Introduction and aim. Sofosbuvir (SOF)-based regimen has been shown to have high efficacy even in patients with decompensated cirrhosis. Treated patients may experience various degrees of hepatic recovery ranging from stabilization of liver function, to removal from liver transplant wait lists. The frequency of these occurrences in larger transplant eligible patient populations is unknown. The aim of this study was to assess the efficacy of SOF-based therapy in HCV infected transplant eligible patients and to evaluate short term changes in liver function and the effect on their liver transplant status.
Material and methods. A retrospective multicenter Canadian study of liver transplant candidates with advanced HCV cirrhosis treated with SOF-based therapy. Outcomes included sustained virologic response (SVR), and liver transplant status.
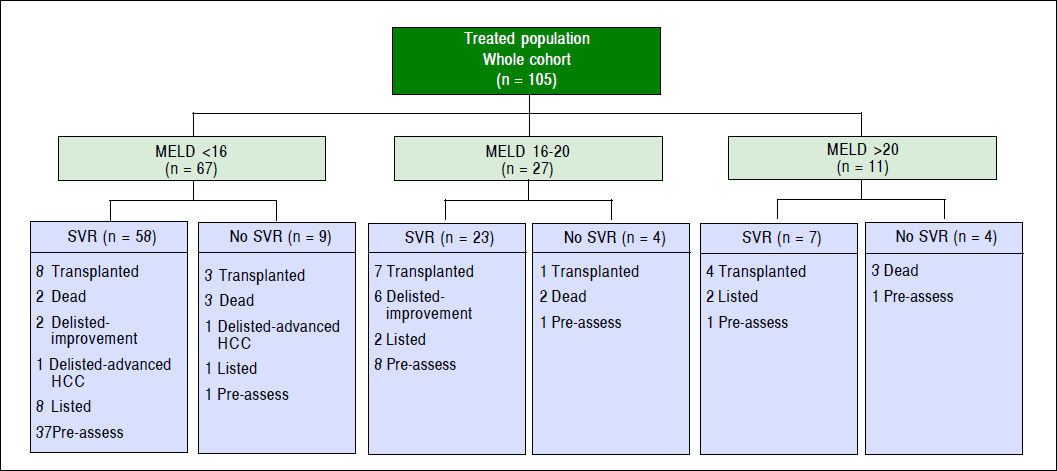
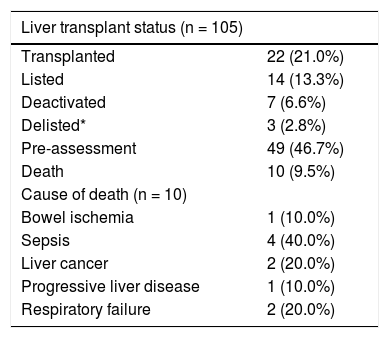
Results. 105 liver transplant candidates with advanced liver disease due to HCV were evaluated. The overall SVR was 83.8%. Hepatocellular carcinoma was diagnosed in 39 (37.1%) prior to transplant evaluation. In short term follow-up, 14 (13.3%) remained active on the list at the time of SVR12, 22 (20.9%) patients underwent liver transplantation, 7 (6.6%) patients were deactivated due to clinical improvement, 3 patients were delisted, and 10 deaths were reported.
Conclusions. SOF-based therapy for patients progressing to liver transplantation leads to high SVR rates, short term stability in liver function, and deactivation from the transplant list.
The prevalence of HCV worldwide is 0.5%-1%, representing 71 million infected individuals.
Patients who are infected with HCV are at a risk of developing serious complications such as liver cirrhosis, hepatocellular carcinoma, and liver failure.1-3 HCV is the leading cause of liver transplantation worldwide, and thus it has had a significant medical and economic burden.4–7 In Canada, despite the evolution of HCV therapy, it had been predicted that there would be a significant increase in the incidence of compensated cirrhosis, decompensated cirrhosis, HCC and liver-related deaths in 2035.8
In the era of interferon-based therapy, management of decompensated liver cirrhosis secondary to HCV was complicated due to suboptimal treatment response with SVR rates ranging between 7%-30% and high rate of adverse events which resulted in dose reduction in 70% of patients and treatment discontinuation in 40% with a risk of worsening decompensation.9,10 Therefore, interferon-based therapy was contraindicated among HCV patients with decompensated liver disease, particularly those patients with advanced Child-Turcotte-Pugh (CTP) or Model for End-Stage Liver Disease (MELD) scores.11,12 However, recently, several studies have shown that oral direct-acting antiviral agents containing sofosbuvir, ledipasvir, daclatasvir and velptasvir, in various combinations, are safe and effective in decompensated cirrhosis.11,13–15
Furthermore, an improvement in MELD and CTP scores have been observed among those patients who achieve SVR.15–17 The magnitude of improvement in their MELD and CTP scores, however, is variable. There are some HCV liver transplant candidates who might have a significant recovery in their liver function following successful therapy to the point that liver transplantation is not warranted, while others could improve slightly, but their lower MELD score may make them less competitive for deceased donor organs.18 The aim of the study is to evaluate the safety and efficacy of sofobuvir-based therapy in HCV patients who have been evaluated for liver transplantation as well as to assess the impact of therapy on liver function and liver transplant wait-list status.
Material and MethodsPatient populationMost of the Canadian adult HCV patients who received sofosbuvir-based therapy in the pre-transplant assessment period between 2014 and 2016 were enrolled in the study, following the approval of the institutional ethics regulation. The study involved the majority of Canadian liver transplant centres.
Study designThis is a multicenter Canadian, retrospective, observational study of liver transplant candidates with advanced HCV cirrhosis treated with SOF-based therapy. Data were collected by the treating center and consolidated into a de-identified dataset by the primary investigator. Treatment regimen was selected and administered by individual treating physicians according to the standard of care and their access to therapy. Some patients received therapy via compassionate access program, others under public coverage or private insurance. Treatment regimens were SOF and simeprevir ± ribavirin (RBV), SOF and daclatasvir ± RBV, sofosbuvir plus ledipasvir ± RBV, SOF and RBV. The RBV dose was adjusted based on renal function and the degree of anemia; however, patients might receive the maximum dosage of RBV if well tolerated. Ribavirin’s usual contraindication of pretreatment hemoglobin < 100 g/L was applied. Subjects with a glomerular filtration rate (GFR) < 30 mL/min were excluded from receiving SOF. Patients with insufficient data, currently on HCV therapy at the time of study analysis, on an interferon-based therapy, or treated after liver transplantation were excluded from the study.
Safety and efficacyHCV RNA plasma concentrations were drawn pretreatment, then again at the end of treatment and 12 weeks after completion of therapy. Plasma HCV levels were quantified by Cobas TaqMan HCV assay, version 2.0 (Roche Molecular Systems, Inc.) with a lower limit of detection 15 IU/mL. MELD-Na and CTP scores were collected in the pre-treatment period, then, 12 and 24-week after completion of therapy.
Data collection included patient age, gender, HCV genotype, viral load, type of therapy, prior treatment history, transplant status, presence of liver-related complications such as ascites, encephalopathy, esophageal varices and the presence of hepatocellular carcinoma. Adverse events were recorded among all recruited patients.
Study endpoints and statistical analysesThe primary end point was the proportion of patients who were deactivated (i.e. patient is placed on hold due to clinical improvement) and delisted (i.e. patients excluded from the waiting list). The secondary endpoint was the proportion of patients who achieved undetectable HCV RNA 12 weeks after completion of therapy, and changes in the MELD-Na and CTP scores after treatment. A decision made to delist or to deactivate a patient was based on the clinical improvement at SVR12. Patients who were transplanted shortly after completion therapy with unknown SVR status were excluded from SVR12 end-point analysis. Univariate comparisons of means and proportions were used to assess for significant differences in the primary and secondary endpoints. Non-parametric tests were utilized in those variables not normally distributed. A p value of 0.05 or less was considered statistically significant. All analyses were performed with SPSS Version 23 (IBM, Armonk, NY).
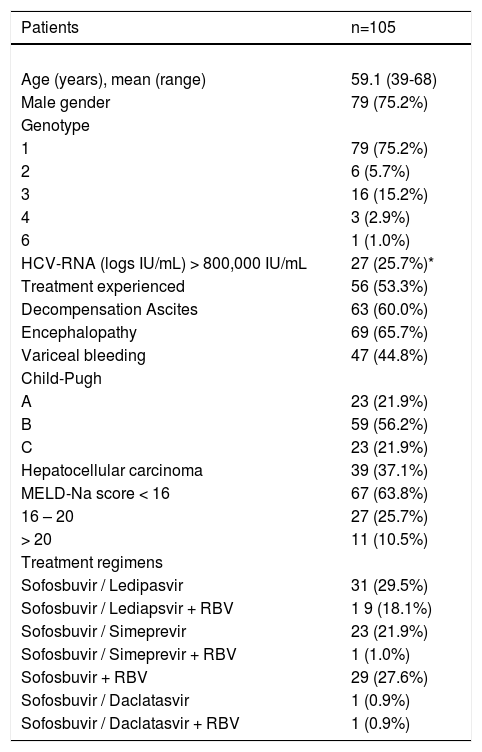
ResultsStudy populationOne hundred and five liver transplant candidate patients with HCV were included in the study. The mean age of the cohort was 59.1 years and 75.2% of patients were male. 79 (75.2%) of patients were infected with genotype 1, 6 (5.7%) with genotype 2, 16 (15.2%) with genotype 3, 3 (2.9%) with genotype 4, and one patient had genotype 6. All patients had advanced liver disease and 21.9% of patients were CTP class C, as shown in table 1. Fifty-six (53.3%) patients were treatment experienced and 39 (37.1%) patients were diagnosed with hepatocellular carcinoma prior to transplant evaluation. The majority, 67 (63.8%) patients had MELD score < 16, 27 (25.7%) had MELD score between 16-20, and 11 (10.5%) had MELD-Na score > 20. All candidates were started on SOF-based therapy, 31(29.5%) received SOF/ledipasvir, 19 (18.1%) received SOF/ledipasvir + RBV, 23 (21.9%) received SOF + SIM, 29 (27.6%) received SOF + RBV, and others received different type of HCV regimens as shown in table 1.
Patient characteristics.
| Patients | n=105 |
|---|---|
| Age (years), mean (range) | 59.1 (39-68) |
| Male gender | 79 (75.2%) |
| Genotype | |
| 1 | 79 (75.2%) |
| 2 | 6 (5.7%) |
| 3 | 16 (15.2%) |
| 4 | 3 (2.9%) |
| 6 | 1 (1.0%) |
| HCV-RNA (logs IU/mL) > 800,000 IU/mL | 27 (25.7%)* |
| Treatment experienced | 56 (53.3%) |
| Decompensation Ascites | 63 (60.0%) |
| Encephalopathy | 69 (65.7%) |
| Variceal bleeding | 47 (44.8%) |
| Child-Pugh | |
| A | 23 (21.9%) |
| B | 59 (56.2%) |
| C | 23 (21.9%) |
| Hepatocellular carcinoma | 39 (37.1%) |
| MELD-Na score < 16 | 67 (63.8%) |
| 16 – 20 | 27 (25.7%) |
| > 20 | 11 (10.5%) |
| Treatment regimens | |
| Sofosbuvir / Ledipasvir | 31 (29.5%) |
| Sofosbuvir / Lediapsvir + RBV | 1 9 (18.1%) |
| Sofosbuvir / Simeprevir | 23 (21.9%) |
| Sofosbuvir / Simeprevir + RBV | 1 (1.0%) |
| Sofosbuvir + RBV | 29 (27.6%) |
| Sofosbuvir / Daclatasvir | 1 (0.9%) |
| Sofosbuvir / Daclatasvir + RBV | 1 (0.9%) |
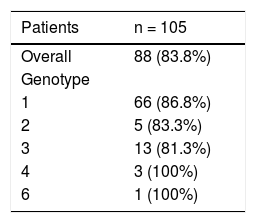
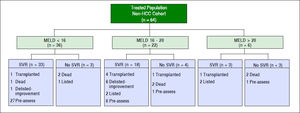
Eighty-eight HCV patients (83.8%) achieved SVR (84.8% for genotype 1, 83.3% for genotype 2, 77.8% for genotype 3, and 100% for genotypes 4 and 6 as shown in table 2. Two patients discontinued therapy, one due to respiratory failure and the other due to bowel ischemia. None of our patients in this cohort underwent liver transplantation while they were on therapy. However, one patient was transplanted after completion of therapy and before SVR could be evaluated. Just under half (46.7%) of the patients were treated in the pre-assessment period (i.e. patients evaluated for liver transplantation but awaiting decision whether or not to list for liver transplantation) compared to 56 (53%) patients treated after activation (placement on the wait list awaiting transplantation). Among patients who were treated while waitlisted for liver transplantation, 14 remained active on the liver transplant list at the time of SVR, 22 patients underwent liver transplantation, 7 patients were deactivated due to mild improvement of their MELD-Na and CTP scores after achieving SVR12, and three patients were delisted as shown in figure 1. Among delisted patients, two had HCC progression outside of Milan Criteria and one patient was delisted based on his request after achieving SVR and being cancer free after treatment with local regional therapy.
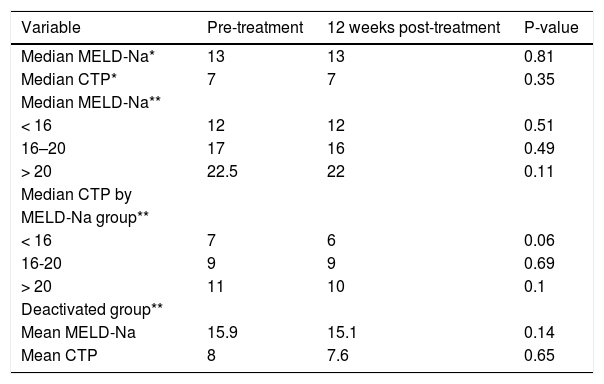
The median MELD-Na (13 vs. 13, p = 0.81) and CTP (7 vs. 7, p = 0.35) scores did not change significantly from baseline to SVR 12 date among the whole cohort (Table 3). Further analysis was performed and patients were divided into three groups based on their MELD-Na scores prior to HCV treatment (< 16, 16-20, > 20) to identify which group would have the greatest benefit of clinical improvement as measured by MELD and CTP scores at SVR12 (Figure 1 and Table 3). After excluding HCC patients from the analysis, there was no significant within group differences in median MELD-Na and CTP scores from baseline to SVR 12 date as is shown in table 3. Seven (10.9%) non-HCC patients were deactivated due to clinical improvement in their MELD-Na (15.9 vs. 15.1) and CTP (8 vs. 7.6) scores after achieving SVR12. However, the result was not statistically significant.
Changes in Model for End Stage Liver Disease-Na (MELD-Na) and Child-Turcotte-Pugh scores from baseline to SVR12.
| Variable | Pre-treatment | 12 weeks post-treatment | P-value |
|---|---|---|---|
| Median MELD-Na* | 13 | 13 | 0.81 |
| Median CTP* | 7 | 7 | 0.35 |
| Median MELD-Na** | |||
| < 16 | 12 | 12 | 0.51 |
| 16–20 | 17 | 16 | 0.49 |
| > 20 | 22.5 | 22 | 0.11 |
| Median CTP by | |||
| MELD-Na group** | |||
| < 16 | 7 | 6 | 0.06 |
| 16-20 | 9 | 9 | 0.69 |
| > 20 | 11 | 10 | 0.1 |
| Deactivated group** | |||
| Mean MELD-Na | 15.9 | 15.1 | 0.14 |
| Mean CTP | 8 | 7.6 | 0.65 |
Two patients did not complete therapy, one died from bowel ischemia and a second patient developed respiratory failure necessitating admission to the intensive care unit. Ten deaths were reported in the study period; the three most common of which were advanced HCC, respiratory failure, and sepsis (Table 4). None of the deaths or discontinuations of therapy were attributed to the HCV treatment.
Outcomes causes of death.
| Liver transplant status (n = 105) | |
|---|---|
| Transplanted | 22 (21.0%) |
| Listed | 14 (13.3%) |
| Deactivated | 7 (6.6%) |
| Delisted* | 3 (2.8%) |
| Pre-assessment | 49 (46.7%) |
| Death | 10 (9.5%) |
| Cause of death (n = 10) | |
| Bowel ischemia | 1 (10.0%) |
| Sepsis | 4 (40.0%) |
| Liver cancer | 2 (20.0%) |
| Progressive liver disease | 1 (10.0%) |
| Respiratory failure | 2 (20.0%) |
The evolution of HCV therapy has led to striking declines in liver disease-related complications from HCV. Between 2003 and 2015, there was a significant drop in HCV patients on the liver transplant waiting-list in the era of protease inhibitors and direct-acting antivirals compared to the era of interferon-based therapy.19 However, patients with HCC might have a different natural disease course and liver transplantation is warranted despite achieving SVR. In our cohort, 39 (37.1%) patients were diagnosed with HCC prior to transplant evaluation. Among HCC patients, 32 (82%) achieved SVR. However, 14/32 (43.7%) HCC patients who achieved SVR underwent liver transplantation and 7 were listed for liver transplantation at the end of the study. Three HCC patients died, two due to advanced HCC and one due to sepsis. The remaining HCC patients were in the pre-assessment phase. Clearly HCC patients would need to be evaluated for liver transplantation regardless of their SVR status. This is not unexpected because it has been shown in several studies that improvement in hepatic function occurs rapidly among patients who achieved SVR. Yet, they remain at risk of HCC and the potential impact of viral therapy on HCC incidence may lag behind in terms of trends in transplant listing.19–22 In the Veterans Affairs study, the incidence of HCC after achieving SVR was 1.4% in cirrhotic patients.23
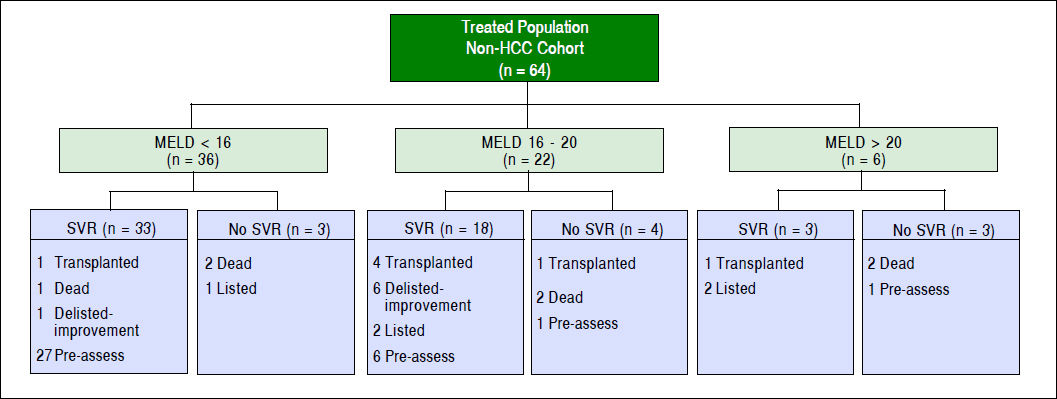
A successful treatment of HCV in the pre-transplant period has the advantage of protecting the graft from recurrent HCV after liver transplantation which may result in graft failure.24–26 In addition, SVR patients with decompensated cirrhosis may have an improvement in their liver function.14–27 In our study, 88 (83.8%) patients achieved SVR. A similar rate was seen after excluding HCC patients from the analysis [54/64 (84%)]. Treated non-HCC patients were divided into three groups based on their MELD-Na (< 16, 16–20, > 20) scores as is shown in figure 2. The median MELD-Na score dropped by almost one point at SVR12 among patients with MELD-Na score of 16 or above (Table 3).
However, the MELD-Na score did not change among patients with median MELD-Na < 16. On the other hand, median CTP dropped by one point at SVR12 among patients with MELD-Na score < 16 and > 20.
The median CTP score at SVR12, however, did not change from baseline among patients with MELD scores.16–20 The magnitude of improvement in the median CTP and MELD-Na scores were not significant in the short-term, though more improvement might be observed in long-term follow-up.
From the expanded access program in the United Kingdom, 409 HCV patients with decompensated cirrhosis received treatment and it has been observed that the improvement of their liver function occurred within 6 months of achieving SVR.28 Several studies have shown that HCV patients with decompensated cirrhosis who were treated with sofosbuvir-based therapy achieved an improvement in their CTP and MELD scores post-treatment.14,15,29–32 Bunchorntavakul, et al. collected data from major trials to evaluate the efficacy of HCV therapy among patients with decompensated cirrhosis (801 patients, 83% SVR rates).27 Many (60%) patients had an improvement in their MELD score from baseline and the magnitude of improvement in the MELD scores ranged from 2-4. However, it’s interesting to observe that a small proportion of patients with decompensated cirrhosis had no clinical improvement and continued to show an increase in the MELD score despite achieving SVR.27 In addition, some patients might have a decline, but not normalization, of their MELD scores (i.e. “MELD purgatory”) which will diminish their access to liver transplantation yet prolong their waiting time for liver transplantation; additionally, most centres (and patients) would refuse to accept a graft from a HCV positive donor after achieving SVR pre-transplant.
In our study, 7/64 (10.9%) of non-HCC patients were deactivated because of improvement in their liver function at SVR12 (Figure 2). However, the result was not significant and a longer duration of follow-up is required to evaluate the magnitude of long-term improvement in MELD and CTP scores (Table 3). Belli, et al., evaluated the magnitude effect of liver function improvement by HCV therapy in 103 liver transplant candidates.33 It was observed that one out of five patients were delisted due to clinical improvement of their MELD and CTP scores at 60 weeks.33 In addition, patients with MELD score < 16 had higher chances of being delisted due to clinical improvement.33 Chhatwal, et al., developed a Markov based microsimulation model to test which treatment strategy (pre- or post-liver transplant) leads to the best outcomes based on MELD score at the time of treatment.20–34 It was observed that treating HCV patients before liver transplantation would increase life expectancy of those with a MELD ≥ 27; but, pre-transplant treatment may have negative impact on life expectancy at higher MELD scores.34 Likewise, a Markov state-transition model was created to evaluate the cost-effectiveness of treating HCV pre-liver transplantation compared to post-liver transplantation. It was found that the most cost-effective strategy is to treat HCV prior to liver transplantation among patients with a MELD score < 25.35 In addition, there has been an over 30% decline in the rate of the liver transplant waiting-list in a population based study after the approval of HCV direct-acting antivirals.19 It has been observed in our organization at London Health Sciences Centre that there is a decline among listing liver transplant candidates with decompensated HCV cirrhosis over the last 3 years.
There are several limitations to our study. First, the present study was a retrospective cohort analysis, with a small sample size. Second, the study follow-up was short and a longer duration of follow-up is required to evaluate the long term impact of HCV treatment in patients with decompensated cirrhosis. Third, there might be selection bias because the study was performed at liver transplant centres and increasingly more clinicians in the community feel comfortable in managing HCV patients with decompensated cirrhosis without being referred for liver transplant evaluation especially those patients with low MELD and CTP scores. Fourth, non-liver transplant candidates (e.g. elderly patients) with decompensated cirrhosis from HCV won’t be referred for a liver transplant evaluation and will likely be managed locally by their providers. In addition, portal pressure measurements were not performed to evaluate the magnitude effect of HCV therapy on pressure gradient reduction among patients who achieved SVR. However, diuretics dosage reduction was achieved in 14/57 (24.5%) patients with ascites who were prescribed diuretics. Moreover, from a clinical perspective, an invasive procedure to document changes in portal pressure, is not necessary as non-invasive clinical features (including MELD and CTP scores) are reasonable surrogate markers and a direct portal pressure measurement will not change patient management.
In conclusion, sofosbuvir-based therapy is efficacious among HCV patients with decompensated cirrhosis referred for liver transplantation, in a “real world” setting. Patients with low MELD scores may have a significant benefit of liver recovery after successful therapy and liver transplantation may not be needed. But, it is unclear whether or not MELD and CTP scores are best predictors of clinical response to antiviral therapy and the optimal cutoffs for treatment are unknown. A proportion of transplant candidates will continue to need liver transplantation after achieving SVR, at least in the short term.