Introduction. Bacterial infection in cirrhotic patients is a severe complication that requires early recognition and specific therapeutic care.
Material and methods. In this review the various aspects of diagnosis and management of infections that may impact survival in cirrhosis are analyzed.
Results. Active search for infections allows early detection and its treatment with suitable antibiotics has reduced mortality rates in spontaneous bacterial peritonitis, the main infection in patients with decompensated cirrhosis. Other common infections, such as bacteremia and septicemia or urinary tract, lung, skin and soft tissue infections must be thoroughly investigated so that antibiotic treatment can be started early. As intestinal bacterial translocation is one of the most important mechanisms for development of bacterial infections, selective intestinal decontamination is able to prevent these infections in populations at risk. After the first episode of spontaneous bacterial peritonitis, poorly absorbed oral antibiotics, such as quinolones, must be started and continued. Moreover, when there is upper gastrointestinal bleeding, infection prevention should be based on oral administration of quinolones or intravenous administration of cephalosporins, both for seven days, to avoid morbidity and early lethality. With the advent of resistance to commonly used antibiotics and recent reports of multiresistant bacteria, there is a need for stricter control when administering antibiotics to cirrhotic patients.
Conclusion. Existing knowledge of therapy and prophylaxis for bacterial infections in cirrhotic patients, which undoubtedly improve survival, should be disseminated and applied in clinical practice for the benefit of the population at large.
Advances in the diagnosis and treatment of chronic liver diseases have significantly changed the natural history of cirrhosis. Although it is a morphological term that describes architectural disrupture of the liver, pathologists nowadays prefer to avoid this term emphasizing etiology of disease, grade of activity and features suggestive of progression or regression.1,2 Clinicians, on the other hand, consider cirrhosis as a dynamic and potentially reversible disease, clearly separated in two stages: compensated and decompensated.3 The compensated phase, divided in two stages without or with gatroesophageal varices, has a good prognosis with a survival probability of more than 10 years. Decompensation, related to portal hypertension, mainly upper gastrointestinal bleeding and ascites or the association of both, result in much lower (around two years) survival probabilities.4
For patients with decompensated cirrhosis the impact of survival is related to the control of the factors leading to the decompensation. It is also known that the different complications of cirrhosis interact. An example is gastrointestinal bleeding, which can trigger the onset of ascites or the development of hepatic encephalopathy. Furthermore, severe complications with an increased risk of short survival, such as hepatorenal syndrome or marked hyponatremia, develop in patients with difficult-to-treat ascites. The onset of bacterial infections in cirrhotic patients with any of these complications significantly increases the risk of lethality, as has been demonstrated in various studies.5–7
The Prevalence and Significance of Infections in CirrhosisThe global prevalence of bacterial infections in hospitalized cirrhotic patients varies from 33 to 47%.8,9 These rates are very high compared with the overall prevalence of < 10% for infections in patients hospitalized due to other conditions. Moreover, the lethality rates in patients with infections are significantly higher than those observed in hospitalized cirrhotic patients with severe liver decompensation who do not develop infections.7 Bacterial infections are currently considered the leading cause of death in decompensated cirrhosis.10
The presence of infections in cirrhotic patients is a factor that triggers other complications, such as hepatic encephalopathy, the persistence of gastrointestinal bleeding and the development of hepatorenal syndrome. However, as cirrhotic patients are frequently in an immunocompromised state, clinical and/or laboratory data that can facilitate the diagnosis of infections is not always available. Hyperthermia or leukocytoses with a left shift occur in less than half of patients with any type of bacterial infection. Even so, early diagnosis and rapid therapeutic intervention are fundamental in preventing morbidity and lethality. It is mandatory to search for infections in any patient that presents with ascites, episodes of upper gastrointestinal bleeding, hepatic encephalopathy or clinically suspected sepsis or septic shock.11
According to a recent investigation of the CLIF consortium, the CANONIC study, bacterial infection is the most common precipitating event of acute-onchronic liver failure (ACLF), corresponding to 33%. ACLF is more frequent in patients with spontaneous bacterial peritonitis or pneumonia than in those with infections in other sites. Risk of death varied from 22 to 77% according to the grade of ACLF. Patients with one organ failure are in grade 1 (with three sub-groups), patients in grade 2 have two organ failures and grade 3 patients have three or more organ feilures.12
The most common bacterial infection in cirrhosis are spontaneous bacterial peritonitis (SBP) and urinary tract infections, followed by respiratory tract infections and soft tissue infections, as well as bacteremias and septicemias.13 Early detection and treatment with suitable antibiotics have reduced mortality rates in SBP from 80 to 20% in the last three decades.14 However, mortality rates for respiratory tract infections (around 50%) and septicemias (70%) remain extremely high.7
While community-acquired infections are easier to control, nosocomial infections have much higher risks of morbidity and lethality. SBP, besides being the most frequent infection in cirrhosis, is exclusive to this disease and, like urinary tract infection, can occur repeatedly. Among community-acquired infections, the most common are those due to Gramnegative bacilli (GNB), especially Escherichia coli, while Gram-positive bacilli (GPB), such as streptococci and staphylococci, predominate in pneumonias and bacteremias associated with procedures.13–15 Those health-care infections are more prevalent than community-acquired or nosocomial infection. This prospective cohort study has shown that potentially preventable second infections are predictors of mortality independent of liver disease severity.16
The Pathogenesis of Bacterial InfectionsThe intrinsic mechanisms that make cirrhotic patients more likely to develop bacterial infections have not yet been fully elucidated. The most widely accepted hypothesis is that cirrhosis impairs the body’s immune defenses and is thus a disease that leads to immunodeficiency. Various factors have been proposed to explain the immunological alterations observed. Portal hypertension, by producing portal-systemic anastomoses, diverts blood that would go to the liver, thus preventing detoxification. In cirrhosis there is also a qualitative dysfunction of the reticuloendothelial system, with monocyte dysfunction and a reduction in complement levels in both the blood and AF. Neutrophil phagocytosis is also adversely affected, particularly in alcoholic cirrhosis.17,18
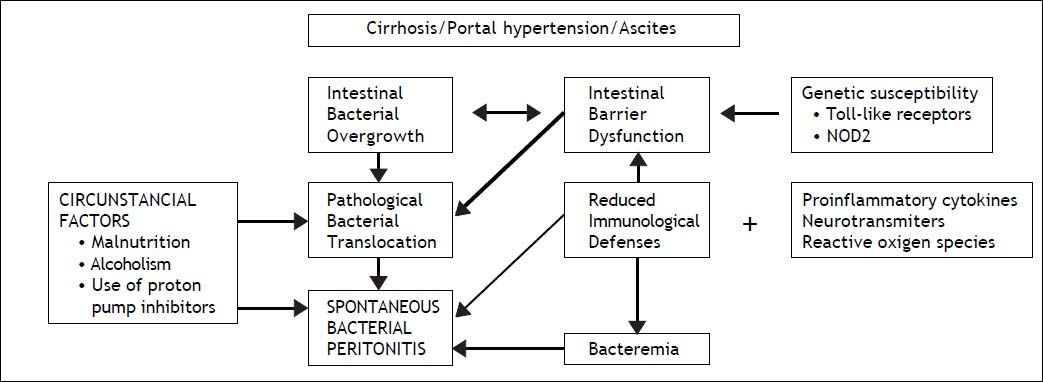
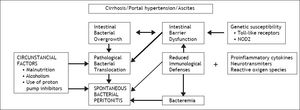
The human gut harbors ten times more microbial cells than eukaryotic cells of the host and bacterial translocation (BT) from the intestinal lumen to regional lymph nodes is a physiological process. Pathological BT that occurs in cirrhosis is related not only to bacterial overgrowth but also to intestinal barrier dysfunction, qualitative parameters of microbiota and immune dysfunction, with no clear evidence of superiority of one factor over the others. Indeed, recent studies have demonstrated in cirrhotic patients with sepsis that BT was not caused by an abnormal small bowel gut microbiota.19 Portal hypertension affects the integrity of intestinal mucosal barrier through induction of mucosal edema and also affects intestinal transit time, leading to luminal bacterial overgrowth. Marked alterations of cirrhosis can accelerate pathological BT mainly due to pro-inflammatory cytokines, as tumor-necrosis factor, neurotransmitters, as norepinephrine and reactive oxygen species.20 Systemic cytokinemia might affect the structural and functional integrity of intestinal tight junctions, increasing paracellular permeability. The state of immunodeficiency found in decompensated cirrhosis induces a persistent activation of immune system cells with production of proinflammatory cytokines. More recently it has been shown that activated intestinal macrophages in patients with cirrhosis release IL6 and NO that may disrupt intestinal barrier function, enhancing permeability to bacterial products.21 Other factors as susceptibility genes may also be envolved. In fact, recent studies indicate that gene variants, as Tolllike receptors and NOD2 (nucleotide-binding oligomerization domain 2) linked to impaired mucosal barrier function represent gentic risk factor for SBP and other infections in cirrhosis.22,23 Although the presence of microorganisms transported by bacterial translocation can be solved by opsonization of bacteria in the AF or by other forms of phagocytosis, when these defense mechanisms are exhausted the infectious process develops.24 Thus, viable and nonviable bacteria and bacterial products such as endotoxins/lipopolysaccharides and bacterial DNA that have crossed the intestinal barrier are found in mesenteric lymph nodes. These are what are known as the intrinsic factors, which are associated with cirrhosis itself.
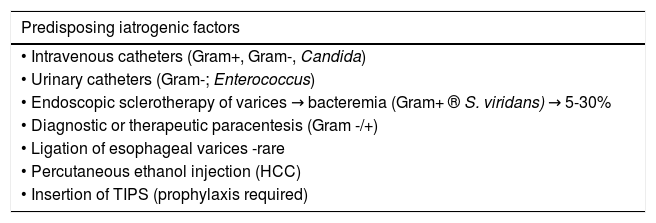
The circumstantial factors that facilitate the development of bacterial infections in cirrhotic patients, especially those with some type of decompensation, include malnutrition, which is very common in cirrhosis of any etiology, chronic alcoholism and more recently the use of acid suppressive therapy has benn associated with development of SBP and other infections. Proton pump inhibitors, widely used in cirrhosis, facilitates enteric bacterial overgrowth and translocation, as demonstrated in clinical studies25 (Figure 1). Medical procedures with iatrogenic potential, such as those involving catheters or probes, as well as other invasive procedures13 are the main iatrogenic factors that can trigger bacterial infections (Table 1).
Infections in liver cirrhosis.
| Predisposing iatrogenic factors |
|---|
| • Intravenous catheters (Gram+, Gram-, Candida) |
| • Urinary catheters (Gram-; Enterococcus) |
| • Endoscopic sclerotherapy of varices → bacteremia (Gram+ ® S. viridans) → 5-30% |
| • Diagnostic or therapeutic paracentesis (Gram -/+) |
| • Ligation of esophageal varices -rare |
| • Percutaneous ethanol injection (HCC) |
| • Insertion of TIPS (prophylaxis required) |
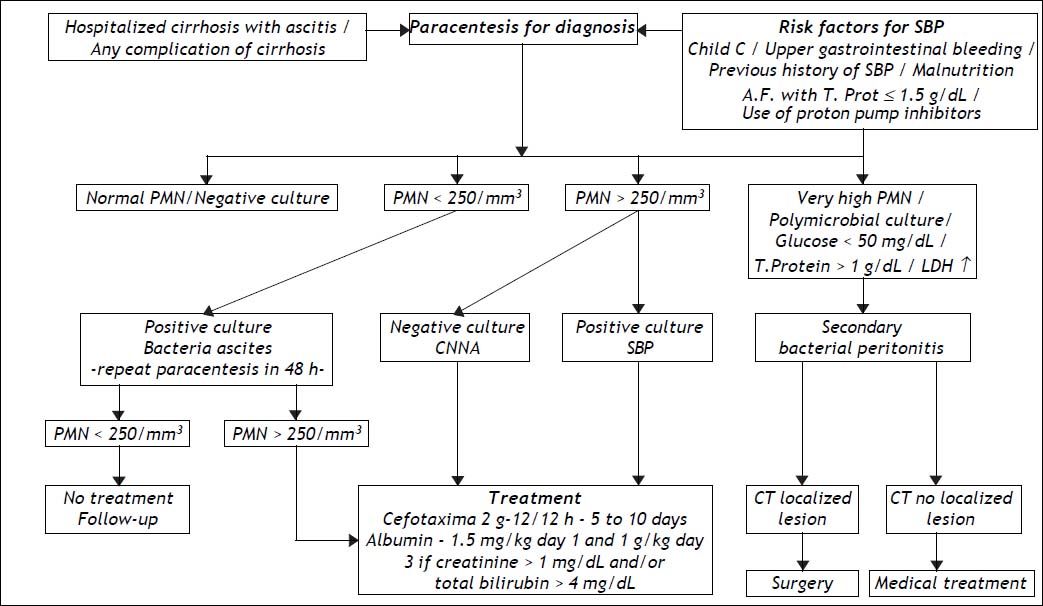
SBP is the most common, severe infection that develops in cirrhotic patients with ascites. SBP is defined as infection of the AF in the absence of an intra-abdominal focus of infection. During a oneyear follow-up, 10% of cirrhotic patients with ascites are likely to develop SBP. Furthermore, after a first episode, SBP can recur frequently if infection prophylaxis is not administered.15,26
DiagnosisThe signs and symptoms of SBP are sometimes unapparent, making it necessary to always bear in mind that this type of infection may be present so that an early diagnosis can be made. Late diagnosis significantly worsens the patient’s prognosis as it delays the start of treatment. In fact, around 10 to 30% of cases of SBP are asymptomatic. It is for this reason that paracentesis of AF is performed in all hospitalized cirrhotic patients with ascites.9 In the community, cirrhotic patients with ascites but without the signs and symptoms of any complications do not need to have this test. The commonest clinical manifestations of SBP include enlargement of ascitis, failure of diuretic treatment, the onset of hepatic encephalopathy or, less frequently, abnormal laboratory findings indicating leukocytosis, metabolic acidosis or kidney dysfunction. Minor increases in temperature (37.8 °C) or abdominal pain, with or without a positive rebound test, may be present. When paralytic ileus, arterial hypotension or hypothermia are present, the infection is at a severe stage and the prognosis is poor.27
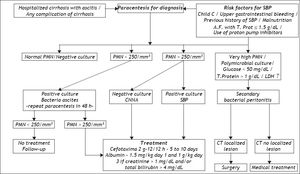
SBP is diagnosed by analyzing AF collected by paracentesis. The mandatory test is a polymorphonuclear(PMN) count, for which values > 250 cell/mm3 indicate a positive diagnosis. If SBP is suspected the flowchart in figure 2 is useful to confirm the diagnosis and criteria for indications of therapy. AF culture is usually positive in 35 to 65% of patients. It should always be collected and put directly into blood culture flasks at the bedside, increasing positivity to 70 or 90%.28 It can be useful to collect peripheral blood for blood cultures at the same time, as the same microorganism is frequently found in AF and peripheral blood. Although various studies involving other laboratory tests have been carried out, these tests do not contribute to a diagnosis of SBP. Hence, the usefulness of measuring AF pH and lactate levels, as well as, more recently, AF lactoferrin in the diagnosis of SBP has not been confirmed.29–31
Procalcitonin (PCT) and C reactive protein (CRP) are two acute-phase serum proteins commonly used as early markers of infection in general population. But as both are produced by hepatocytes, patients with cirrhosis may present reduced levels32 Nevertheless, the predictive power of CRP and PCT for detecting infection has been similar in patients with and without cirrhosis33 Besides that, as a surrogate marker of systemic inflammatory response syndrome (SIRS) CRP was recently shown to predict short-term mortality in patients with cirrhosis, most of them with bacterial infections.34
Various studies suggest the use of urine dipsticks, which indicate leukocyte esterase activity of activated granulocytes by an immediate color change on the strip. Their use to determine the increase in neutrophils in AF has been studied and has proved to have good accuracy. There are various controlled studies showing a sensitivity of around 85%, but these findings have yet to be confirmed.35 Because they are fast and low-cost, dipsticks can be used when the therapeutic decision is made, but neutrophil count in the same fluid continues to be the gold standard for therapy. A new strip calibrated specifically for ascitic fluid was recently tested in 1,304 experiments with a median PMN count of 492 cell/mm3 showing good sensibility and negative predictive value (100%) but specificity of 58% and positive predictive value of 76%.36
With regard of the identification of the bacterial pathogen, real-time PCR assays may be of potential utility, but compared to standard culture techniques the information is not entirely interchangeable.37 More recently, the application of a direct susceptibility testing based on a Matrix Assisted Laser Desorption Ionization-Time of Flight (MALDI-TOF) from positive blood cultures has been proposed for early detection of resistant bacteria and their antibiotic susceptibility.38
It is worth remembering that there are two variants of SBP: culture-negative neutrocytic ascites (CNNA) and bacterascites (BA). In the former, the PMN count is > 250 cell/mm3 but the AF culture is negative. This group of patients presents with the same signs and symptoms and has the same prognosis as those with a positive culture. Other clinical causes of increased AF PMN count, although less frequently associated with ascites and cirrhosis, need to be evaluated, namely, peritoneal carcinomatosis, peritoneal tuberculosis, hemorrhagic ascites and pancreatitis.39,40
In BA, conversely, bacterial growth is observed in AF culture, but PMN counts are below 250 cell/mm3. This group is heterogeneous and may not be infected. If left untreated, spontaneous remission occurs in 60% of cases, particularly asymptomatic ones. If there are symptoms or signs that are compatible with SBP, the patient must be treated, even if there is no increase in PMN count in AF. As BA is diagnosed about two days after paracentesis because of the waiting time for culture results, asymptomatic patients should undergo another paracentesis. If there is an elevated neutrophil count, treatment should be administered. When PMN count remains bellow 250 cell/mm3, frequently the culture is no longer positive and no treatment should be given.41
When infection of AF in cirrhotic patients is secondary to intestinal perforation or abscesses in abdominal organs, the process is known as secondary bacterial peritonitis (2BP), known to correspond to less than 10% of the cases of peritonitis with ascites. The clinical features of secondary bacterial peritonitis may be similar to those of SBP, but local inflammatory response is more severe and mortality is higher.42 While the latter is treatable only with antibiotics, 2BP usually requires surgical intervention (Figure 2). An important factor in the differential diagnosis is that 2BP is usually polymicrobial whereas only one microorganism is isolated in SBP.43 Laboratory findings, such as AF low glucose levels, high LDH and total protein levels are known as the Runyon’s criteria for diagnosis of 2BP44 Elevated alkaline phosphatase or carcinoembryonic antigen levels in the AF analysis, have also been used in this differential diagnosis with varying degrees of reliability.44,45 In cases where 2BP is suspected, antibiotic therapy must also cover anaerobic microorganisms and enterococci. Due to the high mortality rate in less decompensated cirrhosis a more aggressive approach including imaging tests as abdominal computed tomography must be carried out for early surgical approach that can improve prognosis.42
TherapyTreatment of SBP is empirical and should be started immediately following diagnosis. If required, treatment can be modified when the results of the culture and the antibiogram are available. As around 70% of cases of SBP are caused by Gram negative bacteria (GNB) and a minority by gram positive bacteria (GPB), third-generation cephalosporins, which cover around 95% of the germs involved in this condition, are considered the treatment of choice46 (Figure 2). This form of therapy is effective in 77 to 98% of cases described. Various studies investigating dosage and treatment duration have shown that 2 g of cefotaxime every 12 h is as effective as the initially proposed 8/8 h or the administration every 6 h and that 5 days of treatment is similar to treatment for 10 days.47,48 Other cephalosporins such as ceftriaxone and ceftazidine, as well as amoxicillin and clavulanic acid, are effective.49 The quinolones have also been tested, and both ciprofloxacin and ofloxacin yielded good results.50 In uncomplicated SBP, i.e., SBP without gastrointestinal bleeding, hepatic encephalopathy, ileus or renal failure, oral quinolone treatment (ofloxacin) can be considered.51 However, aminoglycosides should be avoided as they are less effective in treating SBP and have high rates of nephrotoxicity.52
The widespread use of quinolones, including for SBP prophylaxis, has led to an increase in quinoloneresistant infections. As greater control was gained over Gram-negative germs, the frequency of SBP due to Gram-positive germs increased. Nevertheless, cephalosporins are still effective in the treatment of infectious episodes.13
Antibiotic resistance has become a problem with the decrease of therapeutic options and poor clinical outcomes.53 Multi-drug resistance (MDR) has developed, including an increased prevalence of methicillin resistant Staphylococcus aureus (MRSA), MDR enterococci, extended spectrum beta-lactamase (ESBL) producing Echerichia coli and Klebsiella penumoniae.54
A recent study found a significant percentage of multiresistant bacteria in cirrhotic patients and proposed the use of broad-spectrum antibiotics such as carbapenems and glycopeptides when treating nosocomial SBP. In this study of 92 episodes of infections, 18% were caused by multi-resistant germs.55 Risk factors for this resistance include having used antibiotics in the previous three months, the presence of diabetes and nosocomial SBP.56
Making an early diagnosis and starting appropriate treatment as soon as possible is not sufficient; in addition, close attention must be paid to the patient’s condition in order to avoid other complications, which could worsen the prognosis. Ascites is the risk factor for the development of bacterial infection, which can lead to renal failure in around 30% of cases. As renal failure can greatly increase the likelihood of mortality in cirrhotic patients, some authors investigated the possibility of reducing this risk by expanding plasma volume with albumin (1.5 g/kg when SBP is diagnosed and a further 1 g/kg on the third day of treatment) in a controlled study with patients who were using cefotaxime. There was a reduction in both renal failure and mortality (from 29 to 10%).57 A more recent study established that the use of albumin as coadjuvant therapy for SBP to prevent renal failure should be limited to more serious cases of cirrhosis, with total bilirubin ≥ 4 mg% or BUN > 30 mg/dL or creatinine > 1 mg/dL58 (Figure 2). In a recent study, hospitalized cirrhotic patients with other infections than SBP were randomized to receive only antibiotics or antibiotics plus albumin in similar doses. Association of albumin has shown beneficial effects on the renal and circulatory function and a potential survival benefit.59
Different Infections in Decompensated Cirrhosis- •
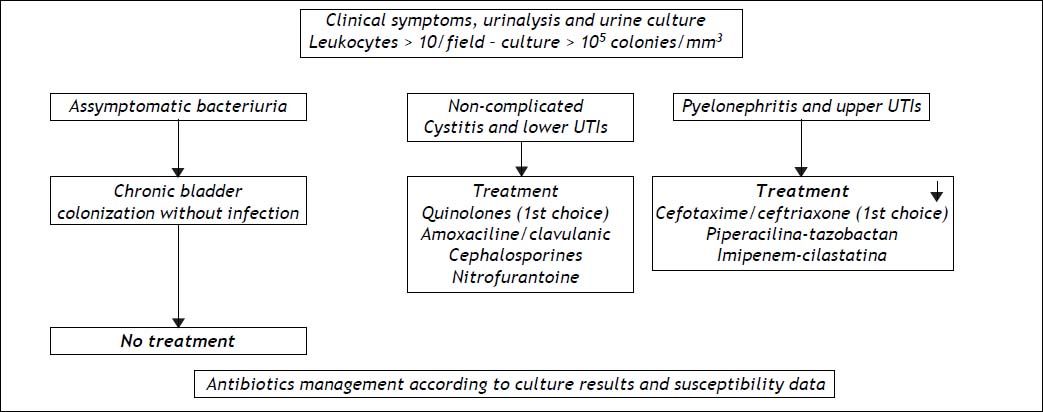
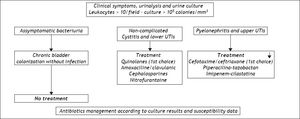
Urinary tract infections (UTI) rival SBP in terms of frequency and share most of the etiopathogenic mechanisms. Like SBP, they usually recur in the same patient and require a variety of different medical measures. Their incidence is higher in females than in males, and patients are usually asymptomatic, presenting only with bacteriuria.9,60,61 Although prophylaxis for SBP is also effective for urinary infections, there is usually cross-resistance to antibiotics because of the predominance of Gram-negative cocci in patients who have not received prophylaxis and the development of Gram-positive infections in patients being treated. The best approach when there is UTI, is to base the choice of therapy on culture results and susceptibility data and monitor its effectiveness in successive tests. Figure 3 shows a flowchart for diagnosis and treatment of both lower and upper urinary tract infections in cirrhosis, with the first choice antibiotics and other possibilities.
- •
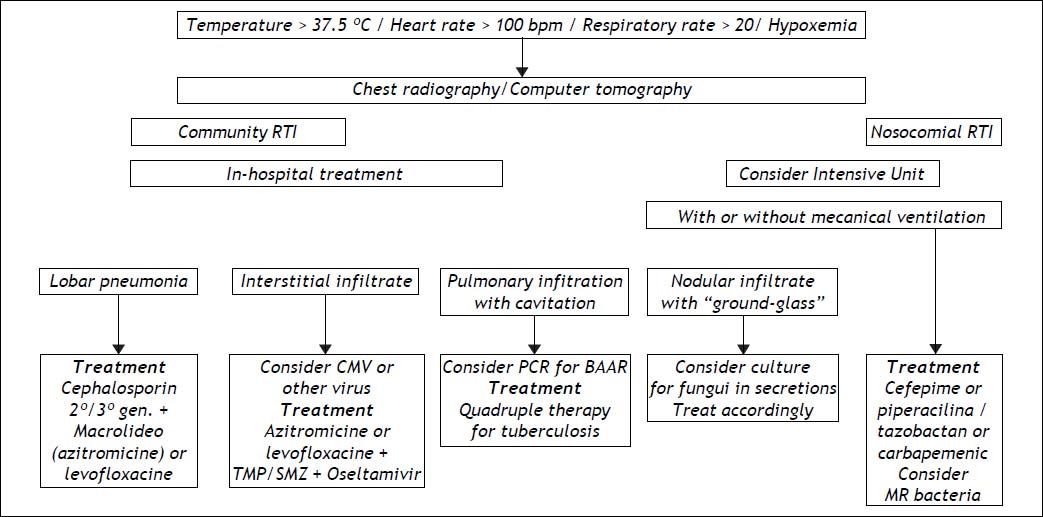
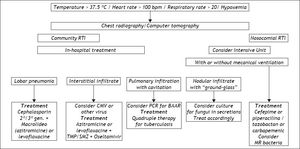
Respiratory tract infections (RTI), although less frequent, are usually more serious, carrying the highest risk of mortality among the prevalent infections in cirrhosis.62 They are diagnosed clinically or by radiological means, since blood cultures are seldom positive and culture of secretions is not easily obtained. A much broader spectrum of microorganisms cause these infections, the main causative organism being Streptococcus pneumoniae. Anaerobic germs or even GNB, such as K. pneumoniae, can also be found. Cirrhotic patients with hydrothorax can develop spontaneous bacterial empyema, which is considered to have the same physiopathological mechanism as SBP.63 Cephalosporins are usually effective, but erythromycin, imipenem or clarithromycin can be used in association with them, depending on the clinical presentation. Figure 4 depicts means of diagnosis, different forms of RTI and their management, emphazising the need for hospital care in these cases.
- •
Skin and soft tissue infections, such as cellulitis and lymphangitis, are relatively frequent, particularly in the lower extremities of cirrhotic patients with edemas. When detected and treated in time, the prognosis is usually good, particularly if they are community acquired. Although such infections are frequently resolved, in a recent retrospective case-control study there was a higher percentage of patients with renal failure (21.3 vs. 5.4%) and hyponatremia (40 vs. 20%), and three-month mortality rates were also higher (23 vs. 4%).64 The most frequently found microorganisms are Staphylococcus aureus and Streptococcus pyogenes. The antibiotics most commonly used in empirical treatment are ceftazidime, amoxicillin with clavulanic acid, and cloxacillin. Other broad-spectrum antibiotics that cover GPB are clindamycin and vancomycin. The choice depends on other factors if the infection is nosocomial.
- •
Infections related to catheters and other procedures can also occur in decompensated cirrhotic patients while they are hospitalized. Measures to avoid these iatrogenic infections must be followed strictly; these include systematic hand hygiene, the use of chlorhexidine for all skin preparations and full-barrier precautions during catheter insertion or other procedures (Table 2). A recent study has shown that second infections, largely preventable as RTI due to aspiration or UTI related to catheters were linked to higher mortality rates.16 Among them fatality rate was higher for Clostridium difficile infections. Difficult of treat infections as Clostridium difficillis have been treated with fecal microbiota transplantation.65 A meta analysis of eleven studies with 273 patients has shown 89.7% of resolutions. So, fecal microbiota transplant holds considerable promise as therapy for these difficult to treat infections but follow-up registries are still needed.66
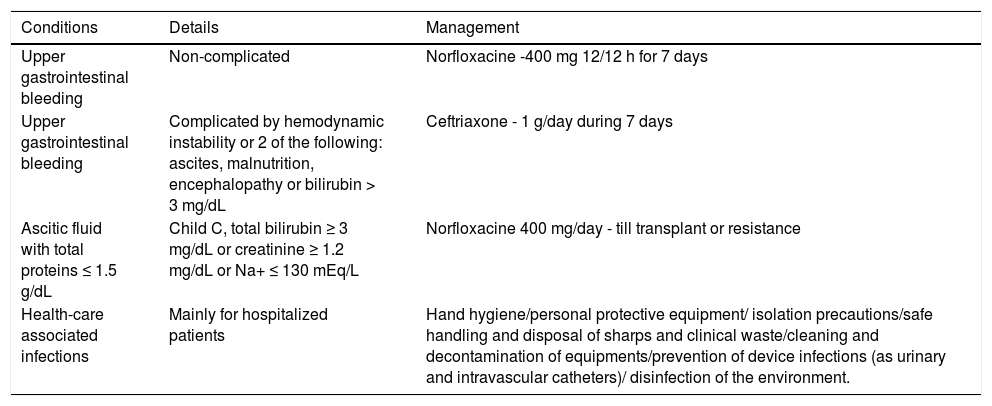
Table 2.Primary prophylaxis of bacterial infections in cirrhosis.
Conditions Details Management Upper gastrointestinal bleeding Non-complicated Norfloxacine -400 mg 12/12 h for 7 days Upper gastrointestinal bleeding Complicated by hemodynamic instability or 2 of the following: ascites, malnutrition, encephalopathy or bilirubin > 3 mg/dL Ceftriaxone - 1 g/day during 7 days Ascitic fluid with total proteins ≤ 1.5 g/dL Child C, total bilirubin ≥ 3 mg/dL or creatinine ≥ 1.2 mg/dL or Na+ ≤ 130 mEq/L Norfloxacine 400 mg/day - till transplant or resistance Health-care associated infections Mainly for hospitalized patients Hand hygiene/personal protective equipment/ isolation precautions/safe handling and disposal of sharps and clinical waste/cleaning and decontamination of equipments/prevention of device infections (as urinary and intravascular catheters)/ disinfection of the environment. - •
Bacteremia and septicemia develop more frequently in decompensated cirrhosis.67 Outbreaks of bacteremia manifest as a minor increase in body temperature and general malaise and are confirmed by blood culture. The clinical and laboratory findings can be those of spontaneous or procedure-related transient bacteremia. However, whenever bacteria are in the general circulatory system, there is a risk of systemic inflammatory response syndrome (SIRS) developing, with a clinical and laboratory picture of septicemia. Sepsis is particularly serious in cirrhotic patients and should always be suspected so that therapeutic measures can be taken immediately, as the condition can progress to multiple organ failure as a result of the immunological dysfunction inherent to cirrhosis.67
Diagnosis of SIRS and severe sepsis can be more difficult in decompensated cirrhotic patients because of their low baseline blood pressure secondary to hyperdynamic circulation in the more advanced stages of cirrhosis. By promoting the release of cytokines into the circulatory system, the bacterial infection worsens splanchnic and systemic vasodilations, which are already present because of portal hypertension. This increased vasodilation leads to a reduction in effective arterial blood volume and activation of the neurohormonal (rennin-angiotensin-aldosterone) system, causing vasoconstriction and renal failure. Renal failure in turn results in elevated levels of inflammatory cytokines such as TNF-α and interleukin-6 and vasodilatory hormones such as nitric oxide (NO). A vicious circle of progressive alterations, including cirrhotic cardiomyopathy, hepatic encephalopathy, coagulopathy and other dysfunctions in organs or systems, is thus established, characterizing multiple organ failure.67 As in non-cirrhotic patients, in cirrhosis there is a direct association between the number of failing organs or systems and lethality.68
Some infected cirrhotic patients with sepsis develop adrenal dysfunction. Its pathogenesis is complex and poorly understood. The majority develop reversible dysfunction due to decreased production of cortisol or ACTH. Although high doses of corticosteroids are detrimental to septic shock patients, low doses remain controversial.69–71
Prophylaxis For Bacterial Infections in Cirrhosis and SBPIf one accepts the bacterial translocation theory, the intestines are the main source of bacteria which migrate to regional lymph nodes, ascites and general circulatory system causing SBP and other GNB infections in cirrhosis. Thus, to avoid these infections, the ideal agent should be safe, minimally absorbed and effective. Additionally, it should eliminate GNB while preserving GPB and anaerobic flora, a process known as selective intestinal decontamination. Oral administration of poorly absorbed antibiotics has been shown to be effective in preventing a relapse of SBP(72) and has been used for around two decades. Nevertheless, such antibiotics should only be indicated for cirrhotic patients who really need them because of the risk of developing resistant bacteria. Below, we discuss three conditions for which the use of prophylactic antibiotics is recommended to prevent the onset of infections and thus improve the survival of patients with decompensated cirrhosis.
Antibiotic therapy in variceal upper gastrointestinal bleeding (UGIB)It is estimated that 20% of cirrhotic patients with UGIB present with bacterial infections on admission to hospital and that around 50% develop them while they are hospitalized.73 Worsening portal hypertension and a greater risk of the recurrence of hemorrhage were observed in patients with UGIB who presented with infectious complications. The development of infections in this group of patients is associated with a fivefold increase in the risk of a recurrence of bleeding, with an adverse impact on survival.74,75
The main bacterial infections that must be systematically investigated in cirrhotic patients with UGIB are urinary tract infection, SBP, respiratory tract infection and spontaneous bacteremia. All cirrhotic patients hospitalized for UGIB must be screened for infections with blood cultures, paracentesis of AF for neutrophil count and culture (for the latter AF is collected directly in blood culture flasks), urine sediment analysis and a chest X-ray.9,76
The use of antibiotic prophylaxis has been associated with a reduced frequency of infectious events and improved survival,76,77 resulting in a decreased risk for infections and mortality in patients treated with this prophylaxis of 58% and 29%, respectively.77 Various prophylactic schemes have been advocated, but the most frequently used are oral quinolones, particularly norfloxacin administered in a 400 mg dose twice a day for seven days.78 Another controlled study was carried out comparing antibiotic prophylaxis with ceftriaxone (1g IV/day) and norfloxacin (400 mg twice daily) for seven days in cirrhotic patients with UGIB with at least two of the following criteria: ascites and/or malnutrition and/or encephalopathy and/or bilirubin > 3 mg/dL. The authors reported a significant reduction in the frequency of infections (11 vs. 26%) and SBP (2 vs. 12%) in the group treated with ceftriaxone.79Table 2 summarizes the main indications and the drugs most commonly used in primary prophylaxis of bacterial infections in cirrhosis.
Prophylaxis for relapse of SBPAfter a first episode SBP frequently recurs, with relapses in 43% of patients in the first 6 months and 68% in a year. Prophylaxis is therefore recommended for any patient recovering from an episode of SBP, as it has been shown that recurrence falls from 68 to 20% and that the probability of developing SBP due to GNB falls from 60 to 3%.72 Quinolones (norfloxacin) are the drug of choice and are indicated for these cirrhotic patients in doses of 400 mg/day to be taken continuously until the ascites resolves or the patient has a transplant.80 As alternative prophylactic drugs, ciprofloxacin or sulfamethoxazole and trimethoprim are also indicated.15,81 More recently the use of rifaximin, a poorly absorbable antibiotic with low risk of bacterial resistance is been indicated,82 and a retrospective analysis comparing its use with norfloxacine has shown better results,83 that should be confirmed in prospective studies.
Ascites with low protein levelsAnother possible condition, for which antibiotic prophylaxis can be indicated in patients with ascites but without a diagnosis of SBP, is patients with AF protein levels ≤ 1.5 g/dL. These low protein levels make opsonization of bacteria in AF difficult, allowing infections to develop more easily. In cases with AF protein levels > 1 g/dL the prevalence of SBP in two years was zero, while the corresponding figure in patients with AF protein levels < 1g/dL reached 20%.84 Although some controlled studies failed to provide convincing evidence to justify this approach in all cirrhotic patients with low AF protein levels, recent investigations recommend prophylaxis with 400 mg/day of norfloxacin when patients with decompensated cirrhosis are Child C, with total bilirubin ≥ 3 mg/dL or creatinine ≥ 1.2 mg/dL or Na+ ≥ 130 mEq/L.85
Alternative non-antibiotic therapies, such as probiotics, prokinetic agents and supplementation with oral bile acids have been proposed. Probiotics would modulate gut microflora, stabilize mucosal barrier function and restor neutrophyl phagocytic capacity.86 It has already been shown to be effective for other clinical conditions,87 but must be evaluated carefully before being adopted. A recent trial has shown that the addition of probiotics to norfloxacin does not improve its efficacy in primary or secondary prophylaxis of SBP.88 As β-adrenergic blockers shorten intestinal transit time with anti-bacterial effects some studies using propranolol have been carried out and a meta-analysis has shown a relative risk reduction of 12% in the development of SBP.89 Combination of norfloxacin with cisapride has also been evaluated.90
In conclusion, there has been great progress in the control of bacterial infections in decompensated cirrhosis in recent decades. Various controlled clinical studies reviewed according to the strict criteria of evidence-based medicine have proved that prophylactic and therapeutic measures have managed to reduce morbidity and increase survival in cirrhotic patients even in the decompensated stages.61,91 However, problems remain to be solved in terms of both therapy and prophylaxis. With the advent of resistance to commonly used antibiotics92 and the recent description of multiresistant bacteria,93 there is a need for stricter control of the administration of antibiotics to cirrhotic patients. Further research in this area is also required. Great care must be taken when using antibiotic prophylaxis and the agreed limits must not be exceeded. To sum up, this body of knowledge about therapy and prophylaxis for bacterial infections in cirrhotic patients, which undoubtedly helps to improve survival, cannot continue to be restricted to specialists but should be disseminated and applied in clinical practice for the benefit of the population at large.