Introduction. Anastomotic biliary strictures (ABS) are a significant clinical problem associated with decreased survival post-liver transplantation (LT). Contributing to the morbidity of ABS is the need for early (i.e. emergent or unplanned) repeat endoscopic retrograde cholangiopancreatographies (ER-ERCPs). Our aim was to determine clinical, operative, and endoscopic predictors of ER-ERCP in patients with ABS.
Material and methods. Medical records of 559 patients who underwent LT at our institution from 2000-2012 were retrospectively reviewed for pertinent data. The primary endpoint was need for ER-ERCP. Seventeen potential predictors of ER-ERCP were assessed in bivariate analyses, and those with p < 0.20 were included in multivariate regression models.
Results. Fifty-four LT patients developed ABS and underwent a total of 200 ERCPs, of which 40 met criteria for ER-ERCP. Predictors of ER-ERCP in bivariate analyses included balloon dilation within 3 months post-LT and donation after cardiac death (both p < 0.05). Balloon dilation within 3 months post-LT was also associated with shorter ER-ERCP-free survival (p = 0.02). Moreover, a significantly higher proportion (67%) of patients who underwent balloon dilation within 3 months post-LT subsequent experienced ≥ 1 ER-ERCP (p = 0.03), and those who experienced ≥ 1 ER-ERCP had lower stricture resolution rates at the end of endoscopic therapy compared to those who did not (79 vs. 97%, p = 0.02). In multivariate analyses, balloon dilation within 3 months post-LT was the strongest predictor of ER-ERCP (OR 3.8, 95% CI 1.7-8.6, p = 0.001).
Conclusions. Balloon dilation of ABS within 3 months post-LT is associated with an increased risk of ER-ERCP, which itself is associated with lower ABS resolution rates. Prospective studies are needed to confirm these findings and their implications for endoscopic management and follow-up of post-LT ABS.
Biliary strictures are a common complication following liver transplantation (LT), occurring in up to approximately 17% of brain dead (BDD) and 50% of cardiac death (DCD) donor LT recipients.1–3 Recent data suggest that the incidence of biliary strictures has in fact increased during the post-Model for End-Stage Liver Disease era.4 The approach to treating post-LT biliary strictures has become progressively less-invasive over the last two decades, with endoscopic treatment now representing the primary therapeutic modality.2,5–8 Although this approach is regarded as having the most favorable balance of safety and efficacy, post-LT biliary strictures continue to be a significant clinical problem and are associated with decreased patient and graft survival.3,5,9–12
It is believed that a considerable proportion of the morbidity and cost associated with biliary strictures stems from frequent and repeated therapeutic endoscopic retrograde cholangiopancreatography (ERCP).11,13,14 While we and others have previously reported on the predictors of final endoscopic treatment outcome (i.e. of achieving stricture resolution),1–3,10,11,13,15,16 the predictors of interval early repeat ERCP (hereinafter “ER-ERCP”) during the endoscopic treatment period remain poorly understood.
Therefore, in this study, we examined demographic, clinical, operative, and endoscopic variables as potential predictors of ER-ERCP among patients undergoing endoscopic treatment of post-LT ABS. In particular, we aimed to determine whether biliary balloon dilation in the early (3 month) post-LT period, a time of relatively greater anastomotic fragility, may be associated with an increased risk of subsequent ER-ERCPs.
Material and MethodsPatientsAfter obtaining institutional review board approval, we performed a retrospective cohort study of adult patients who underwent LT at Johns Hopkins Hospital and required endoscopic therapy for ABS between the years 2000 and 2012. We reviewed electronic medical records of the 559 patients who underwent LT during this interval and excluded patients who:
- •
Received a living-donor LT.
- •
Had a non-choledocho-choledochal anastomosis.
- •
Underwent endoscopic therapy at a different institution or percutaneous management of ABS, and
- •
Were diagnosed with hepatic artery thrombosis.
ABS was defined as a dominant narrowing at the anastomosis with significant impairment of contrast flow during cholangiography.1,2 Ductal narrowing without proximal biliary dilatation or contrast flow impairment was not considered sufficient for the diagnosis of ABS. Nonanastomotic biliary stricture (NABS) was defined as an abrupt luminal narrowing or tapering of the intra and/or extrahepatic ducts proximal to the choledocho-choledochal anastomosis with significant impairment of contrast flow.15,17
All patients included in the study were on a post-LT immunosuppressive regimen consisting of tacrolimus or sirolimus, corticosteroid, and mycophenolate mofetil. The latter two medications were tapered off at approximately 3 months and 1 year post-LT, respectively.
Post-LT stricture managementPatients with cholestatic serum biochemical tests and/or imaging demonstrating dilated intra- or extra-hepatic bile ducts with no alternative clinical diagnosis underwent ERCP for further evaluation. ERCPs were performed with a therapeutic side-viewing endoscope (TJF 160, Olympus America, Inc., Center Valley, PA) with patients in either the prone or supine position by one of several staff advanced endoscopists. Antibiotics were administered peri-procedurally in patients suspected to have cholangitis or incomplete biliary drainage despite endoscopic therapy.
Biliary sphincterotomy, balloon dilation, and stenting were at the discretion of the staff endoscopist. Biliary balloon dilation was primarily with a 4 mm, 6 mm, or 8 mm PET biliary balloon dilator (Conmed, Billerica, MA). Biliary stenting was with a Cotton-Leung or Cotton-Huibregtse biliary stent (> 90% of which were 10 French). Stent exchange was generally performed at 2 month intervals but longer if multiple stents were in place. Endoscopic therapy was continued for up to one year or until improvements in cholangiographic appearance, contrast flow, and serum biochemical tests were noted.
Endpoints and variablesThe primary endpoint of the study was ER-ERCP. This endpoint was defined as an emergent, unplanned, or premature (i.e. occurring prior to the anticipated or scheduled repeat date) accompanied by any one or more of the following signs of biliary obstruction in the absence of an alternative diagnosis (e.g. recurrent viral hepatitis): serum hyperbilirubinemia ≥ 2x upper limit of normal (or ≥ 1.5x the value prior to stent insertion if it was already above normal), temperature > 38.0 oC with acute abdominal pain, or new (i.e. recurrent) bile duct dilatation on imaging.18 Secondary endpoints were:
- •
Time to first ER-ERCP and proportion of patients having experienced at least one ER-ERCP, both as a function of when initial balloon dilation was performed, and
- •
ABS resolution rate at the end of endoscopic therapy as a function of having experienced at least one ER-ERCP.
The following variables were evaluated as potential predictors of ER-ERCP: LT indication, recipient age, sex and race, donor age, race, and type (i.e. BDD- vs. DCD-LT), donor: recipient sex mismatch, cold ischemia time, graft preservation solution, T-tube use, stricture type, sphincterotomy, time to biliary balloon dilation (within 3 months compared to longer periods of time post-LT), balloon dilator size, and number of biliary stents inserted. The 3 month cutoff for time to balloon dilation was based on prior studies using this time point to dichotomize the early post-LT period.19–22
Data analysisDemographic, clinical, operative, and endoscopic treatment characteristics were described as median (interquartile range [IQR]) or proportions. Associations between potential predictors and ER-ERCP were evaluated with bivariate and multivariate logistic regression analyses on a by-procedure basis. Patient level clustering was taken into account using the generalized estimating equation for patients having undergone > 1 ERCP. Variables assessed in the multivariate models were those with p < 0.20 in bivariate analyses as well as age and number of stents inserted given their known impact on outcomes of LT and endoscopic therapy, respectively. For secondary endpoints, analyses were performed on a by-patient basis using Wilcoxon rank-sum and Chi-square tests and the Kaplan-Meier method with log-rank test.
Tests of significance were two-tailed, with an alpha level of 0.05. Analyses were performed using STATA 12.0 (StataCorp LP, College Station, Texas) and JMP Pro (version 9, SAS, Cary, USA) statistical software.
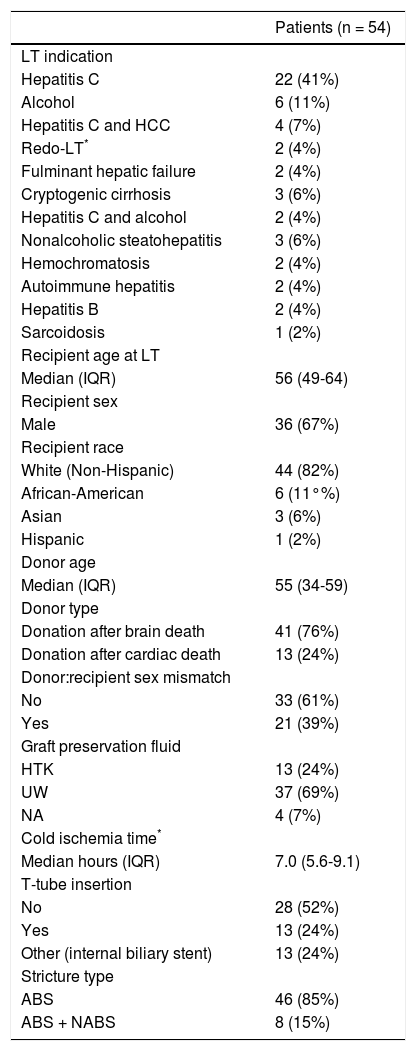
ResultsPatient and ERCP characteristicsFifty-four LT patients developed ABS (median time from LT to ABS diagnosis = 88 days, IQR 35-221) requiring therapeutic ERCP and were thus included in the study. The median sample age was 56 years (IQR 49-64), 67% were male, 82% were non-Hispanic White, and the most common indication for LT was chronic hepatitis C infection (41%). These and other baseline demographic and clinical characteristics are summarized in table 1.
Clinical characteristics of patients who underwent LT from years 2000-2012 and endoscopic treatment of anastomotic biliary strictures.
| Patients (n = 54) | |
|---|---|
| LT indication | |
| Hepatitis C | 22 (41%) |
| Alcohol | 6 (11%) |
| Hepatitis C and HCC | 4 (7%) |
| Redo-LT* | 2 (4%) |
| Fulminant hepatic failure | 2 (4%) |
| Cryptogenic cirrhosis | 3 (6%) |
| Hepatitis C and alcohol | 2 (4%) |
| Nonalcoholic steatohepatitis | 3 (6%) |
| Hemochromatosis | 2 (4%) |
| Autoimmune hepatitis | 2 (4%) |
| Hepatitis B | 2 (4%) |
| Sarcoidosis | 1 (2%) |
| Recipient age at LT | |
| Median (IQR) | 56 (49-64) |
| Recipient sex | |
| Male | 36 (67%) |
| Recipient race | |
| White (Non-Hispanic) | 44 (82%) |
| African-American | 6 (11°%) |
| Asian | 3 (6%) |
| Hispanic | 1 (2%) |
| Donor age | |
| Median (IQR) | 55 (34-59) |
| Donor type | |
| Donation after brain death | 41 (76%) |
| Donation after cardiac death | 13 (24%) |
| Donor:recipient sex mismatch | |
| No | 33 (61%) |
| Yes | 21 (39%) |
| Graft preservation fluid | |
| HTK | 13 (24%) |
| UW | 37 (69%) |
| NA | 4 (7%) |
| Cold ischemia time* | |
| Median hours (IQR) | 7.0 (5.6-9.1) |
| T-tube insertion | |
| No | 28 (52%) |
| Yes | 13 (24%) |
| Other (internal biliary stent) | 13 (24%) |
| Stricture type | |
| ABS | 46 (85%) |
| ABS + NABS | 8 (15%) |
Original indication was hepatitis C (n = 1), alcoholic liver disease (n = 1), and autoimmune hepatitis (n = 1). ABS: anastomotic biliary stricture. HCC: hepatocellular carcinoma. HTK: Histidine-tryptophan-ketoglutarate. IQR: interquartile range. NABS: nonanastomotic biliary stricture. LT: liver transplantation. UW: University of Wisconsin.
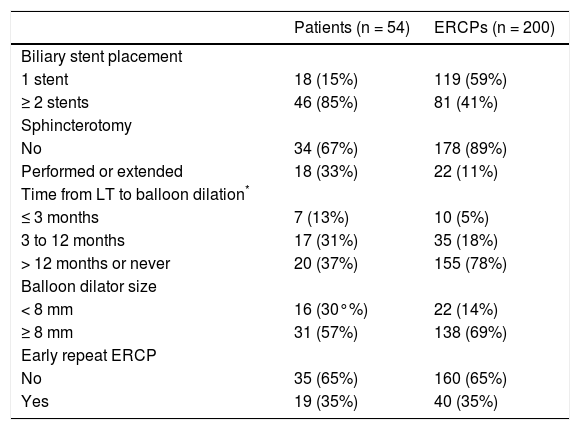
Among the 54 patients in the study sample, a total of 200 ERCPs were performed (not including the final ERCP or upper endoscopy for stent removal) during a median followup of 7.4 years. Endoscopic stenting was performed during all 200 ERCPs, with 119 (59%) consisting of single stent and 81 (41%) consisting of multiple stent (range 2-4) placement. Sphincterotomy was performed or extended in 22 ERCPs (11%), and stricture dilation was performed in 160 ERCPs (80%). Stent occlusion was documented in 19 ERCPs (10%), and stent migration was noted in 8 (4%) These and additional endoscopic treatment characteristics are summarized in table 2.
Endoscopic treatment characteristics.
| Patients (n = 54) | ERCPs (n = 200) | |
|---|---|---|
| Biliary stent placement | ||
| 1 stent | 18 (15%) | 119 (59%) |
| ≥ 2 stents | 46 (85%) | 81 (41%) |
| Sphincterotomy | ||
| No | 34 (67%) | 178 (89%) |
| Performed or extended | 18 (33%) | 22 (11%) |
| Time from LT to balloon dilation* | ||
| ≤ 3 months | 7 (13%) | 10 (5%) |
| 3 to 12 months | 17 (31%) | 35 (18%) |
| > 12 months or never | 20 (37%) | 155 (78%) |
| Balloon dilator size | ||
| < 8 mm | 16 (30°%) | 22 (14%) |
| ≥ 8 mm | 31 (57%) | 138 (69%) |
| Early repeat ERCP | ||
| No | 35 (65%) | 160 (65%) |
| Yes | 19 (35%) | 40 (35%) |
Forty ERCPs (20%) met a priori criteria for the primary endpoint, ER-ERCP. The median time to ER-ERCP was 18 days (IQR 14-36) as compared to 59 days (IQR 48-102) for non-ER-ERCPs (p = 0.002). Notably, the stricture resolution rate upon completion of endoscopic therapy was significantly lower among the 19 patients who experienced ≥ 1 ER-ERCP compared to the 35 patients who did not experience any ER-ERCPs (79 vs. 97%, p = 0.018).
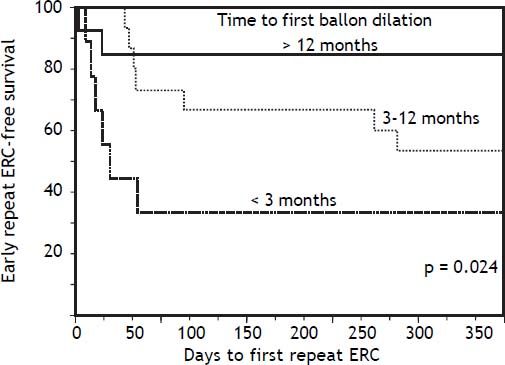
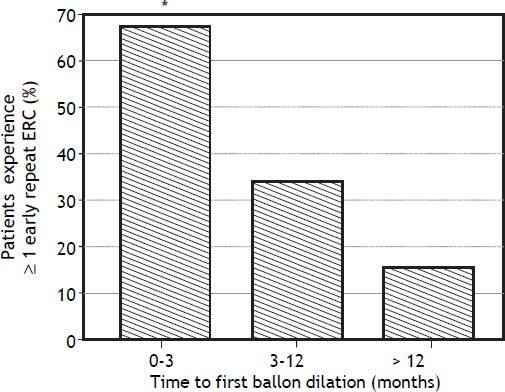
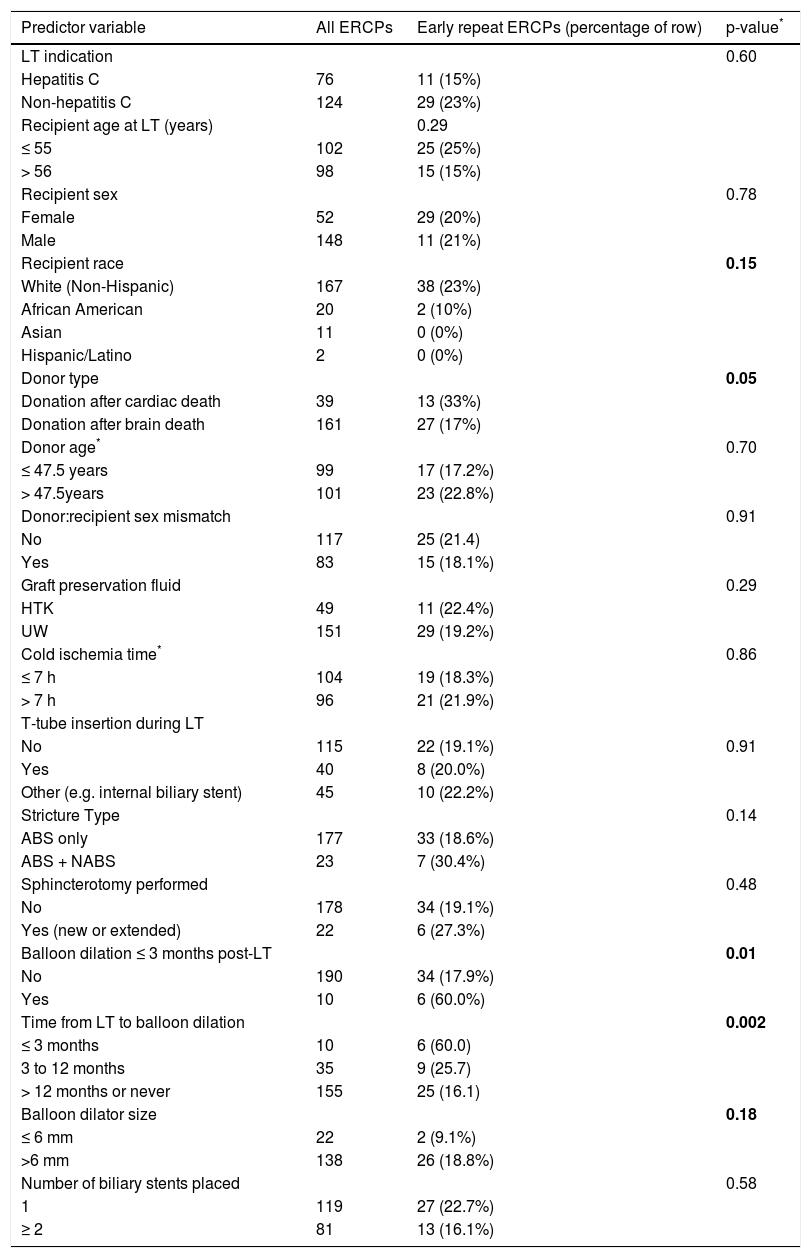
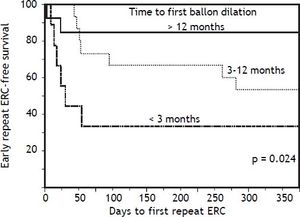
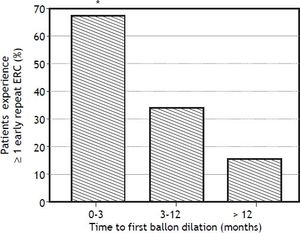
In bivariate analyses, three variables were significantly associated with ER-ERCP: biliary balloon dilation within 3 months post-LT, time from LT to balloon dilation (i.e. as a continuous variable), and DCD-LT (Table 3). Based on these findings, we used the Kaplan-Meier method to better understand the relationship between performance of first (i.e. initial) balloon dilation and time to ER-ERCP; as shown in figure 1, we found significant differences in time to ER-ERCP as a function of when balloon dilation was performed, with patients who underwent initial balloon dilation within 3 months postLT demonstrating the shortest ER-ERCP-free survival (p = 0.024). Moreover, and extending these results, we found that a significantly higher proportion of patients who underwent initial balloon dilation within 3 months post-LT went on to experience at least 1 ER-ERCP (p = 0.028) (Figure 2). Therefore, balloon dilation appeared to be significantly associated with earlier and more frequent ER-ERCP, as evidenced in by-procedure as well as by-patient analyses. In addition, ER-ERCP was associated with a significantly lower stricture resolution rate, as described earlier.
Bivariate analysis of potential predictors of early repeat ERC.
| Predictor variable | All ERCPs | Early repeat ERCPs (percentage of row) | p-value* |
|---|---|---|---|
| LT indication | 0.60 | ||
| Hepatitis C | 76 | 11 (15%) | |
| Non-hepatitis C | 124 | 29 (23%) | |
| Recipient age at LT (years) | 0.29 | ||
| ≤ 55 | 102 | 25 (25%) | |
| > 56 | 98 | 15 (15%) | |
| Recipient sex | 0.78 | ||
| Female | 52 | 29 (20%) | |
| Male | 148 | 11 (21%) | |
| Recipient race | 0.15 | ||
| White (Non-Hispanic) | 167 | 38 (23%) | |
| African American | 20 | 2 (10%) | |
| Asian | 11 | 0 (0%) | |
| Hispanic/Latino | 2 | 0 (0%) | |
| Donor type | 0.05 | ||
| Donation after cardiac death | 39 | 13 (33%) | |
| Donation after brain death | 161 | 27 (17%) | |
| Donor age* | 0.70 | ||
| ≤ 47.5 years | 99 | 17 (17.2%) | |
| > 47.5years | 101 | 23 (22.8%) | |
| Donor:recipient sex mismatch | 0.91 | ||
| No | 117 | 25 (21.4) | |
| Yes | 83 | 15 (18.1%) | |
| Graft preservation fluid | 0.29 | ||
| HTK | 49 | 11 (22.4%) | |
| UW | 151 | 29 (19.2%) | |
| Cold ischemia time* | 0.86 | ||
| ≤ 7 h | 104 | 19 (18.3%) | |
| > 7 h | 96 | 21 (21.9%) | |
| T-tube insertion during LT | |||
| No | 115 | 22 (19.1%) | 0.91 |
| Yes | 40 | 8 (20.0%) | |
| Other (e.g. internal biliary stent) | 45 | 10 (22.2%) | |
| Stricture Type | 0.14 | ||
| ABS only | 177 | 33 (18.6%) | |
| ABS + NABS | 23 | 7 (30.4%) | |
| Sphincterotomy performed | 0.48 | ||
| No | 178 | 34 (19.1%) | |
| Yes (new or extended) | 22 | 6 (27.3%) | |
| Balloon dilation ≤ 3 months post-LT | 0.01 | ||
| No | 190 | 34 (17.9%) | |
| Yes | 10 | 6 (60.0%) | |
| Time from LT to balloon dilation | 0.002 | ||
| ≤ 3 months | 10 | 6 (60.0) | |
| 3 to 12 months | 35 | 9 (25.7) | |
| > 12 months or never | 155 | 25 (16.1) | |
| Balloon dilator size | 0.18 | ||
| ≤ 6 mm | 22 | 2 (9.1%) | |
| >6 mm | 138 | 26 (18.8%) | |
| Number of biliary stents placed | 0.58 | ||
| 1 | 119 | 27 (22.7%) | |
| ≥ 2 | 81 | 13 (16.1%) |
LT: liver transplantation. UW: University of Wisconsin.
Time to early repeat ERCP based on when initial balloon dilation was performed post-LT. Kaplan-Meier curves demonstrate significant differences in early repeat ERCP-free survival (p = 0.024), with patients who underwent initial balloon dilation within 3 months post-LT demonstrating the shortest early repeat ERCP-free survival. ERCP: endoscopic retrograde cholangiopancreatography. LT: liver transplantation.
Timing of initial balloon dilation post-LT is associated with the proportion of patients who experience early repeat ERCP. * There were significant differences in the proportion of patients who went on to experience at least 1 early repeat ERCP (p = 0.028), with patients whose initial balloon dilation occurred within 3 months post-LT having the highest proportion (67%). ERCP: endoscopic retrograde cholangiopancreatography. LT: liver transplantation.
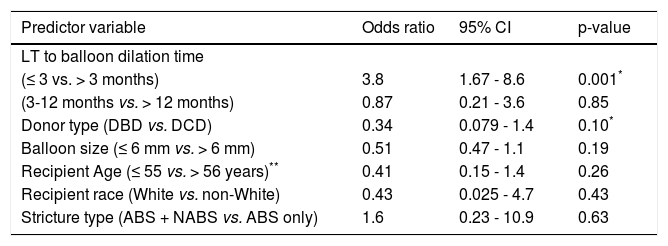
Multivariate logistic regression models were constructed to simultaneously assess multiple potential predictors of ER-ERCP (Table 4). After adjusting for recipient age, donor race, donor type, stricture type, and biliary balloon dilator size, balloon dilation within 3 months post-LT was found to be significantly associated with ER-ERCP, with an odds ratio (OR) of nearly 4 times that of balloon dilation greater than 3 months post-LT (OR 3.8, 95% CI 1.67-8.6, p = 0.001). When number of stents inserted at ERCP was also included in the multivariate model, LT to balloon dilation within 3 months remained statistically significant (p = 0.002) and donor type showed a trend toward statistical significance (p = 0.083), with DCD-LTs conferring an increased risk of ER-ERCP.
Multivariate analysis of predictors of early repeat ERCP.
| Predictor variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| LT to balloon dilation time | |||
| (≤ 3 vs. > 3 months) | 3.8 | 1.67 - 8.6 | 0.001* |
| (3-12 months vs. > 12 months) | 0.87 | 0.21 - 3.6 | 0.85 |
| Donor type (DBD vs. DCD) | 0.34 | 0.079 - 1.4 | 0.10* |
| Balloon size (≤ 6 mm vs. > 6 mm) | 0.51 | 0.47 - 1.1 | 0.19 |
| Recipient Age (≤ 55 vs. > 56 years)** | 0.41 | 0.15 - 1.4 | 0.26 |
| Recipient race (White vs. non-White) | 0.43 | 0.025 - 4.7 | 0.43 |
| Stricture type (ABS + NABS vs. ABS only) | 1.6 | 0.23 - 10.9 | 0.63 |
ABS: anastomotic biliary stricture. CI: confidence interval. ERCP: endoscopic retrograde cholangiopancreatography. NABS: nonanastomotic biliary stricture. LT: liver transplantation.
When number of stents inserted at ERCP was included in the multivariate model, LT to balloon dilation within 3 months remained statistically significant (p = 0.001) and donor type showed a trend toward statistical significance (p = 0.083). Number of stents exhibited a protective effect but was not statistically significant (OR 0.74, p = 0.20).
Biliary strictures remain a costly and challenging clinical problem in the care of LT patients. While ERCP with balloon dilation and stent placement is generally effective for managing post-LT biliary strictures, numerous uncertainties remain regarding optimal therapy and likely account for some of the variable outcomes described in previous reports of endoscopic therapy.8,23 With this in mind, the findings of the present study suggest that although balloon dilation > 12 months post-LT may prolong time to subsequent ERCP and decrease the occurrence of ER-ERCPs, balloon dilation within 3 months post-LT is associated with significantly shorter time to subsequent ER-ERCP as well as more frequent subsequent ER-ERCP. Moreover, this novel finding remained significant after adjusting for multiple other relevant clinical, operative, and endoscopic variables in multivariate analyses. In addition, patients who experienced ≥ 1 ER-ERCP had lower long-term stricture resolution rates compared to those without any ER-ERCPs, suggesting the potential importance of ER-ERCPs on both short- and long-term patient outcomes.
With respect to why biliary balloon dilation during the early post-LT period may be associated with a greater risk of ER-ERCP and other adverse outcomes, there are several possible reasons. The choledocho-choledochal anastomosis remains relatively fragile for 3 or more months post-LT; this may particularly be the case in patients who are still receiving corticosteroids or are on sirolimusbased immunosuppression, which can impair biliary epithelial regeneration.17,18,24 The fragility of the anastomosis during this early post-LT period may lead to further susceptibility to trauma from the rapid expansion of the bile duct diameter during balloon dilation. Such trauma may induce:
- •
Ductal edema leading to impairment of bile flow around biliary stents as well as
- •
Changes in biliary epithelial function and composition27,28 (e.g. with a greater propensity to sludge) leading to impairment of flow through the stents.
Furthermore, balloon dilation during the early post-LT period may lead to persistent biliary injury and inflammation resulting in compromised anastomotic remodeling; these effects may extend well beyond the initial balloon dilation, thereby negatively impacting future ERCPs and therapeutic outcomes, as suggested in part by the significantly lower stricture resolution rate among patients with at least 1 ER-ERCP (79% compared to 97%). While some centers empirically avoid balloon dilation within 3 months post-LT or in patients whose prednisone dose has not been weaned to < 5 mg/day,2 this is the first study, to our knowledge, which provides evidence to support this practice.
In addition to the association between early balloon dilation and ER-ERCP, we also found a modest association between DCD-LT and ER-ERCP. DCD-LT is a recognized risk factor for higher rates of biliary strictures and particularly NABS,3,15 and its association with ER-ERCP (and NABS) is likely related to ischemic injury of the biliary epithelium prior to organ harvesting.3,25 In turn, depending on the location and severity of the strictures arising in DCD-LT, they are often less responsive to endoscopic therapy compared to strictures arising in DBB-LT;1,15,26 thus, it is not surprising that DCD-LT is also associated with ER-ERCP.
The findings of this study should be interpreted in the context of its limitations. First, sample size was small, albeit comparable to or larger than most studies of endoscopic therapy of post-LT ABS; had we used less rigorous criteria (e.g. including patients with hepaticojejunostomy or living-donor LT), our sample would have been larger, although at the expense of less homogeneity and more confounding. Second, the study was retrospective, which precludes inference of causal relationships. Third, we cannot rule out the possibility that the need for balloon dilation in the early post-LT period may have been a marker of disease severity rather than a risk factor for ER-ERCP; this relationship would best be clarified by a prospective study of patients undergoing ERCP for anastomotic stricture during the early post-LT period randomized to either balloon dilation plus stenting versus stenting alone (or alternatively stenting plus tapered catheter or small caliber balloon dilation). Lastly, we recognize that 3 months may be an arbitrary dichotomy for the early post-LT period though it is commonly used; thus, we also analyzed the data herein using a 6 month cutoff and found that balloon dilation remained statistically significant, although with a lower OR (2.1) for ER-ERCP.
In summary, biliary balloon dilation, when performed within 3 months post-LT, appears to be associated with an increased risk of ER-ERCP. Patients who experience ≥ 1 ER-ERCP appear to have significantly lower long-term stricture resolution rates compared to those who do not experience ER-ERCP. Therefore, balloon dilation in the early post-LT period may be a predictor of ER-ERCPs and thus potentially increased morbidity, cost of care, and rate of failure with endoscopic management of ABS. Future studies are needed to further investigate these findings and determine if avoidance of biliary balloon dilation in the early post-LT period may lead to improved short- and long-term outcomes and if patients who have experienced ≥ 1 ER-ERCP, be it with or without early balloon dilation, may benefit from closer clinical follow up.
Abbreviations- •
ABS: anastomotic biliary stricture.
- •
BDD: brain dead donor.
- •
DCD: cardiac death donor.
- •
ER-ERCP: early repeat endoscopic retrograde cholangiopancreatography.
- •
IQR: interquartile range.
- •
LT: liver transplantation.
- •
NABS: nonanastomotic biliary stricture.
- •
OR: odds ratio.
None.