Introduction. The aim of this study is to evaluate the risk factors for acute kidney injury (AKI) and 30-day mortality after liver transplantation.
Material and methods. This is a retrospective cohort of consecutive adults undergoing orthotopic liver transplantation (OLT) at a referral hospital in Brazil, from January 2013 to January 2014. Risk factors for AKI and death were investigated.
Results. A total 134 patients were included, with median age of 56 years. AKI was found in 46.7% of patients in the first 72 h after OLT. Risk factors for AKI were: viral hepatitis (OR 2.9, 95% CI = 1.2-7), warm ischemia time (OR 1.1, 95% CI = 1.01-1.2) and serum lactate (OR 1.3, 95%CI = 1.02-1.89). The length of intensive care unit (ICU) stay was longer in AKI group: 4 (3-7) days vs. 3 (2-4) days (p = 0.001), as well as overall hospitalization stay: 16 (9-26) days vs. 10 (8-14) days (p = 0.001). The 30-day mortality was 15%. AKI was an independent risk factor for mortality (OR 4.3, 95% CI = 1.3-14.6). MELD-Na ≥ 22 was a predictor for hemodialysis need (OR 8.4, 95%CI = 1.5-46.5). Chronic kidney disease (CKD) was found in 36 patients (56.2% of AKI patients).
Conclusions. Viral hepatitis, longer warm ischemia time and high levels of serum lactate are risk factors for AKI after OLT. AKI is a risk factor for death and can lead to CKD in a high percentage of patients after OLT. A high MELD-Na score is a predictor for hemodialysis need.
The development of renal dysfunction before or after liver transplantation remains a complicated, multifaceted, and critical issue that adversely affects patients’ outcomes, which range from increased costs of care to inferior grafts and decreased patient survival.1
Despite lack of standardized definitions, renal dysfunction before liver transplantation is common and may be due to chronic kidney disease (CKD), acute kidney injury (AKI), or their combination. Sharma, et al.2 demonstrated that at the time of liver transplantation, 51% of their patients had an eGFR < 60 mL/min, and 6.3% needs dialysis.
AKI after orthotopic liver transplantation (OLT) is a common complication, with incidences ranging from 12-95%.3–6 Renal function deterioration in this setting is also associated with increased 30-day mortality rate, graft dysfunction and 1-year mortality.7,8 In the last decade, there have been many efforts to improve perioperative management and to enhance the use of intervention drugs with less nephrotoxicity.9 Still, there remains a lack of understanding about the risk factors leading to AKI after OLT.
The aim of this study was to determine the risk factors for AKI during the early post-transplant period and the 30-day mortality in patients undergoing OLT.
Material and MethodsThis is a retrospective cohort of consecutive adults undergoing orthotopic liver transplantation (OLT) at a referral hospital in northeast of Brazil, from January 2013 to January 2014. The study protocol was reviewed and approved by the Committee of Ethics from Walter Cantidio University Hospital, Federal University of Ceara, in Fortaleza, Brazil (protocol 052.07.12).
Information recorded from patients’ charts included demographic characteristics, previous history of hypertension (HTN), diabetes mellitus (DM), heart disease (HD), previous liver disease, hepatic encephalopathy, Child-Pugh classification and MELD and MELD-Na. Preoperative laboratory values were also recorded: serum creatinine, INR and bilirubin. From the intraoperative period, we recorded: volume of blood components transfused, warm ischemia time, cold ischemia time, duration of surgery. Post-operative factors included: lactate levels during the first 24 h after surgery, days of ICU and overall in-hospital stay, and in-hospital mortality rates. The glomerular filtration rate 3 months after surgery was recorded.
AKI was defined according to the Acute Kidney Injury Network (AKIN) as an increase more than two times in serum creatinine (AKIN 2 or 3) in the first 72 h after procedure.10 Reference creatinine was defined as the last lowest creatinine available before the transplantation procedure, measured by the colorimetric kinetic method. The diagnosis of AKI in our study was based on only one of the components of the AKIN since data on urinary output was not available for all patients. A comparison between patients with and without AKI was done, as well as between survivors and non-survivors. Glomerular filtration rate (GFR) was estimated through the modification of diet in renal disease (MDRD) equation,11 and chronic kidney disease was considered as GFR < 60 mL/min/1.73 m2 three months after AKI episode.
Statistical analysisVariables in the study were evaluated by the Shapiro-Wilk W test and distribution plots to test normality of distribution. Data that did not meet normality assumptions are presented as median and percentiles and the Mann-Whitney U test was used to compare groups. Data that met the normality distribution are showed by the t student test. For categorical variables, the Pearson χ2 test or Fisher’s exact test was applied as appropriate. We evaluated the association between baseline characteristics and perioperative factors with the development of AKI within 72 h after transplant. The risk factors for inhospital mortality were analyzed. Multivariate logistic regression analysis was used to evaluate variables that were independently associated with development of AKI and mortality. A backward stepwise elimination algorithm was used with p = 0.05 for predictors to remain in the final model.
ResultsA total of 148 OLT were performed in that period from which 134 were included in the study. A total of 14 patients have been excluded because they had no complete follow-up after transplantation or had been referred to another hospital (n = 6), were younger than 18 years-old (n = 5), had undergone simultaneous kidney and liver transplantation (n = 3). There were 67 (50%) males. The median and interquartile range of age was 56 (48-62) years. The incidence of hypertension was 24%, diabetes mellitus 26% and heart disease 1.5%. The median serum creatinine was 0.9 mg/dL (0.7-1.2 mg/dL) and median calculated MELD and MELD-Na was 19 (15-23) and 22 (18-26), respectively. Most of patients (69%) were classified as Child-Pugh A or B, viral liver disease was an indication for transplant among 67 patients with end stage liver disease and hepatitis C virus was the most frequent etiology (76%) (Table 1). Among the 134 patients, 64 (47%) developed AKI after OLT. Table 2 shows the risk factors for AKI. The following factors were AKI predictors: presence of encephalopathy preoperatively (p = 0.0005), presence of viral liver disease as an indication for hepatic transplantation (p = 0.009). Patients who developed AKI had significantly higher levels of lactate, the warm ischemia time was longer and the measured level of serum sodium was lower.
Socio-demographic, clinical and laboratory characteristics of 134 patients undergoing liver transplantation.
| Variable | 134 patients |
|---|---|
| Gender | |
| Female, n (%) | 67 (50%) |
| Male, n (%) | 60 (50%) |
| Age (years), median (IQ) | 56 (48-62) |
| Transplant indication | |
| Viral liver disease, n (%) | 67 (50%) |
| Alcoholism, n (%) | 80 (59%) |
| Hepatic encephalopathy, n (%) | 62 (46%) |
| Child-Pugh classification | |
| Class A or B, n (%) | 92 (69%) |
| Class C, n (%) | 42 (31%) |
| Hypertension, n (%) | 32 (24%) |
| Diabetes, n (%) | 35 (26%) |
| Heart disease, n (%) | 2 (1.5%) |
| Glomerular filtration rate, | |
| median (IR) | 93 (65.2-115.2) |
| MELD, median (IR) | 19 (15-23) |
| Creatinine (preoperative, mg/dL), | |
| median (IR) | 0.9 (0.7-1.2) |
| INR, median (IR) | 1.5 (1.2-1.7) |
| Bilirubin, median (IR) | 2.8 (1.7-6.3) |
| Sodium, median (IR) | 136 (132-139) |
| MELD-Na, median (IR) | 22 (18-26) |
IR: Interquartil Range. MELD: Model for End-Stage Liver Disease. INR: International Normalized Ratio.
Risk factors for development of acute kidney injury.
| Variable | AKI (n = 64) | Non-AKI (n = 70) | P |
|---|---|---|---|
| Gender (female %) | 14 (21) | 26 (37) | 0.6 |
| Hypertension (%) | 14 (21) | 18 (25) | 0.6* |
| Diabetes (%) | 21 (32) | 14 (20) | 0.1* |
| Heart Disease (%) | 1 (0.01) | 1 (0.01) | 0.1* |
| Alcoholism (%) | 42 (66) | 38 (54) | 0.2* |
| Encephalopathy (%) | 38 (59) | 24 (34) | 0.005* |
| CHILD A or B (%) | 39 (60) | 53 (75) | 0.09* |
| C | 25 (40) | 17 (25) | |
| Trans-operative Bleeding (%) | 33 (51) | 28 (40) | 0.2* |
| Volume of Blood Products (%) | 39 (60) | 33 (47) | 0.1* |
| Viral etiology for underlying end-stage liver disease (%) | 40 (62) | 27 (38) | 0.009* |
| Age - median (IR) (years) | 55 (48.2-62.7) | 56.5 (47.7-62) | 0.2† |
| Preoperative creatinine - median (IR) (mg/dL) | 0.9 (0.7-1.2) | 0.9 (0.6-1.2) | 0.5† |
| INR- median | 1.5 (1.3-1.8) | 1.4 (1.1-1.6) | 0.06† |
| Bilirubin - median (IR) (mg/L) | 3.7 (2-6.8) | 2.2 (1.4-5) | 0.07† |
| Sodium - median(IR) (mmol/L) | 135 (131-138) | 138 (133-140) | 0.02† |
| MELD-Na- median (IR) | 23 (18-28) | 22 (17-24) | 1† |
| MELD- median (IR) | 20 (16.2-23.7) | 18 (14.7-22) | 0.1† |
| GFR - pre-LT- mean (SD) (mL/min) | 94.5±45.2 | 87.7 ± 30 | 0.3‡ |
| Cold ischemia time- mean (SD) (min) | 316.8±74 | 299 ± 61 | 0.1‡ |
| Hot ischemia time - median (IR) (min) | 31 (28-35) | 30 (25-32.2) | 0.02† |
| Surgery duration - mean (SD) (min) | 396±70 | 384 ± 94 | 0.4‡ |
| Lactate - median (IR) (mmol/L) | 2.3 (1.7-3.3) | 1.9 (1.4-2.5) | 0.04† |
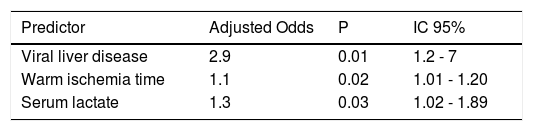
In the multivariate analysis, independent risk factors for AKI were: viral etiology for underlying endstage liver disease (OR 95% = 2.9, 95% CI = 1.2-7), warm ischemia time (OR = 1.1, 95% CI = 1.01-1.20) and serum level of lactate (OR 95% = 1.3, 95% CI = 1.02-1.89) (Table 3).
AKI patients had a longer ICU stay, 4 days (3-7) vs. 3 days (2-4), p = 0.001, as well as longer overall hospital stay, 16 days (9-26) vs. 10 days (8-14), p = 0.001 than non AKI patients, respectively.
Thirty three of the 64 patients (51.5%), who developed AKI, were submitted to hemodialysis. The logistic regression showed that MELD Na ≥ 22 was a risk factor for hemodialysis (OR = 8.4, 95% CI = 1.5-46.5).
Overall in-hospital mortality rate was 15%. After multivariate analyses, AKI was the only variable associated with higher mortality rate (OR = 4.3, 95% CI = 1.3-14.3).
Renal function evaluation three months after OLT evidenced a decrease in eGFR. The eGFR pre-transplant was 81 ± 33 mL/min and, 3 months after transplant, eGFR was 71 ± 23 mL/min (p = 0.004). CKD was found in 36 patients, which corresponds to 56.2% of patients with AKI. All patients with CKD had presented AKI in the post-operative period. No patient in the “non-AKI” group had developed CKD during the first 3 months after transplantation.
DiscussionLiver transplantation is the only option for patients with advanced hepatic failure, and this procedure is growing in Brazil. Our hospital is now one of the biggest hepatic transplant centers in Latin America.12 AKI is a frequent complication after OLT. There are many factors that contribute to AKI development in liver transplantation. Intra-operative factors, such as inferior portal vein clamping, which interrupts venous return, along with cardiac output and blood pressure reduction, decreases renal perfusion and contributes to AKI occurrence (pre-renal injury).13 Cabezuelo, et al.14 demonstrated that the “piggyback” technique decreases the occurrence of AKI in the post-transplant period when compared to standard technique (with or without veno-venous bypass). Aggressive bleeding control in the intra-operative period, cardiac output optimization, hemodynamic stabilization and hydroelectrolytic disturbances control are extremely important measures to AKI prevention in OLT.13 Risk factors for AKI in the post-transplant period included nephrotoxic drugs use, mainly calcineurin inhibitors, such as cyclosporine and tacrolimus, infectious complications, antibiotics use, prolonged hypotension, sepsis and radiologic contrast use.13
AKI was observed in almost half of patients in the post-operative period. Previous studies found a prevalence of AKI after OLT varying from 17 to 95%, which is large dependent on the definition used for AKI.3,13,15–18 The new AKI classifications, such as RIFLE and AKIN, have standardized the AKI definition, and this is important to better compare the study results from different parts of the world.
In the present study there was a significant number of patients in the most advanced AKI stages, and this could be related to the liver disease stage, which was also advanced in the majority of cases (high MELD score). Other studies found a lower prevalence of severe AKI forms after OLT,8,16 but the MELD score was also lower.8
The presence of viral liver disease was a predictor of AKI, and this could be related to an underlying viral glomerulonephritis. McGuire, et al.,19 in a study with 30 patients with hepatitis C virus who underwent renal biopsy during liver transplantation, found a series of glomerular diseases, including membranoproliferative glomerulonephritis (n = 12), IgA nephropathy (n = 7) and mesangial glomerulonephritis (n = 6). In this same study, serum creatinine levels were normal in the majority of cases, evidencing the limitations of this biomarker. It is possible that glomerular changes, induced by viral hepatitis, along with hemodynamic instability, play an important role in the genesis of AKI after OLT.
AKI was associated with high lactate levels. Lactate is a marker of poor tissue perfusion and it is also an indicator of hepatic graft dysfunction in OLT.20 Graft dysfunction is associated with hemodynamic instability, hypotension and, consequently, AKI. This can explain, at least in part, the association between high lactate levels and AKI in the present study.
Interestingly, warm ischemia time was longer in the AKI group, suggesting a possible occurrence of ischemia-reperfusion injury. The longer the liver is exposed to ischemia, the higher will be the release of reactive oxygen species, including superoxide anion, hydrogen peroxide and hydroxy 1 radical generated by xanthine oxidase and hypoxanthine, and this can contribute to kidney injury.21–23
Sodium metabolism is altered in advanced hepatic disease, and hyponatremia is a predictor of worse outcome after liver transplantation.24 In the present study, serum sodium was lower in the AKI group, which can reflect the higher severity of liver disease in these patients.
After OLT, dialysis is required for 8 to 17% of patients,25,26 which is lower than the observed in the present study. Zand, et al.,27 in a study with 743 OLT patients, evidenced that patients with AKI requiring dialysis in the pre and post-operative period had a significantly higher mortality than those with non-dialytic AKI. In the present study, hemodialysis was need in more than half of patients with AKI, and MELD Na ≥ 22 was a risk factor for hemodialysis. The mean MELD score was similar in both groups (AKI vs. non-AKI). When using a cut-off value of 22 (MELD as a qualitative variable or “high MELD vs. low MELD”) we have found that it was associated with hemodialysis need (or with “severe AKI”). The use of means in statistics can generate some confusion as in this case. MELD as a quantitative (continuous) variable presented no significant difference between patients with and without AKI, but when analyzing this as a qualitative variable it was shown to be associated with severe AKI (interpreted here as need for dialysis). MELD is then more useful if used as a qualitative variable. When evaluating patients with liver disease for AKI risk it is more important to classify them as having a “high” or “low” MELD, and possibly the cut-off level of 22 is useful to determine which patients are at high risk of developing severe renal dysfunction. Narciso, et al.,28 found 20% of dialysis need in a group of patients with mean MELD = 13, and in the present study mean MELD was 22, which reflects the worse condition of liver function in our patients. A previous study has found a significant association between MELD score and mortality after liver transplantation,29 and this is expected because a higher MELD score reflects a higher patients’ severity, but this was not observed in our study. The MELD score found in the present study was higher than found in other studies18,29 and the distribution among our 134 patients was homogeneous (i.e. both survivors and non-survivors had similar MELD scores), and did not show association with death. However, MELD was a predictor of dialysis need. Better severity scores should be investigated for patients undergoing liver transplantation.
AKI patients had a longer ICU and hospital stay, and it was the only independent risk factor for in-hospital mortality after OLT. Overall in-hospital mortality rate was 15%, which is lightly lower than observed by others, which is around 17%.20,29,30 In general, AKI increases the length of hospital stay,31 and this also occurs after OLT.32,33 AKI increases mortality in different clinical settings, representing a complication of different clinical conditions.34 In the present study, mortality among patients with AKI was 25%, which is similar to other studies,18 but considering that our patients had a higher MELD score than in other studies, we had similar mortality rates, even with patients presenting higher severity. Several studies evidences AKI as a risk factor for mortality after OLT, and it is also associated with other bad outcomes, including length of hospital stay and development of chronic kidney disease (CKD).25,26,35,36 In a recent study, in which cardiovascular morbidity and mortality was investigated among 389 adult patients after liver transplantation, MELD score and AKI were independent risk factors for cardiac-related mortality.29 In our study AKI was also identified as an independent risk factor for mortality. We can consider AKI development as the most important predictor of mortality after OLT, and efforts to its early diagnosis and adequate treatment are urgently required in an attempt to decrease mortality. New biomarkers which could early identify or predict AKI development have a huge importance in the setting of liver transplantation, including neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C.37–40 New AKI biomarkers, such as N-acetyl-β-D-glucosamininidase (NAG), kidney injury molecule-1 (KIM-1) and Interleukin 18 (IL-18),41 should be investigated in liver transplant recipients in order to stablish its role in the early diagnosis of kidney injury.
The impact of AKI in long-term renal function was evidenced in our study. More than half of patients presenting AKI in the early post-operative period had decreased GFR three months after transplantation, which can be considered as chronic kidney disease (CKD). Recent studies have pointed AKI as an important risk factor for development of CKD.31,42,43 Both AKI and CKD, which are now considered as interconnected syndromes, are important risk factors for cardiovascular diseases,43 and then increase mortality in any patient, including those undergoing OLT. It is important to point out that the early stages of CKD, in which serum creatinine can be within normal limits, has also impact on long-term patients’ survival,36 so that it is important to estimate glomerular filtration rate in every patient after OLT to early diagnose CKD and to adopt measures to slow kidney disease progression.
In summary, AKI is a frequent complication of liver transplantation, which is associated with longer hospital stay and higher mortality. The value of MELD-Na ≥ 22 is a predictor for renal replacement therapy among these patients. AKI has also an important impact on long-term renal function, as a high proportion of patients developed chronic kidney disease.
Study limitations include its retrospective design, so that some important data could be missing, the low number of patients and the short period of time (13 months). Further studies are required to better understand the pathophysiology of liver transplantation-associated AKI and to investigate factors associated to worse outcome. The early recognition and treatment of AKI may decrease post-operative mortality in liver transplantation.
AcknowledgementsWe are very grateful to the team of physicians, residents, medical students and nurses from the Walter Cantídio University Hospital for the exceptional assistance provided to the patients and for the technical support provided to the development of this research. EFD received a grant from the Brazilian Research Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq).