Infections are a frequent complication and a major cause of death among patients with cirrhosis. The important impact of infections in general and especially spontaneous bacterial peritonitis on the course of disease and prognosis of patients with cirrhosis has been recognized for many years. Nevertheless, such importance has recently increased due to the comprehension of infection as one of the most prominent risk factors for patients to develop acute-on-chronic liver failure. Furthermore, the issue of infections in cirrhosis is a focus of increasing attention because of the spreading of multidrug resistant bacteria, which is an emerging concern among physicians assisting patients with cirrhosis. In the present paper, we will review the current epidemiology of infections in patients with cirrhosis and particularly that of infections caused by resistant bacteria, demonstrating the relevance of the subject. Besides, we will discuss the current recommendations on diagnosis and treatment of different kinds of infections, including spontaneous bacterial peritonitis, and we will highlight the importance of knowing local microbiological profiles and choosing empirical antibiotic therapy wisely. Finally, we will debate the existing evidences regarding the role of volume expansion with albumin in patients with cirrhosis and extraperitoneal infections, and that of antibiotic prophylaxis of spontaneous bacterial peritonitis.
spontaneous bacterial peritonitis
European Association for the Study of the Liver
Chronic Liver Failure
acute-on-chronic liver failure
C-reactive protein
procalcitonin
likelihood ratio
Model for End-stage Liver Disease
multidrug-resistant
extended spectrum β-lactamase
European Medicines Agency
Food and Drug Administration
methicillin-resistant Staphylococcus aureus
Cirrhosis is a major cause of death worldwide, appearing among the 10 most frequent causes of death in many countries [1–3]. Bacterial infections are very common complications among patients with cirrhosis, and there are many reasons for a predisposition to infections among such patients: depressed humoral and cell-mediated immunity caused by the liver dysfunction, gut dysbiosis and bacterial translocation exacerbated especially by portal hypertension, and even genetic factors are determinants of the pathophysiology of infections in this population [4].
Studies have shown that 25–35% of patients with cirrhosis present bacterial infection at hospital admission or during the hospitalization period, with high associated mortality rates. Infection is the most important cause of death in individuals with decompensated cirrhosis, representing a four-fold increment in mortality risk when compared to subjects with cirrhosis who are not infected [4].
In a Brazilian study, when 540 hospital admissions of patients with cirrhosis were evaluated, we found that 25% of them were associated with bacterial infections. The most frequent infections were urinary tract infections, followed by spontaneous bacterial peritonitis (SBP), respiratory infections and skin infections [5]. Another study analyzed the role of alcohol consumption in the prognosis of cirrhosis. Among 388 hospitalized subjects, 152 (39.2%) were abusing alcohol before admission, and this population had significantly higher prevalences of infections (prevalence ratio of 2.14), ascites (prevalence ratio of 1.67), hepatic encephalopathy (prevalence ratio of 1.68), upper digestive bleeding (prevalence ratio of 2.51), renal failure (prevalence ratio of 1.62) and death (prevalence ratio of 2.40) than patients who did not abuse alcohol [6].
Bacterial infection alters the natural history of cirrhosis. Dionigi et al. [7] evaluated 501 consecutive patients with cirrhosis and observed that infection was present in 25.6%, with a 12-month survival rate of 41% (compared to 71% for patients without infections). The authors concluded that bacterial infection is an independent predictor of overall survival in this population. In fact, infection represents a different stage in the course of cirrhosis, affecting survival regardless of disease severity, and this assumption was already incorporated in a recent proposal for cirrhosis classification (infection would refer to stage 6 of cirrhosis – end-stage disease) [8].
According to the CANONIC Study [9], developed by the European Association for the Study of the Liver (EASL) Chronic Liver Failure (CLIF) Consortium, bacterial infection and alcohol consumption were considered the most frequent precipitating factors for acute-on-chronic liver failure (ACLF), which is the most severe form of acute decompensation of cirrhosis. In the CANONIC Study, 28-day mortality was 33.9% for individuals with ACLF at admission, 29.7% for patients developing ACLF during hospitalization and only 1.9% for patients who did not fulfill diagnostic criteria for ACLF. In a prospective cohort of 113 patients with cirrhosis, we found even higher mortality rates: 28-day mortality was 61.1% for patients with ACLF at admission, 50.0% for those who developed ACLF during hospitalization and 9.0% for those without ACLF [10]. In a North-American multicenter cohort study, it was demonstrated that patients with infections and ACLF had worse prognosis than patients with ACLF who were not infected [11]. These data reinforce the role of infection on the clinical course of cirrhosis.
2Patient evaluationThe presentation of infections in individuals with cirrhosis may be subtle, so that high clinical suspicion is a key point to reduce their negative consequences. Whenever infections are suspected at the outpatient clinic, patients must be considered for hospitalization due to the severe risks they impose to subjects with cirrhosis. Every hospitalized patient with cirrhosis should be evaluated for infection. Consequently, a thorough workup should be performed at admission and whenever a hospitalized patient deteriorates so that a possible infection can be detected (Table 1). It is advisable to collect blood and urine cultures, and to perform sputum analysis in patients with airway secretion. Chest X ray and abdominal ultrasound can guide additional investigation. In patients with ascites, it is mandatory to perform a diagnostic paracentesis for cytological analysis and culture in order to rule out SBP [12].
Basic diagnostic workup to assess for bacterial infections in patients with cirrhosis.
| (a) General blood tests: WBC, liver and renal tests, serum electrolytes |
| (b) Diagnostic paracentesis (if ascites is present) |
| (c) Cultures: blood, urine, ascites, sputum (if suspicion of respiratory tract infection) |
| (d) Imaging: chest X-ray and abdominal ultrasound |
| (e) Consider biomarkers for acute inflammatory response (CRP or PCT) |
WBC – white blood cell count; CRP – C reactive protein; PCT – procalcitonin.
Leukocyte count is difficult to interpret in patients with chronic liver disease due to hypersplenism with pancytopenia. Therefore, markers for acute inflammatory response, such as C-reactive protein (CRP) and procalcitonin (PCT), can be used to evaluate patients with possible infection. In a systematic review with meta-analysis [13], both CRP and PCT were accurate in distinguishing bacterial from other noninfective causes of inflammatory response in patients with cirrhosis. Positive likelihood ratio (LR) for PCT was high enough for it to be used as a rule-in diagnostic tool (positive LR of 7.38, 95% confidence interval – CI of 4.70–11.58), and the negative LR for CRP was low enough for it to be used as a rule-out diagnostic tool for infection (negative LR of 0.23, 95% CI of 0.13–0.41). More studies are necessary to define if biomarkers for acute inflammatory response can be used to diagnose infection or as a tool to guide antibiotic prescriptions or modifications in patients with cirrhosis.
3Spontaneous bacterial peritonitisSBP is a frequent and severe complication in patients with cirrhosis. SBP is the infection of ascites, in the absence of an apparent intra-abdominal focus, and its diagnosis should be considered when the neutrophil count is above 250cells/μL in the ascitic fluid [12]. The incidence of SBP is 5% to 25% in hospitalized patients with cirrhosis who have ascites [14,15]. Patients with SBP have a poor prognosis, with mortality rates varying from 20% to 30%. The Model for End-stage Liver Disease (MELD) seems useful in predicting its prognosis [16]. Because of its severity, SBP should be treated promptly. Its treatment is based on two cornerstones: antibiotic therapy and volume expansion with albumin. All patients with a history of SBP should also be evaluated for liver transplantation [12].
In a 2002 Brazilian study of 1031 consecutive hospitalized patients with cirrhosis, the prevalence of SBP was 11% and the associated mortality was 22% [17]. In a later study, mortality was 40% [16]. The occurrence of infections due to multidrug-resistant (MDR) bacteria began to gain importance in medical literature, and such infections could partly explain rising mortality rates.
3.1Microbiological profileEuropean authors have shown that MDR bacteria were responsible for 20% of infections, mostly for those of nosocomial origin [18,19]. American authors also have shown a higher prevalence of infections caused by MDR bacteria in patients with cirrhosis [20]. Infections caused by MDR bacteria lead to higher morbidity and death rates, and to longer hospitalizations, ultimately leading to excessive health expenditure. The most important risk factors associated with infections caused by MDR bacteria are a recent hospitalization, especially if the patient was in intensive care setting, and previous utilization of antibiotics like quinolones and β-lactams [18].
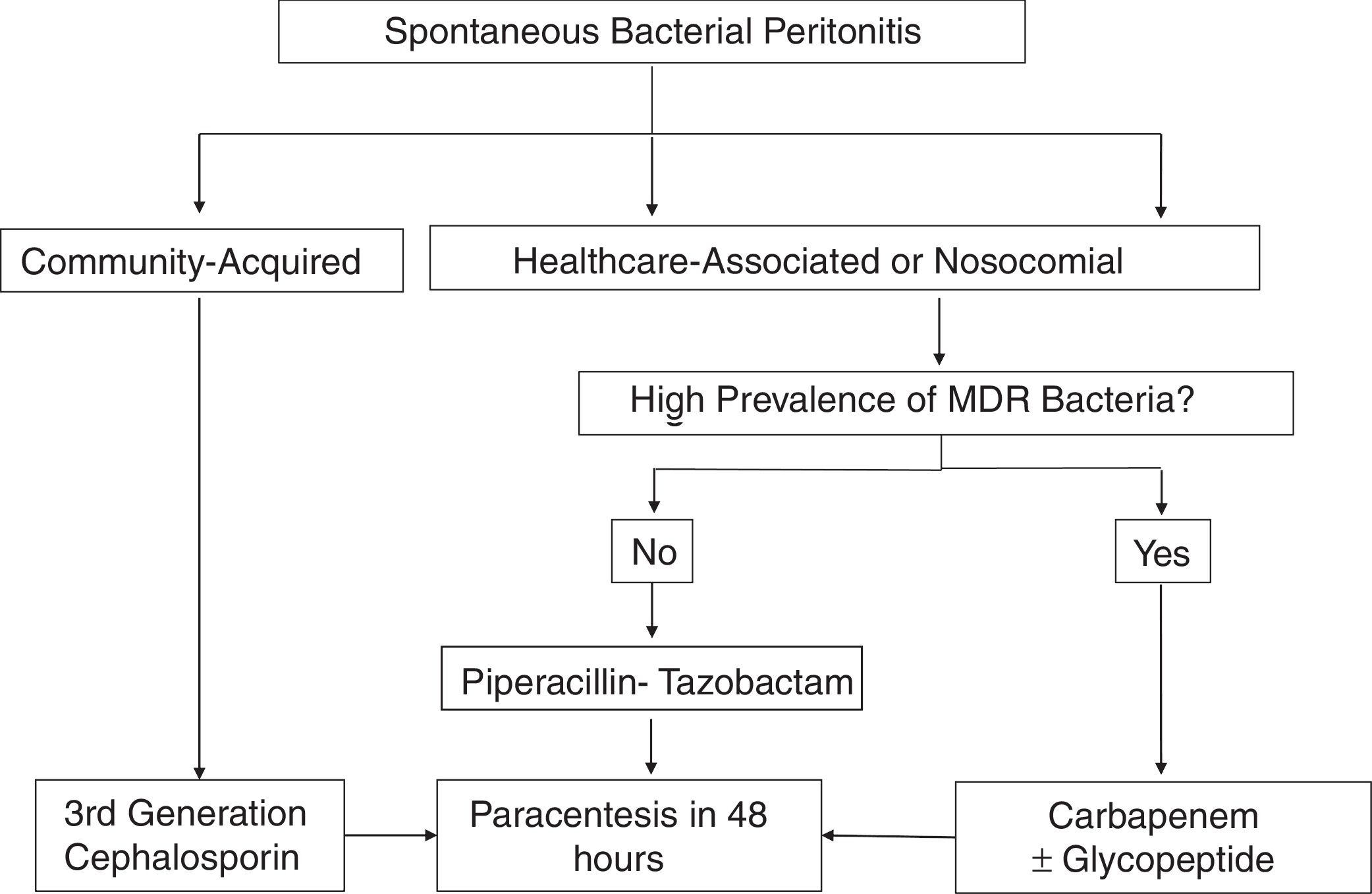
Since hospitalization is a recognized risk factor for MDR bacterial infections, one should take into account the epidemiological classification of infections to assure the best treatment for patients: community infections are those diagnosed within the first 48h of hospitalization, whereas nosocomial infections are those diagnosed after this period. Healthcare-associated infections are the ones detected in the first 2 days of hospitalization of an individual recently attending healthcare facilities. MDR bacteria are less frequent in community-acquired infections, but they occur more frequently in healthcare-associated and mainly in nosocomial infections [19]. Therefore, the traditional empiric treatment with third generation cephalosporins may be effective for community-acquired infections, but not for healthcare-associated/nosocomial infections.
The North American Consortium for the Study of End-Stage Liver Disease has recently highlighted the effects of nosocomial infections on the course of cirrhosis [21]. The study included 2864 hospitalized patients with cirrhosis, 15% of whom developed nosocomial infections. Patients with nosocomial infection were more likely to have advanced cirrhosis and to be infected at admission. Nosocomial infections were associated with higher rates of ACLF, mortality and transplantation. Such patients also had higher rates of respiratory infection, urinary tract infection and infections caused by Clostridium difficile, fungi or MDR bacteria. Age, alcohol abuse, MELD score at admission, lactulose use, diagnosis of ACLF or acute kidney injury, admission to an intensive care unit and nosocomial infection were associated with increased mortality.
SBP is typically a monomicrobial infection, Escherichia coli and gram-positive cocci (streptococci and enterococci) being the most frequent cultured isolates. Despite the “routine” use of third generation cephalosporins (cefotaxime, for instance) in the suspicion of SBP, it is now essential to consider the role of MDR strains in patients with cirrhosis. Extended spectrum β-lactamase (ESBL)-producing enterobacteria are the most frequent MDR strains in patients with cirrhosis who are infected. Infections with these bacteria, isolated in more than 30% of cases of SBP, are associated with higher mortality than infections with less resistant bacteria [18]. We have recently evaluated the susceptibility of 5800 isolates of patients with or without cirrhosis who were hospitalized in southern Brazil. MDR bacteria infected 38% of patients with cirrhosis and 44% of those without cirrhosis. E. coli strains were the most frequent MDR bacteria in both groups. Among patients wih cirrhosis, around 20% of E. coli and Klebsiella sp isolates produced ESBL. In addition, 44% of S. aureus isolates were methicillin-resistant. Moreover, 36% of isolates from individuals with cirrhosis were not susceptible to third-generation cephalosporins [22]. In another Brazilian experience, authors found a higher proportion of MDR germs among patients with SBP, reaching levels of 53.7% when considering only nosocomial infections. Being infected with resistant bacteria did not have a significant impact on important clinical outcomes in that study, which could be explained by the fact that almost 40% of the MDR agents were gram-positive bacteria, while gram-negative enterobacteriacea usually responded to third generation cephalosporins, piperacillin-tazobactam and carbapenems [23].
In a multicenter prospective study, 34% of patients with cirrhosis had infections caused by MDR bacteria and this figure varied according to geographic location. The highest prevalence was observed in Asia, and especially in India. In South America, the highest prevalences were seen in Brazil, Argentina and Chile. Infections associated with MDR bacteria were less frequently cured and led to increased rates of shock, organ failures and death. On the other hand, appropriate empirical antibiotic therapy increased survival [24].
Another study evaluated the role of MDR bacteria in patients with decompensated cirrhosis or ACLF [25]. The authors prospectively evaluated two series of patients. The first was derived from the CANONIC Study [9] and consisted of 1146 patients evaluated in 2011, 39.7% of whom were infected. Among those patients who had positive culture samples, 29.2% had infections caused by MDR bacteria, and nosocomial infection, admission to the intensive care unit and recent hospitalization were identified as factors associated with the presence of such bacteria. The second series of patients regarded 883 patients evaluated in 2018, 32.2% of whom were infected. Among the patients with infections who had positive culture samples, MDR bacteria were identified in 37.9%. The increase of almost 10% in the rate of infections associated with MDR bacteria in less than 10 years highlights the importance of the subject of bacterial resistance in this population of patients [25].
3.2Antibiotic treatmentThe choice of empirical antibiotic therapy for SBP should be based on severity and source of infection, and on local epidemiological data on bacterial resistance (Table 2). Generally, third-generation cephalosporins remain the first-line therapy for community-acquired infections. Empirical treatment of healthcare-associated and nosocomial infections should be guided according to local microbiological profiles (Fig. 1) [26].
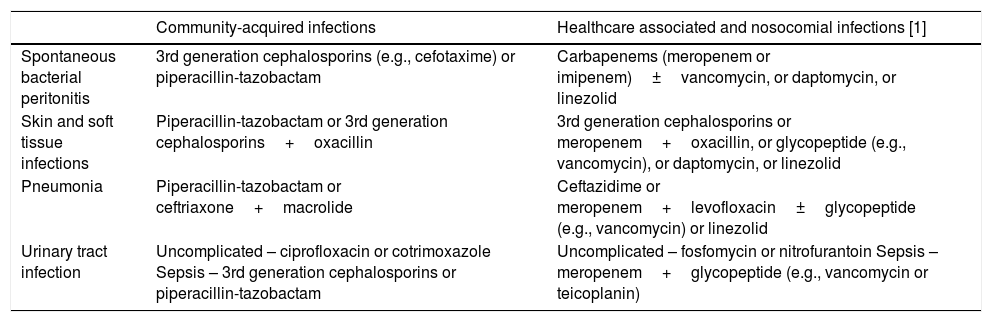
Suggested empirical antibiotic therapy for infections in patients with cirrhosis.
| Community-acquired infections | Healthcare associated and nosocomial infections [1] | |
|---|---|---|
| Spontaneous bacterial peritonitis | 3rd generation cephalosporins (e.g., cefotaxime) or piperacillin-tazobactam | Carbapenems (meropenem or imipenem)±vancomycin, or daptomycin, or linezolid |
| Skin and soft tissue infections | Piperacillin-tazobactam or 3rd generation cephalosporins+oxacillin | 3rd generation cephalosporins or meropenem+oxacillin, or glycopeptide (e.g., vancomycin), or daptomycin, or linezolid |
| Pneumonia | Piperacillin-tazobactam or ceftriaxone+macrolide | Ceftazidime or meropenem+levofloxacin±glycopeptide (e.g., vancomycin) or linezolid |
| Urinary tract infection | Uncomplicated – ciprofloxacin or cotrimoxazole Sepsis – 3rd generation cephalosporins or piperacillin-tazobactam | Uncomplicated – fosfomycin or nitrofurantoin Sepsis – meropenem+glycopeptide (e.g., vancomycin or teicoplanin) |
1-Health-care associated infections in settings with low prevalence of multidrug-resistant bacteria could be treated as community-acquired infections, except if sepsis.
The effectiveness of antibiotic treatment against SBP should be assessed by an analysis of ascites after 48h of therapy. Carbapenems associated or not with glycopeptides are the mainstream choice for healthcare-associated and nosocomial infections. Alternatives, such as piperacillin-tazobactam, could be considered in settings with low prevalence of infections associated with MDR bacteria [12,26–28].
3.3Albumin infusionAcute kidney injury occurs in approximately one third of patients diagnosed with SBP and it is a strong predictor of mortality during hospitalization. In a study conducted in southern Brazil evaluating over 100 episodes of SBP, some degree of renal impairment was found in 24% of the cases. The mortality rate in patients with or without renal impairment was 36% and 6% respectively [29]. Plasma volume expansion with intravenous albumin decreases renal impairment and mortality in patients with cirrhosis and SBP. A 1999 randomized controlled trial at the Hospital Clinic of Barcelona showed that prophylactic albumin infusion (1.5g/kg on day 1 and 1g/kg on day 3) was associated with a lower prevalence of renal impairment and mortality than antibiotic therapy alone [30].
According to the International Club of Ascites, the greatest benefits of prophylactic albumin infusion in SBP are seen in patients with bilirubin levels >4mg/dL or creatinine levels >1mg/dL [31]. However, a meta-analysis concluded that albumin infusion prevented renal impairment and reduced mortality in all patients with SBP, independently of disease severity [32].
3.4ProphylaxisRegarding primary prophylaxis of SBP, two situations must be considered. The first refers to patients with cirrhosis (with or without ascites) with bleeding from any source passing through the digestive tract. Such patients have higher risk of bacterial translocation and infections and should undergo prophylaxis for 7 days with norfloxacin 400mg orally twice daily [12]. A randomized controlled trial has demonstrated that patients who meet at least two of four severity criteria (presence of ascites, hepatic encephalopathy, severe malnutrition and/or bilirubin levels >3mg/dL) develop fewer infections when prophylaxis is performed with intravenous ceftriaxone 1g/day instead of norfloxacin [33].
The second indication for primary prophylaxis concerns individuals who have protein levels under 1.5g/dL in ascites. These patients have increased risk of developing SBP due to the low opsonic capacity of the ascitic fluid. Two randomized controlled trials demonstrated increased survival with prophylaxis in this setting. In the first study, the use of norfloxacin 400mg/day orally was evaluated in patients with low total protein content in ascitic fluid who presented signs of advanced hepatic failure (Child–Pugh ≥9 and bilirubin levels ≥3mg/dL), or some degree of renal impairment (creatinine levels ≥1.2mg/dL, or blood urea nitrogen ≥25mg/dL, or serum sodium levels ≤130mEq/L) [34]. In the second trial, the authors studied the use of oral ciprofloxacin 500mg/day in patients with low total protein content in ascitic fluid as the only risk factor for SBP [35]. The EASL recommendation is to perform prophylaxis according to the first clinical trial: norfloxacin should be administered until the clinical status of the patient has improved and ascites has disappeared [12]. An open-label randomized controlled trial comparing alternating norfloxacin and rifaximin versus norfloxacin or rifaximin alone as primary prophylaxis for SBP in patients with low total protein content in ascitic fluid found that the alternating prophylaxis was more effective than norfloxacin alone regarding SBP prevention. There was no significant difference when comparing the alternating prophylaxis and rifaximin alone, nor when comparing norfloxacin alone and rifaximin alone [36].
Regarding secondary prophylaxis, the use of oral norfloxacin 400mg/day is recommended after an episode of SBP as long as the patient remains with ascites or until transplantation. This is justified by a rate of SBP recurrence of approximately 70% in one year [12]. A recent randomized trial compared oral rifaximin 400mg thrice daily to oral norfloxacin 400mg once daily in the secondary prophylaxis of SBP and found a lower rate of SBP relapse and lower mortality with rifaximin [37]. More studies are still needed in order to recommend rifaximin as the standard of care both for primary and secondary prophylaxis [12,38].
Recently, the European Medicines Agency (EMA), following multiple previous “Drug Safety Communications” from the Food and Drug Administration (FDA), has published a recommendation on quinolone and fluoroquinolone antibiotics, restricting their use because of the possibility of complications: tendon inflammation or rupture, joint or extremity pain, gait disturbance, neuropathies, depression, fatigue, memory loss, sleep disturbances and aortic aneurysm or dissection [39,40]. Moreover, fluoroquinolone use leads to higher rates of resistant microorganisms besides gut microbiome. Tacconelli et al. demonstrated that quinolone administration increased three times the risk of infection by methicillin-resistant Staphylococcus aureus (MRSA) [41].
Such data impose a new challenge for all medical community: revising the choice for quinolones and fluoroquinolones in the treatment of infectious diseases, SBP among them. In this context, there is a pressing need for more studies testing alternative or new antimicrobial classes for SBP prophylaxis, or to reassure the use of norfloxacin as first choice in chronic liver disease patients. Three studies have recently addressed this issue. Piano et al. found no association between bacterial resistance and previous prophylaxis with antibiotics (quinolones) in individuals with cirrhosis [24]. In a randomized controlled trial published by Moreau et al., analyzing the long-term effect of norfloxacin use by individuals with advanced cirrhosis (Child C), there was no benefit regarding mortality at 6 months. On the other hand, an increase in survival was observed in patients with low protein levels in the ascitic fluid (<1.5g/dL) [42]. Finally, in the study by Fernández et al., long-term prophylaxis with norfloxacin was not associated to infections caused by MDR bacteria [25].
There is also a debate regarding the role of proton pump inhibitors as a risk factor for infections. We performed a prospective study evaluating 582 outpatients with cirrhosis and we did not observe an increased risk for SBP among patients under acid suppression [43]. The use of such drugs should be judicious since there are studies that suggest that this association might exist [44–46].
Concerning prophylaxis, the ideal approach would probably be one that spared antibiotics use, in order to decrease the odds of appearance of MDR bacterial strains. Probiotics, prokinetic agents, statins, bile acids, among others have been investigated, but results are not satisfying up to the present time [47]. Also in this context, an interesting meta-analysis including over 1000 patients has recently demonstrated that decreasing portal pressure through the use of β-blockers improves the prognosis of patients with cirrhosis and leads to lower incidence of SBP among those with ascites [48].
3.5Non-selective β-blockers in patients with SBPIn recent years, the “window hypothesis” has been an issue of discussions. According to it, patients with advanced cirrhosis might have their survival impaired by the use of non-selective β-blockers, frequently prescribed for variceal bleeding prophylaxis [49]. Despite initial evidence, especially associating the use of non-selective β-blockers with worse prognosis among patients with refractory ascites [50], this theory do not seem to be supported by more recent studies, particularly a meta-analysis which failed to demonstrate increased mortality among non-selective β-blockers users who had ascites or refractory ascites [51].
Specifically concerning SBP, a retrospective cohort study has shown that, while using non-selective β-blockers was associated to a significant increase in transplant-free survival of patients with cirrhosis and without SBP, it was associated to a significant decrease in transplant-free survival of those with SBP [52]. On the other hand, this was not confirmed in another study when propranolol was used in doses lower than 160mg/day [53]. In this regard, EASL recommends that non-selective β-blockers are discontinued in patients with systolic blood pressure under 90mmHg, acute kidney injury, serum sodium under 130mmol/L, bleeding, SBP or sepsis. If these contraindications persist, variceal banding should be started for primary prophylaxis against esophageal variceal hemorrhage [12].
4Extraperitoneal infectionsEarly broad-spectrum empiric antibiotic therapy is recommended until cultures can guide the targeted use of antibiotics [12]. Prompt empiric antibiotic treatment is particularly important for patients with cirrhosis and septic shock, in whom every 1-h delay in receiving appropriate antimicrobials leads to an increase in mortality (adjusted odds ratio of 1.1) [54]. As in other immunosuppressive states, antifungal therapy should be added to the antibiotic scheme when a clear clinical improvement is not achieved [12]. We emphasize that it is important to recognize local epidemiology of resistance and frequent isolates to guide empirical antibiotic therapy.
EASL has recently made general recommendations regarding the empirical antimicrobial therapy for some extraperitoneal infections. The choice concerning empirical antimicrobial treatment should take into consideration whether the infection is community-associated, healthcare-associated or of nosocomial origin (Table 2). For healthcare-associated infections, in settings with high prevalence of MDR bacteria or in cases of sepsis, treatment should be similar to that recommended for nosocomial infections [12].
4.1Skin and soft tissue infectionsCommunity-acquired skin and soft tissue infections should be managed with piperacillin-tazobactam or the combination of a third-generation cephalosporin and oxacillin. In nosocomial infections, a third-generation cephalosporin or meropenem associated with an antibiotic against gram-positive bacteria (oxacillin, vancomycin, daptomycin, or linezolid) are recommended [12].
4.2Respiratory tract infectionsFor community-acquired pneumonia, EASL suggested piperacillin-tazobactam, or ceftriaxone associated with a macrolide. Respiratory quinolones (levofloxacin or moxifloxacin) are alternatives for patients allergic to betalactamic agents. For nosocomial pneumonia, EASL suggested ceftazidime or meropenem in combination with levofloxacin, with or without a glycopeptide or linezolid [12].
4.3Urinary tract infectionsFor community-acquired urinary tract infections, EASL recommended ciprofloxacin or cotrimoxazole for uncomplicated infections or a third-generation cephalosporin or piperacillin-tazobactam for cases of urinary sepsis. For uncomplicated nosocomial urinary tract infections, fosfomycin or nitrofurantoin are the options, whereas the indication for nosocomial urinary sepsis is meropenem associated with teicoplanin or vancomycin [12].
4.4Albumin infusionThree randomized controlled trials evaluated the role of volume expansion with albumin in infections other than SBP. In the first, there was no significant difference in mortality, but, after adjusting for other prognostic factors, albumin infusion was independently associated with survival [55]. In the second, albumin infusion delayed the development of renal impairment, but there was no significant differences regarding the rate of renal impairment at three months or concerning overall survival [56]. The third trial also failed to demonstrate significant benefits with the administration of albumin for such patients [57]. Regarding albumin infusion for individuals with cirrhosis and infections other than SBP, a meta-analysis recently published by our group [58] has demonstrated the absence of evidence of significant impact of albumin administration on 30-day mortality (risk ratio of 1.62, 95% CI of 0.92–2.84, p=0.09), 90-day mortality (risk ratio of 1.27, 95% CI of 0.89–1.83, p=0.19) and renal dysfunction (risk ratio of 0.55, 95% CI of 0.25–1.19, p=0.13). Thus, the use of albumin in infections other than SBP cannot be formally recommended at this moment [12].
A recent study demonstrated the heterogeneity of extraperitoneal infections. In that study, patients with endocarditis, secondary peritonitis, pneumonia and bacteremia had a worse prognosis. A combination of hepatic and renal function parameters and infection type had a clear association with survival. The authors suggested that the role of albumin infusion could be further explored in these populations at the highest risk for poor outcomes [59].
5ConclusionHigh-risk patients must be treated for infections in a more aggressive way. Patients with cirrhosis who are infected, especially those with SBP, should be treated promptly and properly. Infections caused by MDR bacteria should be a current concern, and new antibiotic strategies are needed for this special population. Individualized antibiotic treatment based on local epidemiology is the key for success, not neglecting the urge to preserve renal function of these complex patients. It is important to keep pursuing new forms of prophylaxis against SBP, so that, in the future, antibiotics might no longer be necessary in this context, decreasing the risk of development of MDR bacteria.
Conflicts of interestThe authors declare that there is no conflict of interest regarding the publication of this paper.