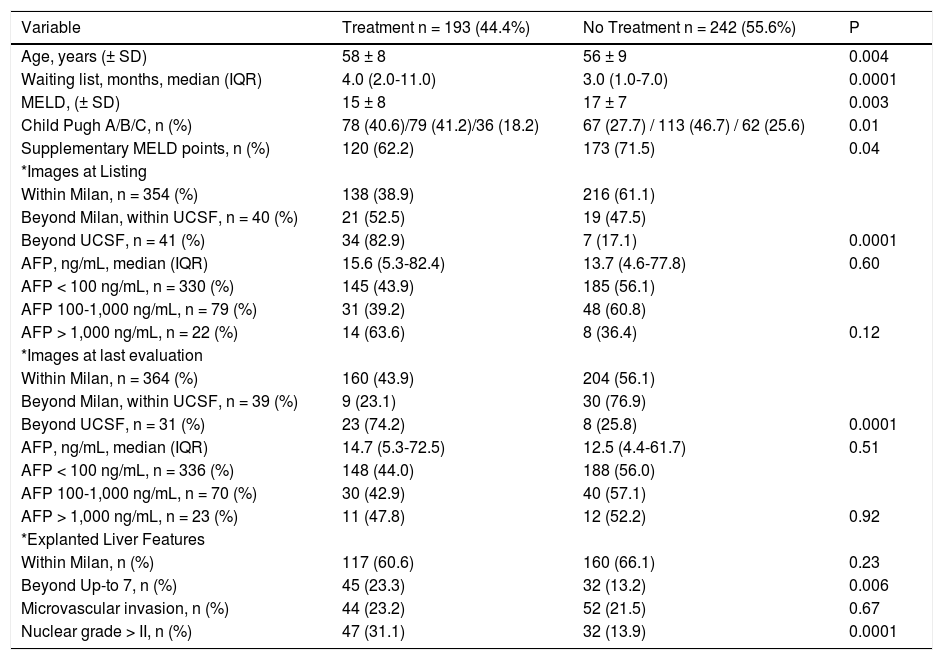
Comparative analysis according to locoregional treatment before liver transplantation.
| Variable | Treatment n = 193 (44.4%) | No Treatment n = 242 (55.6%) | P |
|---|---|---|---|
| Age, years (± SD) | 58 ± 8 | 56 ± 9 | 0.004 |
| Waiting list, months, median (IQR) | 4.0 (2.0-11.0) | 3.0 (1.0-7.0) | 0.0001 |
| MELD, (± SD) | 15 ± 8 | 17 ± 7 | 0.003 |
| Child Pugh A/B/C, n (%) | 78 (40.6)/79 (41.2)/36 (18.2) | 67 (27.7) / 113 (46.7) / 62 (25.6) | 0.01 |
| Supplementary MELD points, n (%) | 120 (62.2) | 173 (71.5) | 0.04 |
| *Images at Listing | |||
| Within Milan, n = 354 (%) | 138 (38.9) | 216 (61.1) | |
| Beyond Milan, within UCSF, n = 40 (%) | 21 (52.5) | 19 (47.5) | |
| Beyond UCSF, n = 41 (%) | 34 (82.9) | 7 (17.1) | 0.0001 |
| AFP, ng/mL, median (IQR) | 15.6 (5.3-82.4) | 13.7 (4.6-77.8) | 0.60 |
| AFP < 100 ng/mL, n = 330 (%) | 145 (43.9) | 185 (56.1) | |
| AFP 100-1,000 ng/mL, n = 79 (%) | 31 (39.2) | 48 (60.8) | |
| AFP > 1,000 ng/mL, n = 22 (%) | 14 (63.6) | 8 (36.4) | 0.12 |
| *Images at last evaluation | |||
| Within Milan, n = 364 (%) | 160 (43.9) | 204 (56.1) | |
| Beyond Milan, within UCSF, n = 39 (%) | 9 (23.1) | 30 (76.9) | |
| Beyond UCSF, n = 31 (%) | 23 (74.2) | 8 (25.8) | 0.0001 |
| AFP, ng/mL, median (IQR) | 14.7 (5.3-72.5) | 12.5 (4.4-61.7) | 0.51 |
| AFP < 100 ng/mL, n = 336 (%) | 148 (44.0) | 188 (56.0) | |
| AFP 100-1,000 ng/mL, n = 70 (%) | 30 (42.9) | 40 (57.1) | |
| AFP > 1,000 ng/mL, n = 23 (%) | 11 (47.8) | 12 (52.2) | 0.92 |
| *Explanted Liver Features | |||
| Within Milan, n (%) | 117 (60.6) | 160 (66.1) | 0.23 |
| Beyond Up-to 7, n (%) | 45 (23.3) | 32 (13.2) | 0.006 |
| Microvascular invasion, n (%) | 44 (23.2) | 52 (21.5) | 0.67 |
| Nuclear grade > II, n (%) | 47 (31.1) | 32 (13.9) | 0.0001 |
Normal values: alpha-fetoprotein 0.6-4.4 ng/mL. AFP: alpha-fetoprotein. HCC: hepatocellular carcinoma.
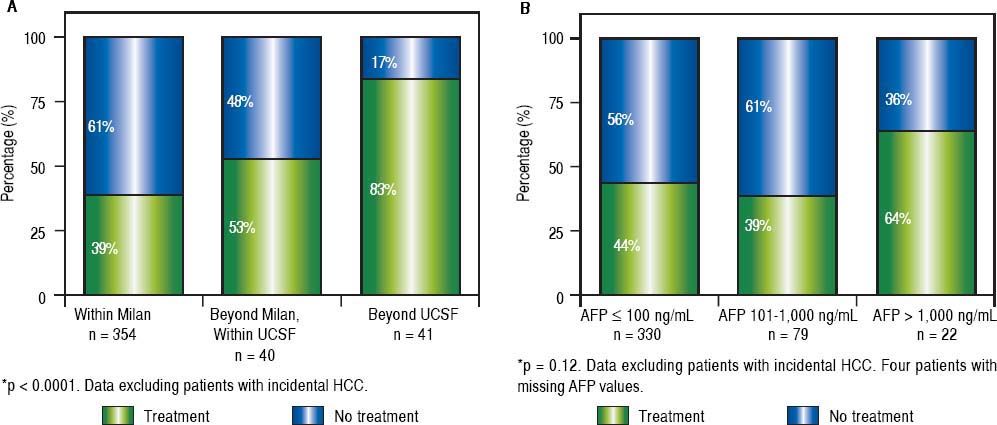
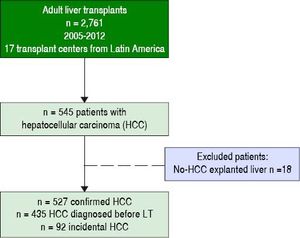
The clinical decision to perform a pre-transplant locoregional treatment was largely assessed according to the diameter and number of nodules by imaging data and according to pre-LT selection criteria (Figure 2A). It was observed that pre-LT treatment was done incrementally when patients were within Milan 39%, beyond Milan/ within UCSF 53% and exceeding UCSF 83% (P < 0.0001). On the other hand, this proportional increase was not observed when considering serum AFP values, particularly in the intermediate risk stratum of 101-1,000 ng/mL (Figure 2B).
Proportion of patients receiving pre-transplantation locoregional tumor treatment considering imaging data (A) and serum AFP values at listing (B). Note: Locoregional treatment prior to LT was performed in 44% of cHCC patients; in 39% of cHCC within Milan, in 53% beyond Milan/within UCSF and in 83% exceeding UCSF (P < 0.0001). However, there was no significant difference in the proportion of patients receiving pre-LT locoregional treatment according to pre-LT AFP values ( ≤ 100 ng/mL 44%, 101-1,000 ng/mL 39%, and > 1,000 ng/mL 64%; p = 0.12).
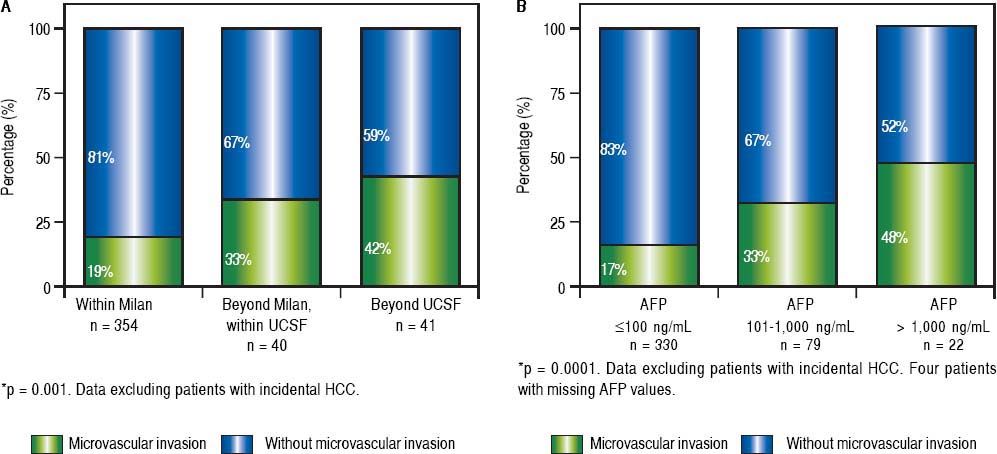
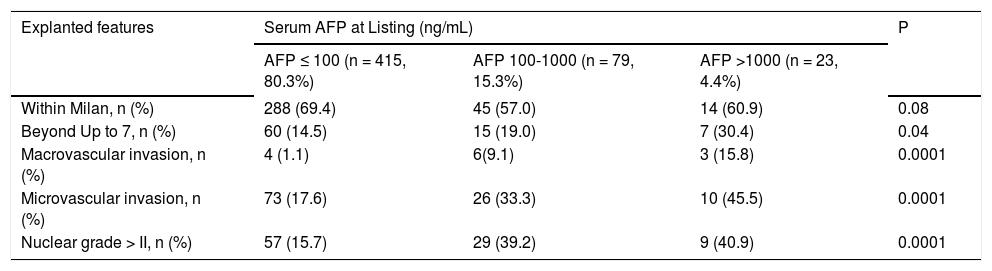
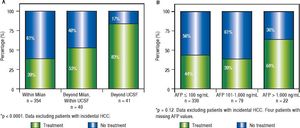
Whereas increasing proportion of microvascular invasion was observed considering MC or extended criteria at listing (Figure 3A), pre-LT serum AFP cut-off values were also associated with increasing proportion of microvascular invasion and undifferentiated tumors (Figure 3B, Table 3).
Relationship between AFP levels and Histopathologic features of hepatocellular carcinoma.
| Explanted features | Serum AFP at Listing (ng/mL) | P | ||
|---|---|---|---|---|
| AFP ≤ 100 (n = 415, 80.3%) | AFP 100-1000 (n = 79, 15.3%) | AFP >1000 (n = 23, 4.4%) | ||
| Within Milan, n (%) | 288 (69.4) | 45 (57.0) | 14 (60.9) | 0.08 |
| Beyond Up to 7, n (%) | 60 (14.5) | 15 (19.0) | 7 (30.4) | 0.04 |
| Macrovascular invasion, n (%) | 4 (1.1) | 6(9.1) | 3 (15.8) | 0.0001 |
| Microvascular invasion, n (%) | 73 (17.6) | 26 (33.3) | 10 (45.5) | 0.0001 |
| Nuclear grade > II, n (%) | 57 (15.7) | 29 (39.2) | 9 (40.9) | 0.0001 |
Normal Values: alpha-fetoprotein 0.6-4.4 ng/mL. AFP: alpha-fetoprotein.
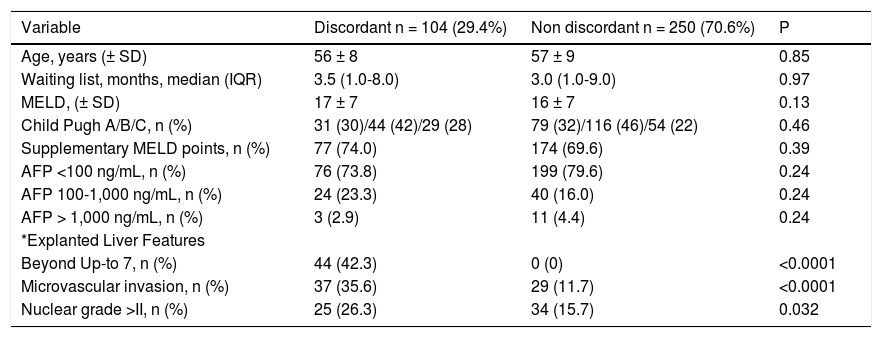
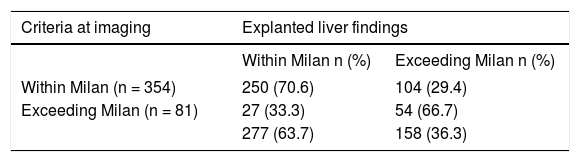
Discordant imaging-explanted data was observed in 104 out of 354 patients (29.4%) (Table 4). Rates of discordance (Milan IN-Milan OUT) according to last imaging modality were as follows: CT alone 34.6% discordance, MRI alone 20.4% discordance and both methods MRI/CT 26.4% discordance (P = 0.031). Although no significant differences were observed regarding serum AFP cut-offs values between discordant and non-discordant HCC patients, discordant HCC presented higher proportion of patients beyond Up-to 7 and higher proportion of micro-vascular invasion and dedifferentiated tumors (Table 4). Regarding assessment of MC, pre-LT imaging data showed a sensitivity of 0.90, specificity of 0.65, accuracy of 0.77, positive Likelihood ratio of 2.57, and negative Likelihood ratio of 0.15, when compared with explanted liver findings (Table 5).
Comparative analysis according to discordant vs. non-discordant imaging-explanted liver data.
| Variable | Discordant n = 104 (29.4%) | Non discordant n = 250 (70.6%) | P |
|---|---|---|---|
| Age, years (± SD) | 56 ± 8 | 57 ± 9 | 0.85 |
| Waiting list, months, median (IQR) | 3.5 (1.0-8.0) | 3.0 (1.0-9.0) | 0.97 |
| MELD, (± SD) | 17 ± 7 | 16 ± 7 | 0.13 |
| Child Pugh A/B/C, n (%) | 31 (30)/44 (42)/29 (28) | 79 (32)/116 (46)/54 (22) | 0.46 |
| Supplementary MELD points, n (%) | 77 (74.0) | 174 (69.6) | 0.39 |
| AFP <100 ng/mL, n (%) | 76 (73.8) | 199 (79.6) | 0.24 |
| AFP 100-1,000 ng/mL, n (%) | 24 (23.3) | 40 (16.0) | 0.24 |
| AFP > 1,000 ng/mL, n (%) | 3 (2.9) | 11 (4.4) | 0.24 |
| *Explanted Liver Features | |||
| Beyond Up-to 7, n (%) | 44 (42.3) | 0 (0) | <0.0001 |
| Microvascular invasion, n (%) | 37 (35.6) | 29 (11.7) | <0.0001 |
| Nuclear grade >II, n (%) | 25 (26.3) | 34 (15.7) | 0.032 |
Normal Values: alpha-fetoprotein 0.6-4.4 ng/mL. AFP: alpha-fetoprotein. HCC: hepatocellular carcinoma.
Precision of Images vs. explanted liver findings.
| Criteria at imaging | Explanted liver findings | |
|---|---|---|
| Within Milan n (%) | Exceeding Milan n (%) | |
| Within Milan (n = 354) | 250 (70.6) | 104 (29.4) |
| Exceeding Milan (n = 81) | 27 (33.3) | 54 (66.7) |
| 277 (63.7) | 158 (36.3) | |
Overall imaging data before transplantation when compared with explanted liver findings showed Sensitivity of 0.90, Specificity of 0.65, Accuracy of 0.77, positive Likelihood ratio of 2.57, and negative Likelihood ratio of. 0.15.
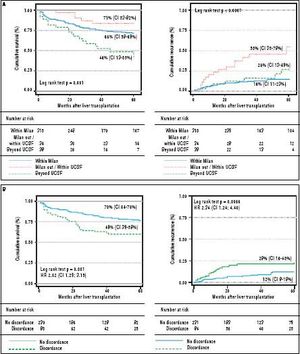
Median follow-up in the overall cohort was 37.0 months (IQR 13.5-64.0 months). Patient survival and recurrence rates at 1, 3 and 5 years were 77.7%, 67.4% and 64.8% (n = 153 deaths) and 7.1%, 12.2% and 14.2% (n = 62 recurrences), respectively. The main causes of death were recurrent HCC 34.2% (n = 50) and sepsis 20.6% (n = 30). Median TTR was 13.0 months (IQR 6.0-28.5 months). Median survival following recurrence diagnosis was 6.0 months (IQR 3.0-14.0 months). Recurrence rates for patients meeting MC, and UCSF are shown on table 6.
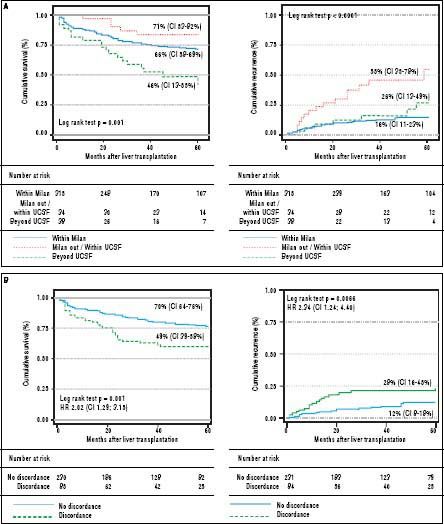
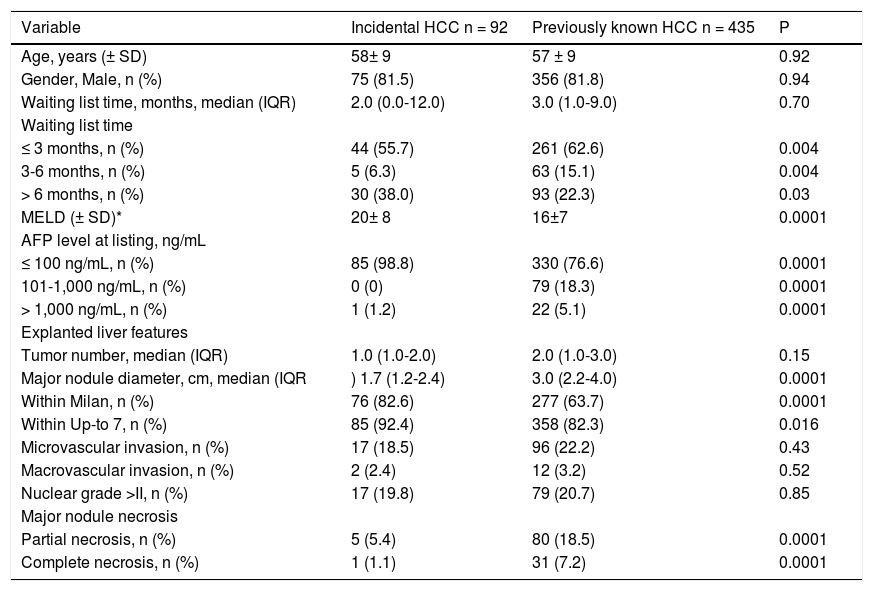
Survival and recurrence rates according to UCSF and MC are shown on Figure 4A. Patients with discordant imaging-explanted data had lower 5-year survival HR 2.02 (CI 1.29; 3.15) and higher cumulative recurrence HR 2.34 (CI 1.24; 4.40) (Figure 4B). Patients who were exceeding Milan criteria at listing and were finally within Milan in the explanted liver (n = 27) had lower risk of recurrence when compared to those patients exceeding both Milan criteria at listing and in the explanted liver (n = 54) HR 0.23 (CI 0.06; 0.78). There was not a significant survival difference comparing iHCC and HCC (60.4% vs. 65%; p = 0.34); however, iHCC had higher proportion of nonHCC related deaths (88.2% vs. 59.3%; p = 0.001) and a lower 5-year recurrence rate when compared to previously known HCC (5.4% vs. 14.3%; p = 0.048) (Table 7).
Tumor recurrence and patient survival rates according to Milan or University of California San Francisco criteria (A) and discordant versus non-discordant imaging-explanted data (B) (Kaplan Meier; log Rank test). Note: Survival was not significantly different between patients within Milan and within UCSF criteria at listing; patients beyond UCSF had higher mortality survival rate with a HR of 1.70 (C1 1.08-2.67; p = 0.03). Patients with discordant imagingexplanted data had lower survival and higher recurrence rates. Data excluding patients with incidental HCC.
Comparative analysis between previously known and incidental HCC.
| Variable | Incidental HCC n = 92 | Previously known HCC n = 435 | P |
|---|---|---|---|
| Age, years (± SD) | 58± 9 | 57 ± 9 | 0.92 |
| Gender, Male, n (%) | 75 (81.5) | 356 (81.8) | 0.94 |
| Waiting list time, months, median (IQR) | 2.0 (0.0-12.0) | 3.0 (1.0-9.0) | 0.70 |
| Waiting list time | |||
| ≤ 3 months, n (%) | 44 (55.7) | 261 (62.6) | 0.004 |
| 3-6 months, n (%) | 5 (6.3) | 63 (15.1) | 0.004 |
| > 6 months, n (%) | 30 (38.0) | 93 (22.3) | 0.03 |
| MELD (± SD)* | 20± 8 | 16±7 | 0.0001 |
| AFP level at listing, ng/mL | |||
| ≤ 100 ng/mL, n (%) | 85 (98.8) | 330 (76.6) | 0.0001 |
| 101-1,000 ng/mL, n (%) | 0 (0) | 79 (18.3) | 0.0001 |
| > 1,000 ng/mL, n (%) | 1 (1.2) | 22 (5.1) | 0.0001 |
| Explanted liver features | |||
| Tumor number, median (IQR) | 1.0 (1.0-2.0) | 2.0 (1.0-3.0) | 0.15 |
| Major nodule diameter, cm, median (IQR | ) 1.7 (1.2-2.4) | 3.0 (2.2-4.0) | 0.0001 |
| Within Milan, n (%) | 76 (82.6) | 277 (63.7) | 0.0001 |
| Within Up-to 7, n (%) | 85 (92.4) | 358 (82.3) | 0.016 |
| Microvascular invasion, n (%) | 17 (18.5) | 96 (22.2) | 0.43 |
| Macrovascular invasion, n (%) | 2 (2.4) | 12 (3.2) | 0.52 |
| Nuclear grade >II, n (%) | 17 (19.8) | 79 (20.7) | 0.85 |
| Major nodule necrosis | |||
| Partial necrosis, n (%) | 5 (5.4) | 80 (18.5) | 0.0001 |
| Complete necrosis, n (%) | 1 (1.1) | 31 (7.2) | 0.0001 |
AFP: alpha-fetoprotein. MELD: Model for End stage Liver Disease.
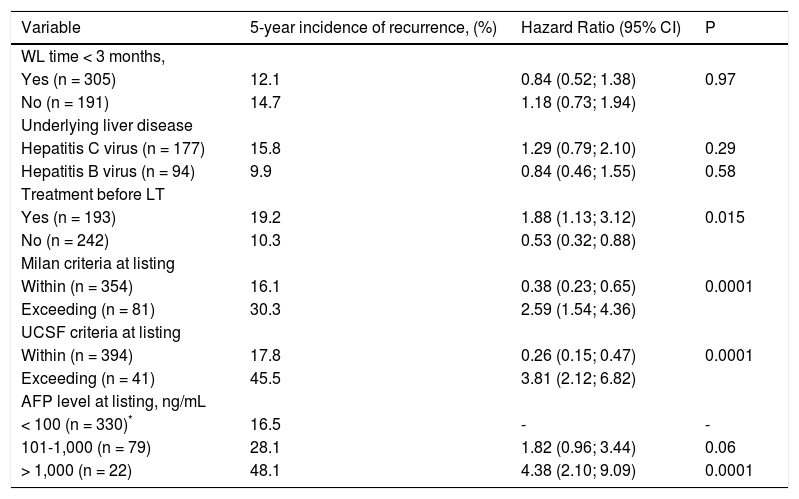
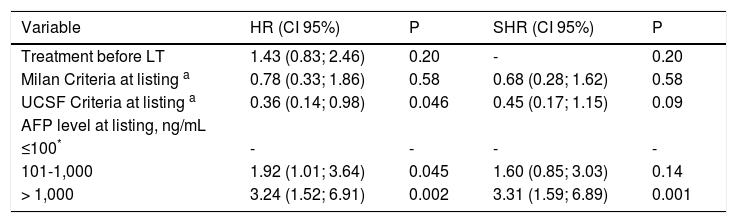
Tables 6 A-B shows results from the Cox regression analysis regarding risk of recurrence including pre LT variables. In univariate analysis, patients exceeding Milan criteria had a 5-year risk of recurrence of 27.2% with a HR of 2.59 (CI 1.54; 4.36). Exceeding UCSF criteria showed the highest risk of recurrence with a crude HR of 3.81 (CI 2.12; 6.82). Dummies for AFP cut-offvalues showed that a serum AFP > 1,000 ng/mL presented 4 more times of recurrence risk in the overall cohort (HR 4.38; p = 0.0001). Adjusted HRs from the multivariate Cox regression model showed that AFP > 1,000 ng/mL at listing was independently associated with 5-year HCC recurrence with an adjusted HR of 3.24 (CI 1.52; 6.91) (Table 6, Panel B). In the Cox regression model, the adjusted HR for treatment before LT was not independently associated with 5-year recurrence.
Pre transplant variables associated with 5-year HCC recurrence after liver transplantation. Univariate Cox regression.
| Variable | 5-year incidence of recurrence, (%) | Hazard Ratio (95% CI) | P |
|---|---|---|---|
| WL time < 3 months, | |||
| Yes (n = 305) | 12.1 | 0.84 (0.52; 1.38) | 0.97 |
| No (n = 191) | 14.7 | 1.18 (0.73; 1.94) | |
| Underlying liver disease | |||
| Hepatitis C virus (n = 177) | 15.8 | 1.29 (0.79; 2.10) | 0.29 |
| Hepatitis B virus (n = 94) | 9.9 | 0.84 (0.46; 1.55) | 0.58 |
| Treatment before LT | |||
| Yes (n = 193) | 19.2 | 1.88 (1.13; 3.12) | 0.015 |
| No (n = 242) | 10.3 | 0.53 (0.32; 0.88) | |
| Milan criteria at listing | |||
| Within (n = 354) | 16.1 | 0.38 (0.23; 0.65) | 0.0001 |
| Exceeding (n = 81) | 30.3 | 2.59 (1.54; 4.36) | |
| UCSF criteria at listing | |||
| Within (n = 394) | 17.8 | 0.26 (0.15; 0.47) | 0.0001 |
| Exceeding (n = 41) | 45.5 | 3.81 (2.12; 6.82) | |
| AFP level at listing, ng/mL | |||
| < 100 (n = 330)* | 16.5 | - | - |
| 101-1,000 (n = 79) | 28.1 | 1.82 (0.96; 3.44) | 0.06 |
| > 1,000 (n = 22) | 48.1 | 4.38 (2.10; 9.09) | 0.0001 |
Normal Values: alpha-fetoprotein 0.6-4.4 ng/mL. AFP: alpha-fetoprotein. HCC: hepatocellular carcinoma. LT: liver transplantation. UCSF: University of California San Francisco. WL: waiting list.
Multivariate Cox Regression Analysis of Pre-Transplant Risk factors associated with 5-year Hepatocellular carcinoma Recurrence and Competing Risk Analysis - Confidence Intervals.
| Variable | HR (CI 95%) | P | SHR (CI 95%) | P |
|---|---|---|---|---|
| Treatment before LT | 1.43 (0.83; 2.46) | 0.20 | - | 0.20 |
| Milan Criteria at listing a | 0.78 (0.33; 1.86) | 0.58 | 0.68 (0.28; 1.62) | 0.58 |
| UCSF Criteria at listing a | 0.36 (0.14; 0.98) | 0.046 | 0.45 (0.17; 1.15) | 0.09 |
| AFP level at listing, ng/mL | ||||
| ≤100* | - | - | - | - |
| 101-1,000 | 1.92 (1.01; 3.64) | 0.045 | 1.60 (0.85; 3.03) | 0.14 |
| > 1,000 | 3.24 (1.52; 6.91) | 0.002 | 3.31 (1.59; 6.89) | 0.001 |
HR: Hazard ratio. SHR: subhazard ratio. Schoenfeld’s residual test, proportional hazard assumption p = 0.43. Harrel’s concordance statistic 0.62. Competing risk analysis (SHR) was considered: competing event, death.
A second Cox regression model including post-transplant data showed that microvascular invasion decreased survival for each stratum assessed in the explanted liver (Table 8). According to explanted liver data, patients exceeding Milan criteria within Up-to 7 and without microvascular invasion had similar 5-year recurrence rate when compared to patients within MC in the explanted liver (HR 0.91; CI 0.32-2.62). Patients exceeding Up-to 7 criteria without microvascular invasion had higher risk of recurrence with a HR of 2.98 (CI 1.39-6.39) when compared to patients within MC; and lower risk than those patients either within or exceeding Up-to 7 criteria with microvascular invasion with corresponding HR of 6.45 (CI 2.79-14.88) and 12.71 (CI 7.11-22.71). Survival decreased significantly for patients beyond Up-to criteria and with the presence of microvascular invasion for each stratum.
A.Explanted liver and post-transplant variables associated with 5-year HCC recurrence after liver transplantion. Cox regression.
| Variable | 5-year incidence of recurrence,(%) | Hazard Ratio (95% Cl) | P | |
|---|---|---|---|---|
| Incidental HCC | ||||
| Yes (n = 92) | 5.4 | 0.39 (0.16; 0.99) | 0.048 | |
| No (n = 435) | 14.3 | 2.51 (1.01; 6.23) | ||
| Milan criteria | ||||
| Within (n = 353) | 11.1 | 0.25 (0.15; 0.41) | 0.0001 | |
| Exceeding (n = 174) | 36.8 | 4.02 (2.46; 6.58) | ||
| Up-to 7 criteria | ||||
| Within (n = 443) | 13.5 | 0.18 (0.11; 0.29) | 0.0001 | |
| Exceeding (n = 84) | 47.8 | 5.46 (3.34; 8.78) | ||
| Microvascular invasion | ||||
| Presence (n = 113) | 31.9 | 5.42 (3.35; 8.78) | 0.0001 | |
| Absence (n = 411) | 7.5 | 0.18 (0.11; 0.29) | ||
| Nuclear grade > II | ||||
| Presence (n = 96) | 19.8 | 2.23 (1.29; 3.86) | 0.004 | |
| Absence (n = 372) | 11.0 | 0.45 (0.26; 0.77) | ||
| B. Multivariate Cox Regression Analysis of Explanted Liver Risk factors associated with 5-year Hepatocellular carcinoma Recurrence and Bootstrapped bias corrected - Confidence Intervals. | ||||
| Variable | β | HR (CI 95%) | Bootstrapping CI 95% | P |
| Up to 7 criteria b | -0.804 | 0.45 (0.22; 0.91) | 0.20; 0.86 | 0.027 |
| Microvascular invasion b | 1.397 | 4.04 (2.39; 6.85) | 2.42; 6.92 | 0.0001 |
| Nuclear grade >II b | 0.610 | 1.84 (1.05; 3.22) | 1.04; 3.20 | 0.031 |
HCC: hepatocellular carcinoma. MMF: sodium/mophetil micophenolate. mTOR: mammalian target of Rapamycin inhibitors. **Immunosuppression at the 3rd month after transplant (patients dead were not included).
This is the largest multicenter cohort to describe the selection criteria, treatment during waiting list and overall results of LT for HCC in Latin America. First, in this cohort, most of the LT centers transplanted patients within MC at listing. Overall survival and recurrence rates were similar than that reported in other multicenter European or North American studies.1,2,21 Second, pre-transplant tumor treatment was most frequently done according to imaging rather than serum AFP values at listing, although serum AFP values correlated with presence of microvascular invasion, poor survival and higher recurrence rates. Discordant imaging-explanted data was observed in 29%, showing lower survival and higher recurrence rates in discordant patients. Finally, AFP > 1,000 ng/mL at listing was independently associated with 5-year recurrence.
Different series have been published related to LT and for HCC in Latin America.8–17,24,25 A wide range of recurrence rates has been previously reported in this region.8–17 Participating centers included those with the highest number of procedures in each country, as previously shown.7 Although data from Cuba, Ecuador, Costa Rica, Dominican Republic, Venezuela and Panama are lacking; the number of transplants in these countries is very small.7 Consequently, in this study regional data of LT for HCC are very well represented.
Recently published data confirmed that there might be other related factors beyond tumor size and number associated with worse tumor biology and higher recurrence rate.26–28 Moreover, discordant pre-transplant imaging together with the explanted liver findings in some series was higher than 30%.26,27 In addition, we found that discordant patients presented worst outcomes in terms of survival and recurrence. Consequently, there is a need to refine selection criteria of patients undergoing LT for HCC when considering imaging data only. While tumor differentiation and microvascular invasion potentially can be can be assessed by a tumor biopsy prior to transplantation, the risk of tumor seeding and biopsy complications generate a safety limit.26,27
Pre transplant AFP is a renewed biological marker in clinical practice,5,29,30 although application of this marker for organ allocation policies in Latin America has not been systematically implemented. In this study, elevated serum AFP was associated with higher recurrence and lower survival and correlated with known pathological risk factors, suggesting a more aggressive tumor. More recently, another extended criteria has been proposed in a large HBV-related HCC cohort from China including different cut-offs of AFP.31 In the United States, some changes in the UNOS allocation policy have been agreed upon eligible candidates for a standardized MELD exception including AFP values.
Time on waiting list has been reported as a poor prognostic factor,32 considering the pre-transplant period as a key tool for tumor biology observation. However, other authors have not observed this finding comparing wait time of less than 180 days.33 Probably, time on the wait list might be a potential confounder factor. Time on the waiting list has many caveats, including treatment before transplantation; number and diameter of tumor nodules, serum AFP values; and moreover, regional differences related to donor scarcity and organ allocation policies. Although, locoregional treatment before transplant was associated with a double risk of recurrence, patients who were treated exceeded MC at listing more frequently, showing that this data should be cautiously interpreted (potential confounder factor). A crucial finding was that regarding clinical decision-making, locoregional tumor treatment was performed mainly considering imaging data rather than serum AFP values. This biological marker should be taken in consideration when deciding to treat or not treat patients during the wait list.
We recognize that this study has limitations including first, that in cohort studies with no control group, prognostic factors might be biased. In addition, different pre and post LT treatment algorithms in different LT centers in this region could have leaded a potential selection bias. However, a strict revision of the data was centrally requested, and investigators who performed the final analysis did not participate in the data collection to avoid differential outcome assessment on exposure. A complete follow-up and outcome assessment was available for all patients included. Second, imaging re-evaluation after local/regional treatment was not centrally reviewed; reason for which we only evaluated pre transplant data at time of listing, and additionally at most recent evaluation in treated patients. Third, prognosis, progression and delisting while on the waitlist for all the patients with HCC who were listed for LT were not considered in this study. Unfortunately, our original clinical question of this large registry did not include evaluation of mortality or delisting while on the waitlist. However, we consider that these are key points to address in the coming years in Latin America.
In conclusion, in the largest multicenter Latin American cohort so far published we observed that the majority of LT centers considered Milan criteria as the gold standard for transplantation. However, treatment during transplant waiting list was considered predominantly by imaging rather than serum AFP values at listing. From a regional perspective, this clinical-decision making should be reviewed in the daily practice, as serum AFP predicts recurrence and survival independently from imaging data.
Abbreviations- •
AFP: alpha-fetoprotein.
- •
cHCC: previously known HCC or diagnosed before LT.
- •
CI: confidence interval.
- •
CT: computerized tomography.
- •
HCC: hepatocellular carcinoma.
- •
HR: hazard ratio.
- •
HV: high volume center.
- •
iHCC: incidental HCC.
- •
IQR: interquartile range.
- •
LT: liver transplantation.
- •
MC: Milan criteria.
- •
MELD: Model for End-stage Liver Disease.
- •
MRI: magnetic resonance imaging.
- •
MVI: microvascular invasion.
- •
NAFL: Non-Alcoholic Fatty Liver.
- •
PEI: Percutaneous ethanol injection.
- •
RFA: radiofrequency ablation.
- •
TACE: trans-arterial chemoembolization.
- •
TTR: time to recurrence.
- •
WL: waiting list.
This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Conflicts Of Interest and DisclosuresThe authors of this manuscript have no conflicts of interest to disclose as described by Annals of Hepatology.
AcknowledgmentsWe thank C Podesta (native English speaker) for her assistance with editing of this paper. Argentina: Oscar Andriani, Eduardo de Santibañes, Octavio Gil, Martín Barrabino, Martín Maraschio. Brazil: Elaine Cristina de Ataide, Ana Carolina Portugal. Chile: Juan Carlos Diaz, Cristian Montenegro, Jose Ibarra, Nicolas Jarufe. Colombia: Juan Carlos Restrepo, Isabel Arenas Hoyos, Luisa Santos, and Martín Garzón. Peru: Carlos Rondon, Jose Chaman. Uruguay: Solange Gerona.