Pegylated interferon (Peg-INF) and ribavirin (RBV) based therapy is suboptimal and poorly tolerated. We evaluated the safety, tolerability and efficacy of a 24-week course of sofosbuvir plus daclatasvir without ribavirin for the treatment of hepatitis C virus (HCV) recurrence after liver transplantation (LT) in both HCV-monoinfected and human immunodeficiency virus (HIV)-HCV coinfected patients.
Material and MethodsWe retrospectively evaluated 22 consecutive adult LT recipients (16 mo-noinfected and 6 coinfected with HIV) who received a 24-week course of sofosbuvir plus daclatasvir treatment under an international compassionate access program.
ResultsMost patients were male (86%), with a median age of 58 years (r:58-81y). Median time from LT to treatment onset was 70 months (r: 20-116 m). HCV genotype 1b was the most frequent (45%), 55% had not responded to previous treatment with Peg-INF and RBV and 14% to regiments including first generation protease inhibitors. Fifty-six percent of the patients had histologically proven cirrhosis and 6 had ascites at baseline. All patients completed the 24-week treatment course without significant side effects except for one episode of severe bradicardya, with only minor adjustments in immunosuppressive treatment in some cases. Viral suppression was very rapid with undetectable HCV-RNA in all patients at 12 weeks. All 22 patients achieved a sustained virological response 12 weeks after treatment completion.
ConclusionThe combination of sofosbuvir plus daclatasvir without ribavirin is a safe and effective treatment of HCV recurrence after LT in both monoinfected and HIV-coinfected patients, including those with decompensated cirrhosis.
HCV infection is the leading indication for LT in the Western world.1 Among patients with detectable HCV-RNA at the time of transplantation, recurrent infection is universal and leads to cirrhosis 5 years after LT in up to 30% of patients.2,3 Several studies have shown that HCV eradication after LT improves both graft and patient sur-vival.4,5 Hence, it is of primary importance to achieve sustained viral response (SVR) to improve survival after-LT. The availability of interferon free regimens has improved tolerability and rates of SVR, however the new paradigm has not yet been defined.
Until recently, however, SVR rates using Peg-IFN plus RBV based treatment in the LT setting were very limited, ranging between 10 and 35% of patients owing to poor tolera-bility and high rates of premature discontinuation.6-8 On the other hand, among HIV-coinfected patients, the limited effectiveness of Peg-IFN-based treatment along with the more severe recurrence associated with higher mortality and graft loss rates, have had a negative impact on the willingness of LT programs to enter HIV-infected patients in LT lists.
The advent of interferon-free antiviral regimens with increased efficacy and tolerability offers radically new opportunities to prevent or manage HCV recurrence in both mono- and HIV-coinfected patients and improve long-term survival.
The aim of the current study was to report the efficacy and safety of a 24-week course of sofosbuvir (SOF) and daclatasvir (DCV) without ribavirin in LT HCV monoin-fected and HCV-HIV coinfected LT recipients treated under an international compassionate access program between March and November 2014.
Material and MethodsPatientsEligible patients were ≥ 18 years, had received a LT, and were also required to have severe, life-threatening HCV recurrence. Treatment regimen consisted on sofosbuvir and daclatasvir without ribavirin for 24 weeks. Sofosbuvir was provided by Gilead Sciencies (Cambridge, UK) and daclatasvir by Bristol-Myers Squibb (Princeton, NJ) as part of an international compassionate use protocol under individual approval.
Patients were approved by the sponsors on a case-by-case basis, following review of submitted information on the patient’s medical history, clinical status and laboratory values. All patients gave written informed consent.
Study follow-up scheduleThere was no planned number of patients. All but one patient received treatment with SOF (400 mg once daily) and DCV (60 mg once daily) without ribavirin for 24 weeks. In one of the six HIV-coinfected patients, DCV dose was reduced to 30 mg once daily, due to concerns on potential drug-drug interactions with ritonavir-containing antiretroviral regimen. Clinical history, physical examination and laboratory tests, including complete blood count (with CD4 T-cell counts, in HIV infected patients), baseline liver function tests and viral loads, were collected weekly during the first month of treatment and monthly thereafter during treatment and 12 weeks after treatment completion. Child-Pugh (CP) and Model for End Stage Liver Disease (MELD) scores were calculated at treatment onset and at weeks 12 and 24 (end of treatment) and at 12 weeks after the end of therapy.
HCV RNA testing and genotypingSerum HCV RNA was tested at baseline, during treatment (weeks 1, 2, 3, 4, 8, 12 and 24) and 4 and 12 weeks after treatment completion (SVR4 and SVR12, respectively). HCV RNA levels were tested using a real-time PCR-based assay (Cobas Ampliprep/Cobas TaqMan; Roche Molecular Diagnostics, Barcelona, Spain; lower limit of quantification 15 IU/mL, and lower limit of detection 10 IU/mL). HCV genotyping was determined using a commercially available line probe assay (Inno-LiPA II®; In-nogenetics, Antwerp, Belgium).
IL28B genotypingInterleukin IL28B polymorphism rs12979860 (ILB28) of recipients was performed using a real-time PCR with allele-specific Taq-Man probes, as previously de-scribed.9,10
Liver histologyStaging of post-LT HCV recurrence was performed in all patients based on liver biopsy. A single pathologist (TS), blinded to the clinical data, scored biopsy specimens according to the Ishak classification system.11
Study assessmentsClinical and laboratory data were prospectively collected for all patients throughout therapy and 12 weeks after the end of therapy. Standard definitions for adverse events (AEs) and serious adverse events (SAEs) were used. Adverse events data were collected in all patients during their treatment period and during their follow-up until the 12th week after the planned end of treatment.
Statistical analysisNo sample-size calculations were performed, and no inferential statistics or statistical comparisons were planned.
Quantitative variables are presented as medians and interquartile range (IQR), and categorical variables as frequency and percentage. Differences between categorical variables were assessed by χ2 or Fisher’s exact test. Continuous variables were compared using Mann-Whitney’s test (for umpaired data) or by the t-test or Wilcoxon’s test (for paired data). STATA® v13 (College Station, Texas) was used for all statistical analyses.
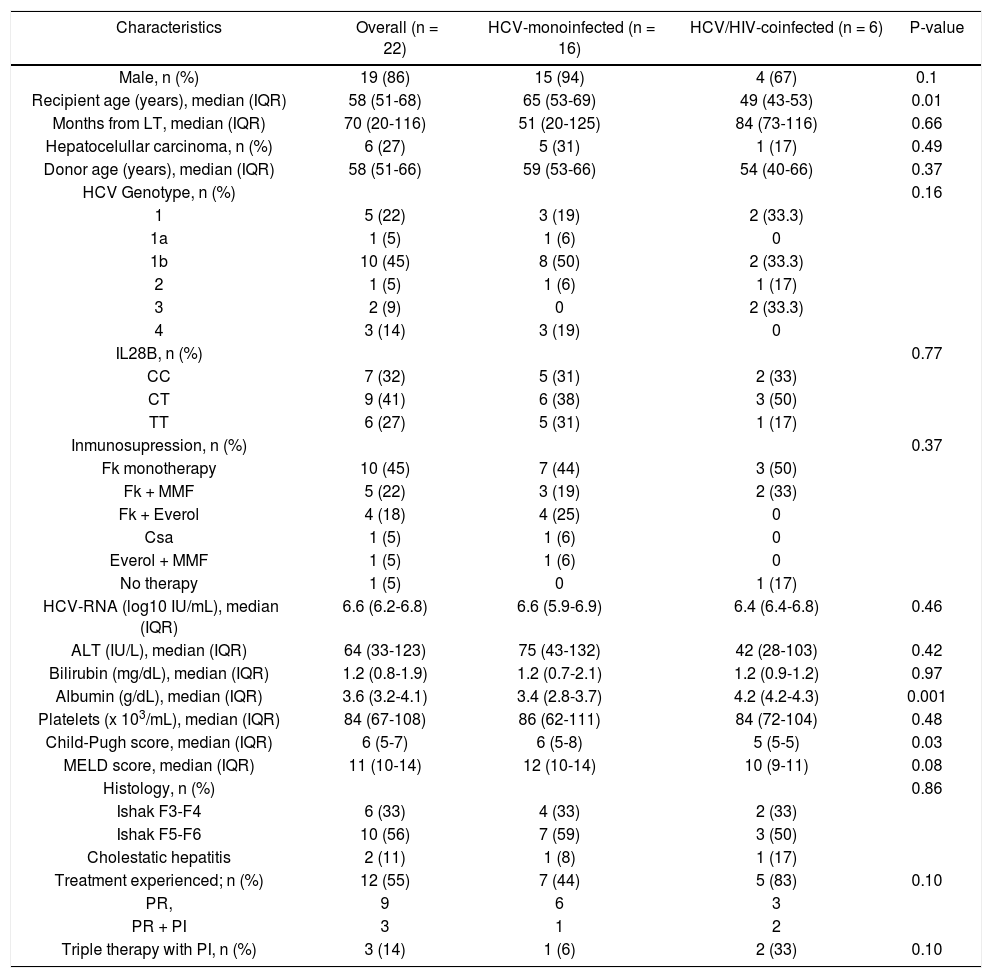
ResultsBaseline characteristicsBaseline characteristics of the 22 patients according to HIV serostatus are summarized in table 1. Six patients (27%) were coinfected by HIV. Overall, most patients were male (86%), had HCV genotype 1b infection (45%), 12 (55%) had failed previous antiviral therapy with PegINF and RBV alone (9 patients) or in combination with a first generation protease inhibitor (3 patients). Median age was 58 years (IQR, 51-68), although the six HIV-HCV coinfected patients were significantly younger (49 vs. 65 years, p = 0.01). HCV monoinfected patients tended to have higher ALT levels (75 vs. 42 IU/mL), higher MELD scores (12 vs. 10), and more advanced fibrosis or cirrhosis (59% vs. 50%) than coinfected patients although the differences were not significant. Albumin levels were higher in monoinfected than in coinfected patients (4.2 vs. 3.4 g/dL, p = 0.001). Immunosuppressant regimen was based on tacrolimus combinations (19 out of 22 patients). The median time from LT to current antiviral therapy was 70 months (IQR, 20-116). At the time of treatment onset, six patients (all HCV-monoinfected) presented decom-pensated cirrhosis with ascites and 2 of them also had hepatic encephalopathy.
Baseline demographic characteristics of the entire cohort and by HIV serostatus.
| Characteristics | Overall (n = 22) | HCV-monoinfected (n = 16) | HCV/HIV-coinfected (n = 6) | P-value |
|---|---|---|---|---|
| Male, n (%) | 19 (86) | 15 (94) | 4 (67) | 0.1 |
| Recipient age (years), median (IQR) | 58 (51-68) | 65 (53-69) | 49 (43-53) | 0.01 |
| Months from LT, median (IQR) | 70 (20-116) | 51 (20-125) | 84 (73-116) | 0.66 |
| Hepatocelullar carcinoma, n (%) | 6 (27) | 5 (31) | 1 (17) | 0.49 |
| Donor age (years), median (IQR) | 58 (51-66) | 59 (53-66) | 54 (40-66) | 0.37 |
| HCV Genotype, n (%) | 0.16 | |||
| 1 | 5 (22) | 3 (19) | 2 (33.3) | |
| 1a | 1 (5) | 1 (6) | 0 | |
| 1b | 10 (45) | 8 (50) | 2 (33.3) | |
| 2 | 1 (5) | 1 (6) | 1 (17) | |
| 3 | 2 (9) | 0 | 2 (33.3) | |
| 4 | 3 (14) | 3 (19) | 0 | |
| IL28B, n (%) | 0.77 | |||
| CC | 7 (32) | 5 (31) | 2 (33) | |
| CT | 9 (41) | 6 (38) | 3 (50) | |
| TT | 6 (27) | 5 (31) | 1 (17) | |
| Inmunosupression, n (%) | 0.37 | |||
| Fk monotherapy | 10 (45) | 7 (44) | 3 (50) | |
| Fk + MMF | 5 (22) | 3 (19) | 2 (33) | |
| Fk + Everol | 4 (18) | 4 (25) | 0 | |
| Csa | 1 (5) | 1 (6) | 0 | |
| Everol + MMF | 1 (5) | 1 (6) | 0 | |
| No therapy | 1 (5) | 0 | 1 (17) | |
| HCV-RNA (log10 IU/mL), median (IQR) | 6.6 (6.2-6.8) | 6.6 (5.9-6.9) | 6.4 (6.4-6.8) | 0.46 |
| ALT (IU/L), median (IQR) | 64 (33-123) | 75 (43-132) | 42 (28-103) | 0.42 |
| Bilirubin (mg/dL), median (IQR) | 1.2 (0.8-1.9) | 1.2 (0.7-2.1) | 1.2 (0.9-1.2) | 0.97 |
| Albumin (g/dL), median (IQR) | 3.6 (3.2-4.1) | 3.4 (2.8-3.7) | 4.2 (4.2-4.3) | 0.001 |
| Platelets (x 103/mL), median (IQR) | 84 (67-108) | 86 (62-111) | 84 (72-104) | 0.48 |
| Child-Pugh score, median (IQR) | 6 (5-7) | 6 (5-8) | 5 (5-5) | 0.03 |
| MELD score, median (IQR) | 11 (10-14) | 12 (10-14) | 10 (9-11) | 0.08 |
| Histology, n (%) | 0.86 | |||
| Ishak F3-F4 | 6 (33) | 4 (33) | 2 (33) | |
| Ishak F5-F6 | 10 (56) | 7 (59) | 3 (50) | |
| Cholestatic hepatitis | 2 (11) | 1 (8) | 1 (17) | |
| Treatment experienced; n (%) | 12 (55) | 7 (44) | 5 (83) | 0.10 |
| PR, | 9 | 6 | 3 | |
| PR + PI | 3 | 1 | 2 | |
| Triple therapy with PI, n (%) | 3 (14) | 1 (6) | 2 (33) | 0.10 |
All six coinfected patients were under antiretroviral therapy (ART) and had undetectable HIV-RNA levels (< 20 IU/mL) at baseline and remained undetectable throughout the treatment in all patients. Median CD4 and CD8 at baseline were 210 (IQR, 150-400) and 565 (IQR, 450-640) cells/mm3, respectively.
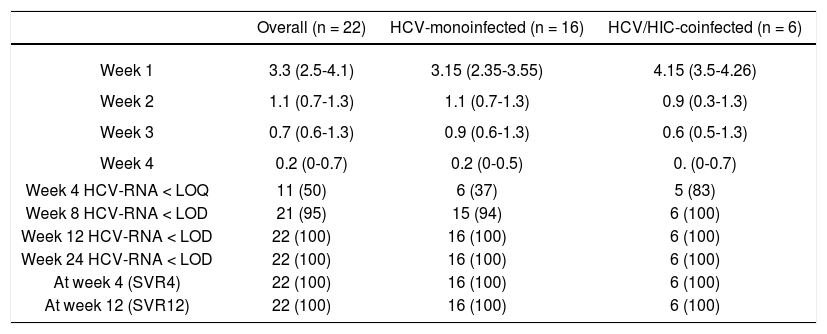
EfficacyIn the current study, all 22 patients achieved an SVR 12 weeks after end of treatment regardless of HIV status. As shown in table 2, treatment resulted in rapid suppression of circulating virus with a median decrease in HCV-RNA of 3.3 log10 IU/mL at one week (IQR, 2.5-4.1). At week 4, 11 of the 22 patients had HCV-RNA below the limit of quantification (< 15 IU/mL) and at week 8 all but one patient had undetectable HCV-RNA (< 10 IU/mL).
Response (HCV RNA < 15 IU/mL) during and after antiviral therapy by HIV serostatus.
| Overall (n = 22) | HCV-monoinfected (n = 16) | HCV/HIC-coinfected (n = 6) | |
|---|---|---|---|
| Week 1 | 3.3 (2.5-4.1) | 3.15 (2.35-3.55) | 4.15 (3.5-4.26) |
| Week 2 | 1.1 (0.7-1.3) | 1.1 (0.7-1.3) | 0.9 (0.3-1.3) |
| Week 3 | 0.7 (0.6-1.3) | 0.9 (0.6-1.3) | 0.6 (0.5-1.3) |
| Week 4 | 0.2 (0-0.7) | 0.2 (0-0.5) | 0. (0-0.7) |
| Week 4 HCV-RNA < LOQ | 11 (50) | 6 (37) | 5 (83) |
| Week 8 HCV-RNA < LOD | 21 (95) | 15 (94) | 6 (100) |
| Week 12 HCV-RNA < LOD | 22 (100) | 16 (100) | 6 (100) |
| Week 24 HCV-RNA < LOD | 22 (100) | 16 (100) | 6 (100) |
| At week 4 (SVR4) | 22 (100) | 16 (100) | 6 (100) |
| At week 12 (SVR12) | 22 (100) | 16 (100) | 6 (100) |
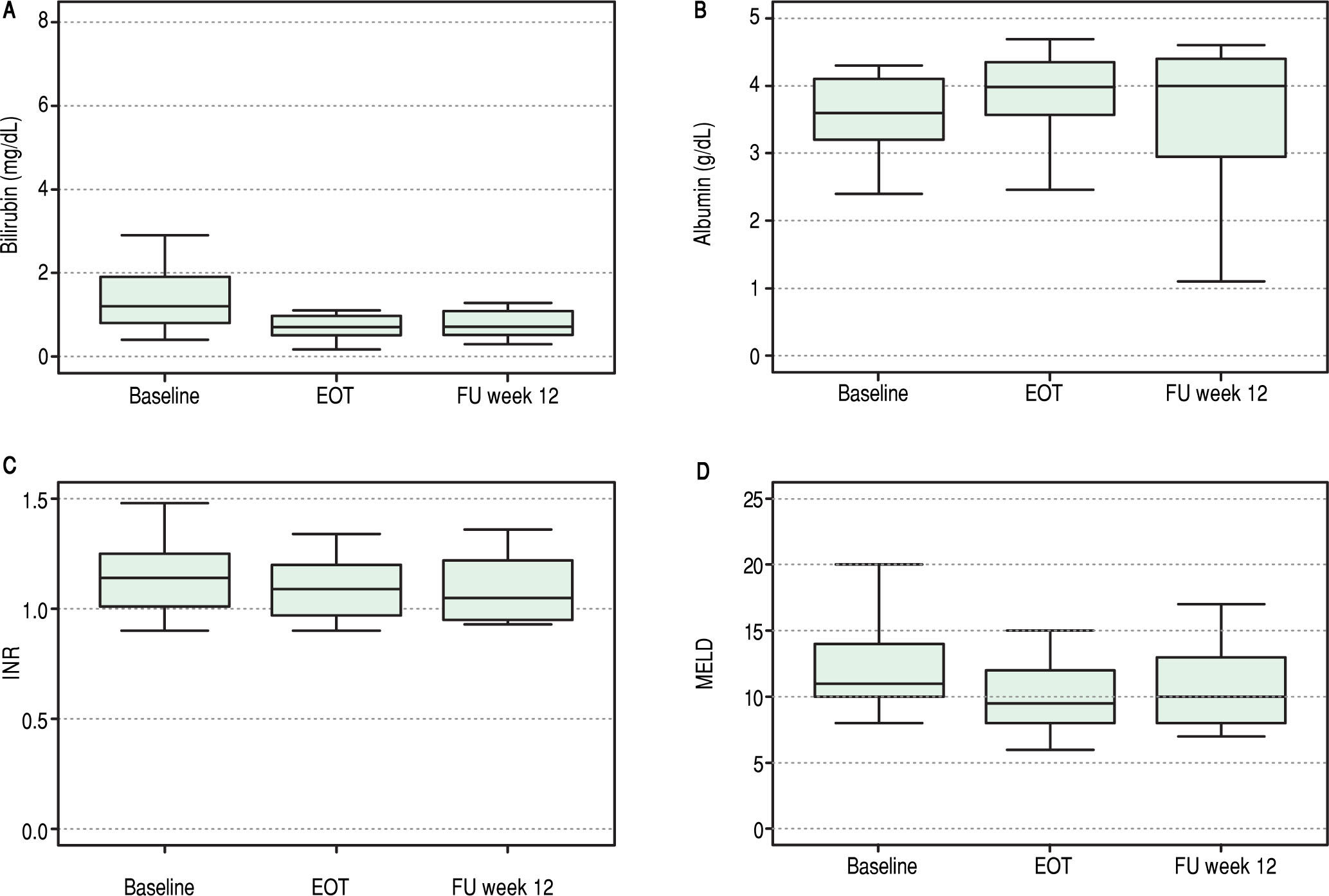
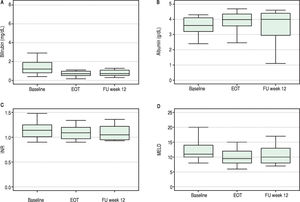
Overall, liver function tests improved significantly over time. Levels of bilirubin, albumin, INR and MELD are shown in Figure 1. Median serum total bilirubin levels decreased significantly from 1.2 mg/dL (0.8-1.9) at baseline to 0.7 mg/dL (p = 0.003) at 12 weeks after end of treatment. Albumin levels increased from 3.6 g/dL to 4 g/dL (P = 0.02). Platelets values increased from 84 to 118 x 103/ mL (p = 0.03). Improvement in MELD score (decreased from 11 to 10 from baseline to follow-up week 12, p = 0.04) was mostly accounted for by a decrease in bilirrubin levels while improvement in Child-Pugh scores (decreased from 6 to 5, p = 0.003) was mainly due to an increase in albumin levels. No change in international normalized ratio (INR) or creatinine was observed.
At the end of therapy, ascites had resolved in four of the six patients with decompensated cirrhosis at baseline.
Safety and clinical complicationsOverall, seven episodes of clinical complications requiring hospitalization occurred in six patients during treatment. Only one episode of atrioventricular blockade, in a patient receiving amiodarone, was considered related to antiviral therapy. The other episodes were, immune thrombocytopenia (n = 1) which resolved with steroid therapy; tuberculous pleuritis (n = 1), cholangitis due to biliary stricture corrected with stenting (n = 1), post-transplant lymphoproliferative disease (n = 1) and recurrent hepatic encephalopathy with refractory ascites (n = 1). All of them were resolved and didn’t require stopping treatment. After the end of treatment and during follow-up, 2 patients presented biopsy proven mild acute rejection, 2 and 4 weeks after the end of therapy, and both episodes were managed with adjustment of inmunosu-pression therapy.
There was no evidence of treatment associated haema-tological or cutaneous toxicity. Two patients required minor tacrolimus and everolimus dose modification during therapy. During treatment, there were no significant changes either in CD4 or CD8 count in HIV coinfected patients. None of the patients required the use of colony-stimulating agents, erythropoiesis-stimulating agents or blood transfusions.
Although there were no deaths during treatment or immediate follow-up, two patients died (four and eight months after end of therapy) due to complications of end-stage liver disease (due to hepatorenal syndrome and upper gastrointestinal bleeding from ruptured esophageal varices, respectively), despite viral eradication.
DiscussionIn the current report, a 24-week course of SOF plus DCV successfully eradicated HCV infection in 22 patients with severe HCV recurrence after LT, including patients with cholestatic hepatitis or decom-pensated cirrhosis, irrespective of HIV status.
HCV recurrence has long been recognized as a major cause of patient and graft loss among HCV-infected liver transplant recipients and limitations in treatment options have represented a major drawback in the transplant set-ting.1-3 The issue is of special relevance in HIV-HCV coinfected patients undergoing LT whose long-term survival has consistently been found to be shorter than that of monoinfected patients6-8 due to their accelerated fibrosis progression and lower response rates to interferon-based treatment.5 Hence, although prevention of recurrence holds the key to improving long-term survival, safe and effective treatment options are required for those with recurrent infection.
The advent of direct acting antiviral agents (DAAs) given as interferon-free combinations has dramatically changed treatment options in the liver transplant setting. Several recent reports have shown that interferon-free DAA combinations are safe and effective for the treatment of HCV recurrence after LT.12,13 In this regard, SVR12 rates among LT recipients treated with the NS3 protease inhibitor simeprevir plus the NS5B polymerase inhibitor SOF, with or without ribavirin for 12 or 24 weeks, have ranged between 88 and 90%.12,14-16 Similarly, the combination of SOF with the NS5A inhibitor DCV with or without ribavirin for 12 or 24 weeks has also been reported to eradicate infection in 90-95% of mo-noinfected liver transplant recipients.12,17 Although reports using triple therapy with pegylated interferon, ribavirin and first generation protease inhibitor (bo-ceprevir or telaprevir), among HIV-HCV coinfected liver transplant recipients was shown to eradicate infection in 60% of patients,18 poor tolerability was still a major issue. More recently, SVR rates of 90% or higher has been reported among coinfected LT recipients treated with sofosbuvir-based DAA combinations.19,20 although information in this patient group remains scarce.
Several findings of the current report deserve further mention. First, the majority of patients might be considered a “hard-to-treat” population with advanced, and sometimes decompensated liver disease, and prior failure to interferon-containing antiviral therapy. Yet viral suppression was remarkably fast with a mean decline in serum HCV-RNA of > 3 logs, just one-week after treatment onset. The observation that viral dynamics and SVR rates were equivalent in coinfected and HCV monoinfected patients is in accordance with the notion that, at least for genotype 1 and 4 infections, response rates to interferon-free DAA combinations are identical in mono and HIV-infected patients, as long as potential drug-drug interactions with antiretroviral agents are carefully avoided.21
Second, combination of sofosbuvir and daclatasvir was not only effective but also very well tolerated in most patients, even those with decompensated disease. The only severe adverse event was an atrioventricular block in a patient under amiodarone therapy requiring pacemaker placement. The episode occurred before a specific warning was issued regarding the risk of life-threatening bradycardia in patients under amiodarone treatment given sofosbuvir-containing DAA combina-tions.22 The fact that our patients were given a ribavirin-free DAA combination might have had an impact on tolerance and suggests that well tolerated ribavirin-free combinations for 24 weeks might have advantages over shorter RBV-containing regimens.
Third, the observed virological response in our patients was associated with a remarkable improvement in liver function tests and clinical status. Dramatic clinical improvement associated with viral clearance has already been reported in HIV-coinfected LT recipients receiving sofosbuvir plus daclatasvir treatment for fibrosing cholestatic HCV recurrence.23 Indeed, recurrent ascites resolved during treatment in four of the six decompen-sated patients. However, two patients continued to have ascites and recurrent episodes of hepatic encephalopathy and eventually died with complications of end-stage liver disease despite viral eradication. This is in agreement with other reports showing that viral eradication does not necessary correlate with clinical improvement,17,24,25 and strongly supports the importance of early consideration for treatment in all patients with HCV recurrence after LT.
Finally, except for the patient who was receiving amio-darone, few drug-drug interactions were observed in our patients. Only two patients had biopsy-proven mild acute rejection at the end of treatment which resolved after minor dose adjustments of immunosuppressive medications. This attests for the importance of systematic and frequent monitoring of immunosuppressant drug though levels throughout treatment, since improvement in liver function may increase hepatic drug metabolism and increase the risk of acute rejection. On the other hand, although no evidence of drug-drug interaction between anti-HCV and antiretroviral medication was seen in our coinfected patients, and sofosbuvir/daclatasvir does not appear to interact with most ARV drugs,26 the risk of interactions may be higher with other DAA combination and warrant close monitoring of HIV suppression.
Because of its single-center observation nature and the limited number of patients included, our results have to be interpreted with caution and may not be applicable to other populations with different genotypes or more advanced disease. Furthermore, although the 20 responding patients remains clinically compensated more than one year after treatment end, the long-term clinical and histo-logical outcome of responder patients remains to be established.
In conclusion, our single center experience shows that a 24-week course of SOF plus DCV without RBV, is safe and effective for LT recipients with HCV recurrence, including HIV/HCV coinfected patients without significant DDIs. These encouraging results provide hope for the improvement of long-term graft and patient survival of either HCV monoinfected or HIV coinfected LT recipients.
Abbreviations- •
ART: antiretroviral theraphy.
- •
CP: Child-Pugh.
- •
Csa: cyclosporine.
- •
DAAs: direct acting antiviral agents.
- •
DCV: daclatasvir.
- •
Everol: everolimus.
- •
Fk: tacrolimus.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
ILB28: interleukin IL28B polymorphism rs12979860.
- •
INR: International Normalized Ratio.
- •
IQR: interquartile range.
- •
LT: liver transplantation.
- •
MELD: Model for End Stage Liver Disease.
- •
MMF: mofetil mycofenolate.
- •
Peg-IFN: pegylated interferon.
- •
PI: protease inhibitor.
- •
RBV: ribavirin.
- •
SAEs: serious adverse events.
- •
SOF: sofosbuvir.
- •
SVR: sustained virological response.
L.C, I.C-V and J.I.E. participated in making the study design, interpretation of data and writing the article; J. L; I.B; M. C; O. L; F.R-F, T.S. participated in performing the research, R.C; and R.E. critical revision of the manuscript for important intellectual content and study supervision.
Conflict of InterestDr Rafael Esteban has attended advisor meetings with Abbott, Boehringer Ingelheim, Bristol Myers-Squibb, Glaxo, Gilead, Janssen Merck, and Novartis; has provided lectures on behalf of Abbott, Boehringer Ingelheim, Bristol Myers-Squibb, Glaxo, Gilead, Janssen, Merck, No-vartis and Roche; and has served on a Data Safety Monitoring Board for Novartis. The authors have no other potential conflicts of interest to disclose regarding the manuscript.
Financial SupportNone.
AcknowledgementsThe authors thank the patients and their families. We also wish to acknowledge those at Gilead and Bristol-Myers Squibb who made the sofosbuvir and daclatasvir compassionate access program available for liver transplant patients.