Antiviral therapy in patients suffering from chronic hepatitis C virus (HCV) infection and rare comorbidities cannot be easily started, as it can reduce the likelihood of a good therapeutic response with an increased frequency of side effects. We report two patients presenting unusual comorbidities associated with chronic C hepatitis: one with the Ehlers-Danlos Syndrome (EDS), a rare genetic disease caused by a defect in collagen synthesis, the other one with the Charcot Marie Tooth (CMT) disease, an uncommon but severe form of demyelinating peripheral neuropathy. Both patients were successfully treated with pegylated Interfe-ron (Peg-IFN) and ribavirin (RBV) combined therapy, with the achievement of a sustained viral response (SVR) and a low occurrence of adverse effects. Up to now there are no reports of patients suffering from chronic C hepatitis associated with these uncommon but severe comorbidities treated with antiviral therapy. In conclusion, in such clinical situations, anti-HCV therapy may be started and tailored, especially if the patient is highly motivated and if optimal predictors of response (i.e. young age, favourable genotype and low baseline viraemia) do exist.
Chronic hepatitis C affects almost 170,000,000 people in the world. Now it’s widely recognized that the standard of care for this infection is a combination of Pegylated Interferon (Peg-IFN) and ribavirin with weight-related doses.1 The only real indication of treatment is represented by activity of infection that means persistently detectable HCV RNA. Such a treatment scheme is successful in patients with HCV genotypes 2 and 3 infections, achieving HCV eradication rates of 75-90%, but it is much less effective in patients with genotypes 1 and 4 with eradication rates ranging between 45 and 52%.2 In addition, the concomitant presence of several other diseases (especially cardiovascular, psychiatric, oncological and endocrinological contraindications) further limits efficacy and applicability of therapy in an appreciable number of patients with chronic HCV-induced liver disease.
Therefore, although any patient could be eligible for treatment, in some conditions antiviral therapy can’t be easily started, in particular the presence of severe comorbidities can reduce the likelihood of response with an increased incidence of side effects.
In such difficult clinical situations, anti-HCV therapy may also be undertaken, especially if the patient is highly motivated and if, as in our case, other predictors of response are present (young age, genotype 2 or 3, recently acquired infection, low vi-raemia at baseline, etc.).3
We report two cases with chronic hepatitis C admitted as outpatients to the Section of Infectious Diseases of Ferrara: one affected by EDS, a rare genetic disease caused by a defect in collagen synthesis and the other one affected by CMT, an uncommon but very severe form of demyelinating peripheral neuropathy. In both cases a SVR was achieved. These patients were successfully treated with antiviral therapy, with a relatively low frequency of side effects.
Case reportsCase 1In July 2006, a 24 year-old male was admitted at Department of Infectious Diseases because of chronic HCV infection. The patient reported that in 1983 he underwent a blood transfusion for foetal anaemia, present at birth. HCV infection had been detected during a routine check carried out a few months before our observation. In addition, the patient was suffering from hereditary EDS Type III, with cutaneous involvement (keloids, velvety smooth skin, atrophic striae) and loose, unstable joints. He also suffered from ulcerative colitis, hiatal hernia, bronchial asthma and hyperinsulinism. He assumed salbutamol as needed and in past he was treated with metformin.
At admission, clinical examination was essentially negative except for a mild heart murmur. The echo-cardiography showed a mitral valve prolapse, while a liver ultrasonography was negative. The erythro-cyte sedimentation rate and the C-reactive protein were negative. Complete blood count appeared to be normal. Blood test showed elevation of serum ALT (61 UI/l) and quantitative detection of HCV-RNA (Real time PCR, COBAS TaqMan Test) was 26,100 IU/mL; HCV genotype was 2a/2c. The patient was seronegative for hepatitis A virus (HAV) infection and had evidence of past hepatitis B virus (HBV) vaccination. Liver biopsy was not performed. An excellent treatment motivation was recorded.
In view of the basal hereditary pathology, we directed the patient to undergo consultation by different specialists including dermatologists, cardiologists, psychiatrists and geneticists who did not find contraindications to antiviral treatment of HCV infection.
We started treatment with Peg-IFN α-2a (180 mg once weekly, subcutaneously) and RBV (800 mg/day, orally). The treatment was well tolerated and effective, but with a slow viral response considering the favourable genotype (loss of 1 log 10 of HCV-RNA after 4 weeks and negativity only after 12 weeks). We thus decided to continue the antiviral treatment at the same dosage for 36 weeks overall. At the end of treatment and after six months of follow up, HCV-RNA was undetectable (with achievement of SVR) and ALT decreased to normal values.
Monitoring was carried out for 12 months in our unit. No detectable residual viraemia was observed and ALT remained normal.
Case 2In April 2010, a 25 years-old male was admitted because of recently discovered HCV infection (the first positive test for HCV occurred in July 2009, with negative controls in previous years, associated with persistent elevation of ALT to 10 times the upper limit of normal values).
His pathological history revealed a short but steady period of drug addiction in 2005, that continued, only sporadically, also in following years. The patient confirmed his abstinence from intravenous drug abuse in the last 4 months, but he continued to take methadone for his previous drug addiction.
His history also showed that he was positive for obsessive-compulsive disorder, for which he was followed by the local mental health centre and he took specific drugs (sertraline, lamotrigine, loraze-pam). We suspected the patient was suffering from Charcot Marie Tooth disease, as it had been already genetically diagnosed in his mother and his brother and considering his clinical features.
At admission, physical examination was negative, apart from mild pain at dorsiflexion of the left foot and a minimal swelling of the ipsilateral medial malleolus. Complete blood count was normal; liver blood test showed ALT 198 IU/l and HCV RNA (Real time PCR, COBAS TaqMan Test) was 19.100 IU/ml. HCV genotype was 3a. Tests for HIV, HAV and HBsAg were negative.
With the purpose of starting treatment and considering clinical family history, the patient was first addressed to a Genetic consultancy, that confirmed the presence of CMTX1 (the X-linked dominant form of Charcot Marie Tooth type I) with homozygous genotype for the pathological mutation Gly59Arg in the gap junction protein beta 1 gene. He also underwent Neurological tests, that highlighted the presence of a sensory-motor neuropathy (atrophy of the lower limbs, hypoexcitability of tendon reflexes, vibratory and tactile hypoesthesia), confirmed by an Electromyography. A Psychiatric consultancy was required, in order to evaluate his obsessive-compulsive disorder, and it did not contraindicate antiviral therapy.
Considering his young age, the presumed recent infection, the favourable viral genotype and the low viraemia at the baseline, Peg-IFN α-2a (180 mg once weekly, subcutaneously) and RBV (1,400 mg/day, orally) combination therapy was commenced. The treatment was mildly tolerated, except for the development of an important anaemia (Hb loss > 5 g/dL and nadir value of 8.8 g/dL) and a mild worsening of the psychiatric symptoms with a consequent necessity of an admission to a protected structure, without any pharmacological measure, in absence of neurological decompensation. However it was extremely effective, as negative HCV RNA was achieved 2 weeks after the beginning of therapy.
Considering the presence of a rapid viral response (RVR), low baseline viraemia and patient’s clinical conditions, treatment was shortened to 12 weeks. A 6 months-follow up after the end of the therapy confirmed no detectable HCV RNA and normal ALT values.
DiscussionWe searched on MedLine for articles on Chronic Hepatitis C or HCV infection and rare comorbidi-ties, as EDS and CMT disease, using keywords: “HCV and Ehlers-Danlos Syndrome”, “HCV and Charcot Marie Tooth Disease” and numerous additional keywords including “hereditary genetic disorder”, “hereditary motor and sensory neuropathy”, “obsessive-compulsive disorder”, “muscular hypotonia”. All these keywords have not produced results. This testifies the unusualness of management of HCV infection associated to these rare diseases.
EDS is a rare hereditary genetic disorder affecting humans and is caused by a defect in collagen synthesis. There is no known cure and treatment is only supportive. The prevalence of EDS is estimated to be between 1/10,000 and 1/25,000, with no ethnic predisposition.4,5 EDS presents with characteristic features, including loose and unstable joints, stretchy and delicate skin, ecchymoses and haematomas after minor trauma, slow healing of wounds with the formation of abnormal scars.6,7 There are 11 subtypes of EDS, occurring to a specific molecular defect, almost all with autosomal dominant transmission. Symptoms vary widely based on which type of EDS the patient has. Our patient was suffering from a prevalent subtype III with skin and joints involvement. Mutations in either of two separate genes (COL3A1 and TNXB) may lead to this variant.8
The muscular hypotonia, with consequent functional limitations and pain, represents the biggest problem for these patients. Considering the possible toxicity of drugs currently used for HCV-treatment and the frequent occurrence of side effects,9,10 we carefully evaluated whether to treat the patient and any possible complications that may occur including worsening of the basic pathology. Only after a thorough examination and taking into account the strong motivation of the patient, we started anti-HCV treatment and we obtained a complete early virological response (cEVR: not detectable HCV-RNA after 12 weeks of therapy), without occurrence of side effects.
According to latest guidelines favourable genotypes that do not achieve RVR, seem to be considered as difficult to treat. Although there has been no scientific confirmation of this point, due to the few reported cases of such responses in genotypes 2 or 3, there is a strong recommendation to extend treatment to 36-48 weeks.1
Therefore, in view of the slow kinetics of the bio-molecular response, treatment was extended to 36 weeks, in order to increase the likelihood of maintaining a long-term virological response. This slow kinetic (considering the genotype and the low baseline viraemia) could be partially explained by limited absorption and distribution of Peg-INF-α2a administered subcutaneously, because of the severe tissue collagen changes typical of EDS. We could also consider a too low dosage of RBV (800 mg daily: about 10 mg/kg) as a cause of this slow viral response, even if also the more recent guidelines recommend a weight-based dose of RBV (15 mg/kg per day) only for HCV genotypes 1. However the patient obtained a SVR, without any adverse effect during the treatment.
CMT disease or hereditary motor and sensory neuropathy (HMSN) is a spectrum of genetical disorders that affect the peripheral nervous system. The disease is characterized by degeneration or abnormal development of peripheral nerves and exhibits a range of patterns of genetic transmission, with an estimated prevalence of 40 per 100,000.
In the majority of cases, CMT first appears in infancy, and its manifestations include clumsiness of gait, predominantly distal muscular atrophy of the limbs, and deformity of the feet in the form of foot drop. It can be classified according to the pattern of transmission (autosomal dominant, autosomal recessive, or X linked), according to electrophysiolo-gical findings (demyelinating or axonal), or according to the causative mutant gene. HMSN type 1, also known as CMT type 1 disease, is a demyelina-ting condition of peripheral nerves; on the other hand HMSN type 2 is an axonal form of CMT. Diagnosis of CMT disease is based on clinical, electro-physiological and genetical data. It is crucial to know which subtype affects each patient in order to provide optimal clinical treatment, effective diagnosis of family members, and valid prognosis.11,12 The disease follows a slow but progressive course. At present, there is no etiological treatment.
As previously stated, our patient was affected by a CMTX1 form that is caused by a mutation in the GJB 1 gene (on chromosome Xq13.1) encoding con-nexin 32, which acts as a gap junction at the level of compact myelin.13,14
Both sexes are affected by this mutation, but symptoms are more prominent in males; the patient became symptomatic in his teens suffering from gait difficulties. Characteristically, electrophysiological studies reveal nerve conduction velocities in the intermediate range. Our patient exhibited a typical CMTX1 form with deformity of the feet, partial lost of reflexes and reduction of nerve conduction velocities on EMG.
Peripheral neuropathy is a possible complication of HCV infection, for which several pathogenetic mechanisms have been proposed: a direct neurotoxic effect of the virus; vasculitis caused by cryoglobu-lins; treatment with interferon.15,16 Our patient was not screened for cryoglobulins that were expected to be negative, considering the recent infection.
Peripheral neuropathy is a rare and uncommon side effect of Peg-IFN. Several types of peripheral neuropathies may be associated to Peg-IFN, including sensory neuropathy, autonomic neuropathy, Bell’s palsy.17,18 In these cases, treatment discontinuation is suggested. By contrast, some studies have demonstrated the efficacy of Interferon in the treatment of several types of Chronic Inflammatory Demyelinating Polyradiculoneuropathy.19
We used PEG-IFN-α2a for treating our patient under neurological control during the therapy, without any worsening of this clinical condition.
According to the latest guidelines for treatment of chronic C hepatitis,1 we shortened duration of treatment (12 weeks instead of 24). In fact, our patient fulfilled all conditions for a short-therapy including low baseline viraemia, the presence of RVR, absence of any factor/morbidity known to reduce the efficacy of antiviral therapy and adequate adherence to Peg-IFN and RBV. We also considered the presence of anaemia and comorbidities (mental disorders and CMT) as factors which should be expected to worsen during continuing treatment.
The occurrence of recent infection represented another reason to reduce treatment duration, although we used combination therapy (IFN-α plus RBV at dosage of 18 mg/kg), as in chronic hepatitis it’s mandatory to do.
Antiviral therapy of chronic hepatitis C with Peg-IFNα is associated with several neuropsychiatric side effects and its employment would be contraindi-cated in those patients with severe mental illness.20
In clinical practice the use of psychiatric drugs (i.e. sertraline, lamotrigine) for management of these side effects permits symptom relief without the need for cessation of Peg-IFN, without any documented drug interaction. The worsening of our patient’s psychiatric symptoms, during anti-HCV therapy, was probable due to Peg-IFN (he had taken his psychiatric therapy for many years without side effects), and a careful monitoring of his condition was necessary to successfully complete a full course of antiviral treatment.
In conclusion, despite the small number of patients, we have evidence to support the following considerations: first, anti-HCV therapy may also be successfully undertaken (with documented SVR) in difficult clinical situations, such as the concomitant presence of rare hereditary diseases, especially if the patient is highly motivated and if we determine good prognosis; second, a multidisciplinary approach and close monitoring are indispensable to the management of critical clinical conditions, such as the treatment of hepatitis C in the presence of comorbidi-ties; third, anti-HCV therapy must be “tailored” to individual patient in terms of duration and dosage, on the basis of patient and viral characteristics (Table 1).
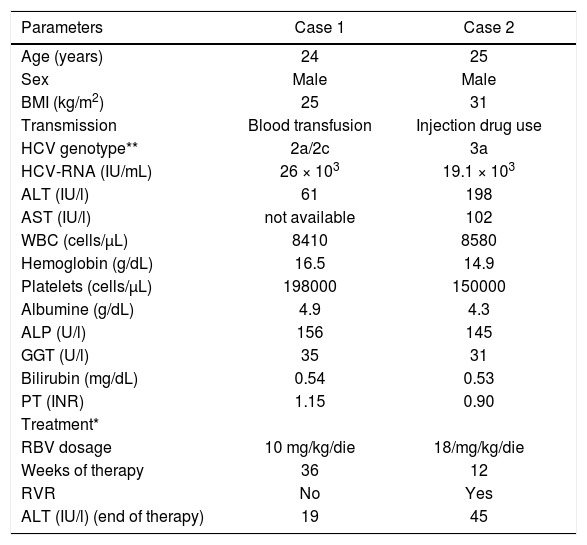
Laboratory findings of HCV patients with EDS and CMT observed at baseline, effectuated treatment and outcomes.
| Parameters | Case 1 | Case 2 |
|---|---|---|
| Age (years) | 24 | 25 |
| Sex | Male | Male |
| BMI (kg/m2) | 25 | 31 |
| Transmission | Blood transfusion | Injection drug use |
| HCV genotype** | 2a/2c | 3a |
| HCV-RNA (IU/mL) | 26 × 103 | 19.1 × 103 |
| ALT (IU/l) | 61 | 198 |
| AST (IU/l) | not available | 102 |
| WBC (cells/μL) | 8410 | 8580 |
| Hemoglobin (g/dL) | 16.5 | 14.9 |
| Platelets (cells/μL) | 198000 | 150000 |
| Albumine (g/dL) | 4.9 | 4.3 |
| ALP (U/l) | 156 | 145 |
| GGT (U/l) | 35 | 31 |
| Bilirubin (mg/dL) | 0.54 | 0.53 |
| PT (INR) | 1.15 | 0.90 |
| Treatment* | ||
| RBV dosage | 10 mg/kg/die | 18/mg/kg/die |
| Weeks of therapy | 36 | 12 |
| RVR | No | Yes |
| ALT (IU/l) (end of therapy) | 19 | 45 |
* PegIFN α-2a, at dosage of 180 μg/week was used in both patients, in combination with ribavirin. Genotyping technique: InnoLiPA-HCV genotype assay. BMI: body mass index. ALT: alanine transferasi. AST: aspartate transferasi. WBC: white blood cells. ALP: alkaline phosphatase. GGT: gamma-glutamyl transferase. PT: prothrombin time. RBV: ribavirin. RVR: rapid virologic response.
Although combination therapy with RBV and Peg-INF-α is the standard of care for patients with chronic HCV infection, careful evaluation and several considerations need to be taken into account before starting treatment in patients with rare diseases.
Abbreviations- •
HCV: hepatitis C virus.
- •
EDS: Ehlers Danlos Syndrome.
- •
CMT: Charcot Marie Tooth.
- •
Peg-IFN: pegylated interferon.
- •
RBV: ribavirin.
- •
SVR: sustained viral response.
- •
RVR: rapid viral response.
PegIFN α-2a, at dosage of 180 μg/week was used in both patients, in combination with ribavirin.
Genotyping technique: InnoLiPA-HCV genotype assay. BMI: body mass index. ALT: alanine transferasi. AST: aspartate transferasi. WBC: white blood cells. ALP: alkaline phosphatase. GGT: gamma-glutamyl transferase. PT: prothrombin time. RBV: ribavirin. RVR: rapid virologic response.