We examined how different media composition of rewarming solutions affected ammonium detoxification function, urea synthesis and the viability of hepatocytes after 72 hs of cold storage in UW solution. Freshly isolated rat hepatocytes were incubated at 37 ºC in a cell culture medium (MEM-E) with 3 mM glycine, 5 mM fructose and 2.5 mM adenosine (group 1) and in Krebs-Heinseleit buffer with 3 mM glycine, 5 mM fructose, 2 mM ornithine, 10 mM lactate and adenosine, that was used in two different concentrations: 2.5 mM (group 2) and 10 mM (group 3). We found that freshly isolated cells produced ammonium in group 1 and 2 but the cells were able to diminish ammonium extracellular concentration in group 3. Urea synthesis and ammonium extracellular concentration in group 1 was higher than in group 2. As a result of this observations, we used the Krebs-Heinseleit solution with addition of 10 mM adenosine to determinate the effect of hypothermic preservation on ammonium detoxification and urea synthesis ability of cells. In conclusion the addition of 2.5 mM adenosine into the rewarming medium interfered with the detection of ammonium detoxification of hepatic cells.
Lists abbreviations
MEM-E: enriched Minimum Essential Medium Eagle
KHR: Krebs-Henseleit rinsing
KHS: Krebs-Henseleit resuspension
HC: freshly isolated hepatocytes
HP: cold preserved hepatocytes
This study was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) Argentina, PICT 05-06434, BID 1201 OC/AR.
IntroductionThe improvement of cold preservation conditions of isolated hepatocytes has allowed to extend the horizons of application for bioartificial liver devices and hepatocellular transplantations, which are alternative therapies to the orthotopic liver transplantation. Hypothermic preservation in University of Wisconsin solution (UW) is a widespread and well-accepted method of maintaining livers and hepatocytes. 1,2 UW solution was designed to prevent cell swelling, intracellular acidosis, injury from oxygen-free radicals and ATP depletion.3 Nevertheless, when the cells are rewarmed to 37 ºC a natural situation that occurs when the organ or the cells are transplanted, they may undergo damage as the result of metabolic changes occurred during storage. Therefore, the analysis of metabolic functions of stored hepatocytes is important to evaluate its therapeutic uses. The ammonium detoxification is a specific characteristic of hepatocytes which has important influence on the prevention of hepatic encephalopathy in patients with acute or chronic liver failure.4 Our group5 and others6-8 have examined the effects of cold preservation on hepatocyte viability and function. Hepatocytes were cold stored (0°) for up 72 hs, cell viability and function was then assessed either immediately after a period of normothermic incubation in oxygenated media (37 °C, 120 minutes) or after transplantation.9 Little attention in these studies was given to the composition of the rewarming media, which were Krebs-Henseleit solution or tissue culture media as Minimum Essential Medium or Liebovitz-15.8 Moreover, in these investigations ammonium removal activity was not determined. At present, it is not knew, if the rewarming solutions have any compound could interfere with the study of this metabolic hepatocyte function. For this reason, we focus our research in the determination of the adequate rewarming media composition to asses hepatocyte ammonium detoxification and urea synthesis that can guarantee a good cell viability after a cold storage preservation period.
Material and methodsAnimalsMale Wistar rats weighing 250-300 g were used in all experiments. The rats were allowed free access to 5800 Formula Diet (LabDietâ,USA) and water ad libitum prior to the experiment and received care according to the international regulations. The National Council Committee approved the experimental protocol.
Hepatocyte isolationThe animals were anesthetized with an intraperitoneal injection of sodium thiopental (70 mg/kg, b.wt., i.p.). Hepatocytes were isolated by collagenase perfusion using the procedure described by Seglen10 and modified by us.11 Rapid cell viability was tested by the exclusion of 0.4% trypan blue (TBE) in phosphate-buffered saline. Preparations with a TBE greater than 85% were considered suitable for the experiments and this viability was not affected by the cold storage step.
Hepatocyte preservationIsolated hepatocytes were washed twice and resuspended in freshly prepared UW solution as modified by us11 at 0 °C. Modified UW solution contained 100 mM lactobionic acid, 25 mM KH2PO4, 5 mM MgSO4, 30 mM raffinose, 2.5 mM adenosine, 3 mM GSH, 1 mM allopurinol, 5% polyethylene glycol (MW: 8000), 15 mM glycine, 0.25 mg/mL streptomycin, 10 UI/mL penicillin G; pH: 7.40. The solution was bubbled with 100% N2 for 15 minutes at 0 °C before use. The hepatocyte suspensions (1.2 x 107 cells in 40 mL UW solution) were allowed to settle to the bottom of 50 ml screw cup polycarbonate tubes and were left unstirred at 0 °C for up to 72 hs.
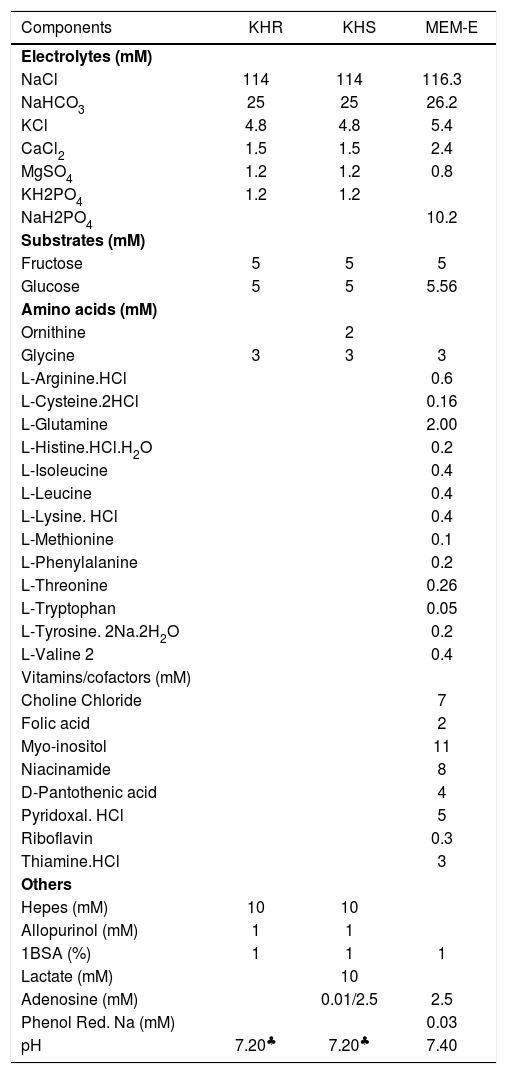
Hepatocyte rewarmingAfter 72 hs of cold storage, the hepatocytes were washed twice with Krebs-Henseleit rinsing solution (KHR) or enriched Minimum Essential Medium Eagle (Sigma-Aldrich Chem Co, cat # M4767) (MEM-E): and sedimented (50 g, 3 min). The MEM-E medium was used to rinse the cells to be rewarmed in MEM-E medium. We used two types of Krebs-Henseleit media, one in the rinsing step (KHR),5 and the other (KHS) in the rewarming step. The compositions of KHR, KHS and MEM-E are shown in (Table I). Hepatocytes (2-3 X 106 cells/mL) were then incubated for 120 min at 37 °C in MEM-E or KHS medium containing 10 mM or 2.5 mM adenosine and 0.2 mM ammonium chloride, under carbogen atmosphere in a Dubnoff metabolic shaker.
Composition of media for rinsing and normothermic incubation of hepatocytes.
| Components | KHR | KHS | MEM-E |
|---|---|---|---|
| Electrolytes (mM) | |||
| NaCl | 114 | 114 | 116.3 |
| NaHCO3 | 25 | 25 | 26.2 |
| KCl | 4.8 | 4.8 | 5.4 |
| CaCl2 | 1.5 | 1.5 | 2.4 |
| MgSO4 | 1.2 | 1.2 | 0.8 |
| KH2PO4 | 1.2 | 1.2 | |
| NaH2PO4 | 10.2 | ||
| Substrates (mM) | |||
| Fructose | 5 | 5 | 5 |
| Glucose | 5 | 5 | 5.56 |
| Amino acids (mM) | |||
| Ornithine | 2 | ||
| Glycine | 3 | 3 | 3 |
| L-Arginine.HCl | 0.6 | ||
| L-Cysteine.2HCl | 0.16 | ||
| L-Glutamine | 2.00 | ||
| L-Histine.HCl.H2O | 0.2 | ||
| L-Isoleucine | 0.4 | ||
| L-Leucine | 0.4 | ||
| L-Lysine. HCl | 0.4 | ||
| L-Methionine | 0.1 | ||
| L-Phenylalanine | 0.2 | ||
| L-Threonine | 0.26 | ||
| L-Tryptophan | 0.05 | ||
| L-Tyrosine. 2Na.2H2O | 0.2 | ||
| L-Valine 2 | 0.4 | ||
| Vitamins/cofactors (mM) | |||
| Choline Chloride | 7 | ||
| Folic acid | 2 | ||
| Myo-inositol | 11 | ||
| Niacinamide | 8 | ||
| D-Pantothenic acid | 4 | ||
| Pyridoxal. HCl | 5 | ||
| Riboflavin | 0.3 | ||
| Thiamine.HCl | 3 | ||
| Others | |||
| Hepes (mM) | 10 | 10 | |
| Allopurinol (mM) | 1 | 1 | |
| 1BSA (%) | 1 | 1 | 1 |
| Lactate (mM) | 10 | ||
| Adenosine (mM) | 0.01/2.5 | 2.5 | |
| Phenol Red. Na (mM) | 0.03 | ||
| pH | 7.20♣ | 7.20♣ | 7.40 |
1 Bovine Serum Albumin (ICN 810035)
KHR: Krebs-Henseleit rinsing
KHS: Krebs-Henseleit resuspension
KHS:MEM-E: Enriched Minimum Essential Medium Eagle
The activity of LDH in cell suspension (total activity) and in the supernatant (extracellular LDH) was determined as described previously.12 Results were expressed as the percentage of total enzyme activity in the extracellular medium.
Determination of extracellular ammonium concentrationAliquots of the hepatocytes were removed at different times and centrifuged (12000 g – 15 seconds). The supernatants were keep at -10 °C until the enzymatic determination. Cellular pellets were deproteinized by addition of 300 mL of cold 3% HClO4. After centrifugation, the protein-free supernatant was neutralised with NaHCO3 and assayed. Ammonium was determined enzymatically according to the method by van Anken et al.13 The assay mixture (0.8 mL) contained 66.7 mM phosphate buffer pH: 8.30, 0.14 mM NADPH, 6.5 mM sodium a-ketoglutarate, 2.5 mM ADP, 120 UI/mL glutamate dehydrogenase.
Urea measurementUrea was spectrophotometrically determined in the supernatants with diacetyl monoxime and thiosemicarbazide in the presence of sulphuric acid, phosphoric acid and ferric chloride as previously described.14
Statistical analysisResults are presented as the mean ± SD and the number of preparations analyzed are indicated in each figure. Statistical significance of the differences between values was assessed by Student’s t test. A p value less than 0.05 was considered statistically significant (Statgraphics, Statistical Graphics System, USA).
ResultsCell viability during cold storage in UW solution.Viability of suspensions of hepatocytes stored in modified UW solutions was assessed by LDH release from the cells into the cold storage solution. At the start of the experiments, the LDH release was 0.65 ± 0.2 % of total LDH. Hepatocyte viability was well maintained during cold storage of cells suspensions for up 72 hs. The final LDH released was 3.65 ± 0.45 %; n = 3.
On the basis of LDH release from the cells, we consider that 72 hs of cold storage is the limit in cold preservation-time, values around 3.50 ± 0.51% correlates with good morphology and rewarming performance. LDH release after 96 hs of cold storage was 6.90 ± 0.62 %; n = 3 and the cells show some tendency to form blebs.11
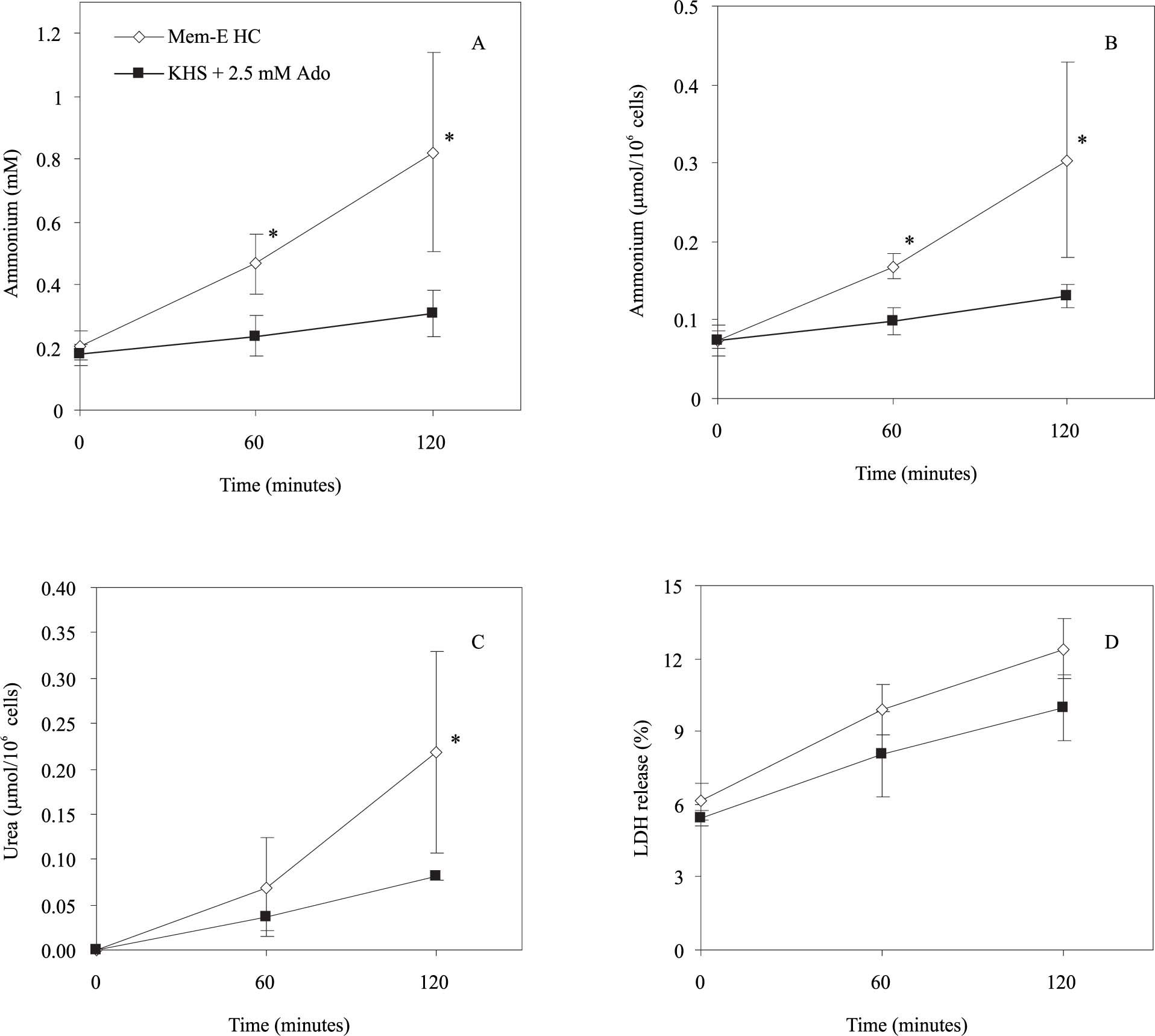
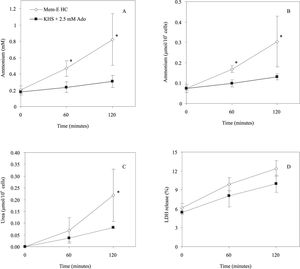
Effect of rewarming media composition on the ammonium detoxification and urea synthesis ability of freshly isolated hepatocytes.In a first set of experiments, ammonium chloride (0.2 mM) is added in both suspension media (KHS and MEM-E) to measure the capacity of cell to eliminate ammonium and convert it to urea. As shown in figures 1A and 1B, there was an increase in the ammonium extracellular concentration or extracellular ammonium content normalized to 106 cells during the normothermic incubation of freshly isolated hepatocytes in MEM-E (t120: 0.820 ± 0.316 mM; 0.300 ± 0.124 mmol/106 cells; n = 3). The same result is shown in KHS + 2.5 mM adenosine (t120: 0.311 ± 0.074 mM; 0.130 ± 0.015 mmol/106 cells, n = 3) but the ammonium extracellular level at 120 min in MEM-E is greater than in KHS. Figure 1C shows the urea synthesis of freshly isolated hepatocytes. A higher production of urea was observed in MEM-E (t120: 0.219 ± 0.112 mmol/106 cells; n = 3) than in KHS + 2.5 mM adenosine (0.082 ± 0.005 mmol/106 cells, n = 3). As shown in figure 1D there were not statistical differences in LDH released in both conditions (t120: 12.57 ± 1.31% MEM-E; 9.71 ± 1.21% KHS + 2.5 mM adenosine). Therefore, this parameter was not dependent on the composition of the rewarming media.
Effect of rewarming medium composition on metabolic functions and cell viability. A: the time course of extracellular ammonium concentration (mM); B: extracellular ammonium content normalized by 106 cells (mmol/106 cells); C: synthesis of urea (mmol/106 cells); D: LDH release (%) from freshly isolated hepatocytes incubated in MEM-E and in KHS + 2.5 mM ado (adenosine) at 37 ºC for 120 minutes. Results are expressed as the mean ± SD of n = 3 separate cell preparations. The symbol (*) indicate that the analysed parameter in MEM-E is statistically different than in KHS + 2.5 mM ado at P < 0.05.
We were able to measure ammonium detoxification of freshly isolated hepatocytes when we decreased the adenosine concentration from 2.5 mM to 10 mM in KHS in presence of 0.2 mM ammonium chloride.
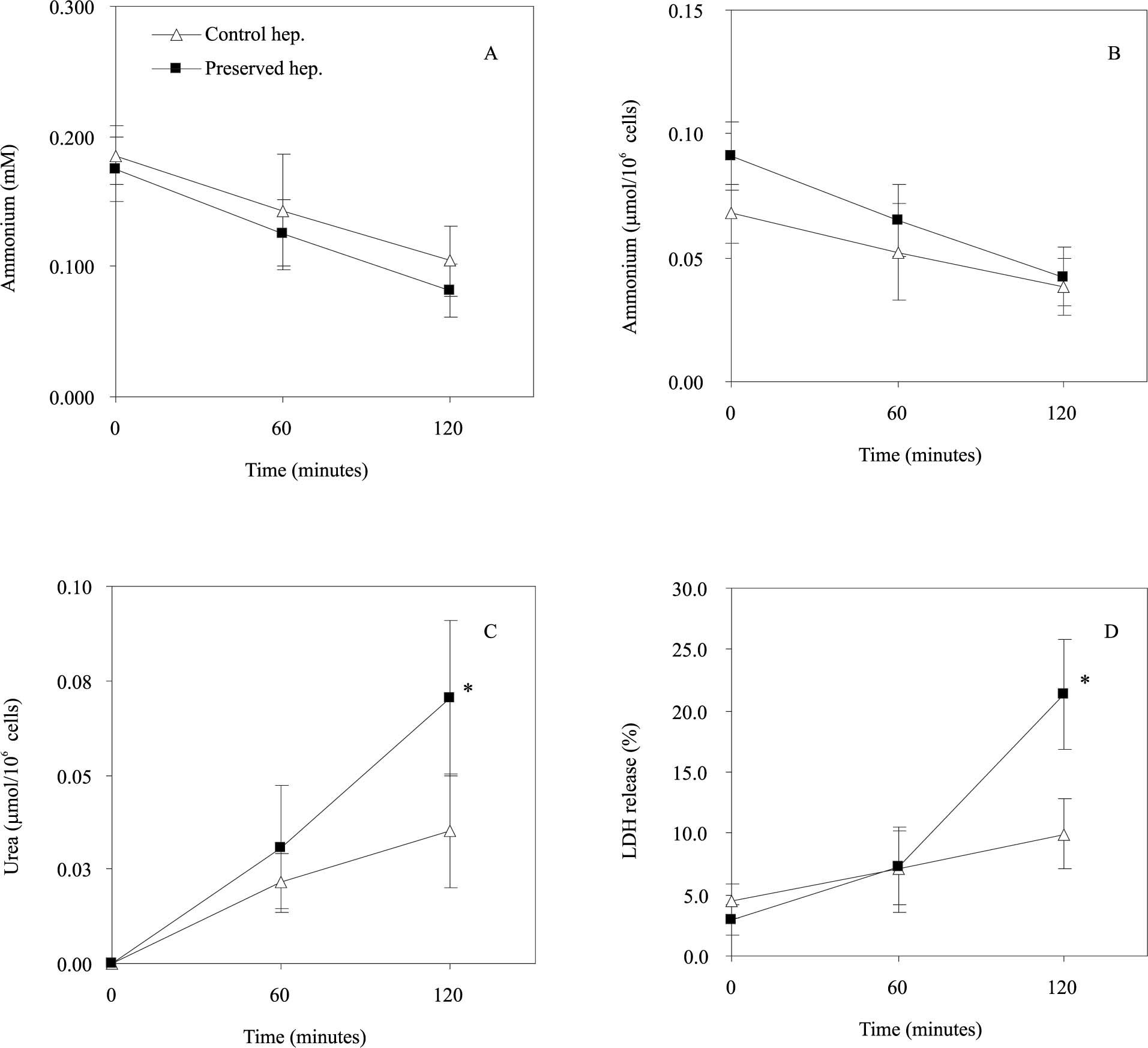
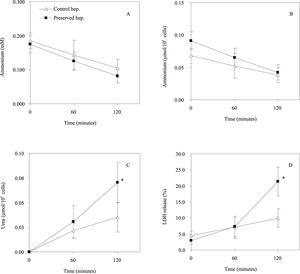
Figures 2Aand2B show the evolution of extracellular ammonium concentration (t120, HC: 0.104 ± 0.027 mM; HP: 0.081 ± 0.020 mM; n = 3) and the extracellular ammonium content normalized to 106 cells (t120, HC: 0.038 ± 0.011 mmol/106 cells; HP: 0.042 ± 0.012 mmol/106 cells; n = 3) respectively during 120 min of rewarming. The ammonium removal rate of preserved hepatocytes was comparable to control cells, both after 60 and 120 min of rewarming. In contrast, the preserved cells shown a higher LDH release (see Figure 2C), particularly after 120 min (t120, HC: 9.93 ± 2.89%; HP: 21.40 ± 4.47%; n = 3). Figure 2D shows that urea synthesis from cold preserved cells was significantly higher than freshly isolated hepatocytes (t120, HC: 0.035 ± 0.015 mmol/106 cells vs HP: 0.070 ± 0.021 mmol/106 cells; n = 3).
Effect of cold preservation on the ammonium detoxification and urea synthesis ability of rat hepatocytes. during rewarming. A: The evolution of extracellular ammonium concentration (mM); B: extracellular ammonium content normalized by 106 cells (µmol/106 cells); C: urea synthesis (µmol/106 cells); D: LDH release (%). Freshly isolated hepatocytes and preserved cells were incubated at 37 ºC up to 120 minutes in KHS + 10 mM ado (adenosine). Data represents the mean (SD for three different hepatocytes preparations. The symbol (*) indicate that the analysed parameter in preserved cells is statistically different from freshly isolated hepatocytes at P < 0.05.
To elucidate the effect of adenosine in the detection of ammonium detoxification, we hypothesised that this nucleoside was converted to inosine and ammonium by the cytosolic enzyme adenosine deaminase (EC 3.5.4.4). In this way, we suggest that the adenosine deaminase is released into resuspension media and it degrades the extracellular adenosine to produce ammonium. This hypothesis is supported by the fact that during normothermic incubation -120 minutes-the membrane integrity of cells was changed (HC: 5.4% and HP: 20.0%), demonstrated by the LDH release (t0, HC: 4.50 ± 1.35%; HP: 2.93 ± 1.20% vs t120, HC: 9.93 ± 2.89%; HP: 21.40 ± 4.47%).
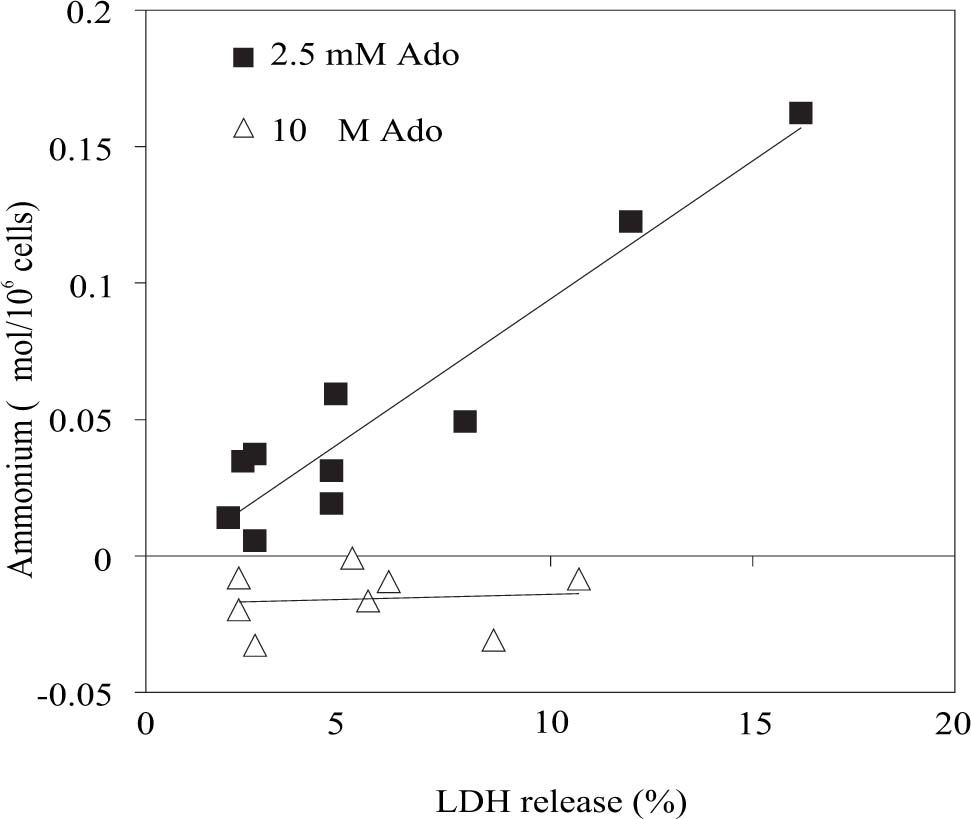
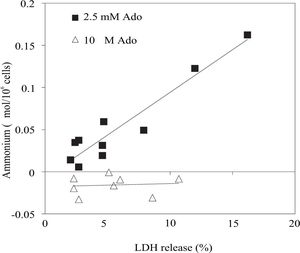
With regard to the hypothesis above stated, we used the data of figures 1B,1D,2Band2D to create a new graph shown in figure 3. The y axis represents the difference between final (60 and 120 min) and initial extracellular ammonium content (mmol/106 cells) and the x axis represents the difference between final and initial LDH release (%).
Correlation of net ammonium production represented as Δ Ammonium (mmol/106 cells) and net LDH released represented as Δ LDH release (%) during a definite period of time (60 and 120 min) with respect the beginning of the experiment. The data of figure 1B, 1D, 2Band2D were used to built this graph. To see details of these experiments look at legends of figures 1 and 2.
The linear relationship between Δ Ammonium (|mmol/ 106 cells) and Δ LDH release (%) is shown in figure 3. The correlation coefficient was 0.8988 in the presence of 2.5 mM adenosine and it was 0.0009 when adenosine concentration is reduced.
DiscussionWe started our research with MEM-E because its complex composition allowed us to maintain cell viability during the normothermic incubation. However, we included 2.5 mM adenosine, 3 mM glycine and 5 mM fructose to enhance its quality. Adenosine was added to increase the intracellular level of ATP.3 This effect was demonstrated in metabolic studies using isolated rat hepatocytes.15 Moreover, the University of Carolina solution, which is used to protect rat isolated hepatocytes in suspension at 37 °C, contains adenosine as a precursor of ATP.6 Fructose was added because it is an alternative carbohydrate source for glycolysis and induces hepatocytes protection when ATP synthesis is not enough.16 Glycine was added as a cytoprotector during hepatocellular injury from ATP depletion.17
All media that were used in this study had got 0.2 mM ammonium due to the fact that it is approximately the plasma ammonium concentration in patients with liver failure.18 This initial ammonium concentration did not affect the cell viability measure by LDH release and ammonium detoxification ability.19
We could not observe ammonium detoxification when hepatocytes were incubated normothermically in MEM-E (see Figures 1Aand1B). Therefore, we used a more simple resuspension medium like Krebs-Heinseleit buffer, but maintaining the three compounds mentioned before. Also, we included ornithine and lactate to stimulate urea cycle.20 In spite of the changes in media composition we were not able to detect ammonium detoxification again (see Figures 1Aand1B).
During the rewarming, the ammonium extracellular concentration increased in both media, but it was larger in MEM-E. This difference could be provided from a higher amino acid deamination in the culture media, (MEM-E containing ornithine and glutamine). Even, this higher ammonium production in MEM-E could be the cause for the increase of urea synthesis with respect to KHS + 2.5 mM adenosine (see Figure 1C). In contrast, the cells viability assessed with LDH release test is similar in both conditions (see Figure 1D). Thus we continued our rewarming solution studies with the KHS medium.
In KHS media, the only substances whose degradation could generate ammonium are: ornithine and adenosine. We decided to decrease adenosine concentration from 2.5 mM to 10 mM with the aim of promoting urea synthesis.21 We found that this was a good condition to evaluate ammonium detoxification and urea synthesis of cold preserved hepatocytes in UW solution during 72 hs (see Figures 2Aand2B).
As shown in Figures 2Aand2B the hypothermic preservation did not alter ammonium detoxification and urea synthesis functions of cold stored hepatocytes. However, there was a significant increase on membrane permeability of preserved hepatocytes after 120 min of rewarming (see Figure 2D) probably due to the hypoxia suffered during the cold storage.22 Therefore injury to hepatocytes after cold storage occurred during the rewarming and was characterized by increase in the permeability of the plasma membrane to LDH, potassium and succinate.8 Another conclusion from this study is that the cold preservation period sensitises hepatocytes to reoxygenation injury and that injury does not affect the cell ability to detoxify ammonium.
In our study, like other authors,23 we saw an increase of urea synthesis after cold preservation (see Figure 2C). The stimulation of urea synthesis may be caused by adenosine or its breakdown product, inosine.21 During hypothermic preservation, hepatocytes were stored in a solution containing adenosine, this nucleoside remaining in the cells on resuspension may be have activated ureagenesis.
To understand the mechanism of adenosine interference on ammonium removal activity, we built a graph (see Figure 3) that shows a correlation of net ammonium production and net LDH release at 60 and 120 min of rewarming. We suggest that adenosine is degraded to inosine and ammonium by the enzyme adenosine deaminase which is released during normothermic incubation. Also, we can not rule out the probability that, during rewarming, adenosine could enter the cells for a specific nucleoside transports24 and its conversion may be done in the cells. In fact, we think about the possibility that both phenomena take place during the rewarming.
However, we don’t know whether KHS is the best rewarming solution to examine hepatocyte metabolic functions, but we are sure that this medium is appropriate to garantee a good cell viability and to maintain specific cell functions. At the present, we work in a better rewarming solution to improve hepatocytes performance by adding others compounds like carnitine, which has been studied in the prevention of hepatic encephalopathy in hyperammonemic conditions.25,26
In conclusion the composition of the rewarming medium is an important factor in assessing the quality of preservation of hepatocytes and the metabolic cells analysis to improve cellular applications in bioartificial liver or hepatocyte transplantation.
AcknowledgementThe authors are very grateful to Wiener Lab Group Argentina for the kind supply of glutamate dehydrogenase.