Bile acids (BAs), the end products of cholesterol catabolism, are essential for the absorption of lipids and fat-soluble vitamins; but they have also emerged as novel signaling molecules that act as metabolic regulators. It has been well described that the enterohe-patic circulation, a nuclear (FXR) and a cytoplasmic (TGR5/M-BAR) receptor aid in controlling hepatic bile acid synthesis. Modulating bile acid synthesis greatly impacts in metabolism, because these receptors also are implicated in glucose, lipid, and energy expenditure. Recent studies had revealed the way these receptors participate in regulating gluconeogenesis, peripheral insulin sensitivity, glycogen synthesis, glucagon like peptide 1 (GLP-1) and insulin secretion. Nowadays, it is demonstrated that enhancing bile acid signaling in the intestine contributes to the metabolic benefits of bile acid sequestrants and bariatric surgery on glucose homeos-tasis. This paper discusses the role of bile acid as regulators of glucose metabolism and their potential as therapeutic targets for diabetes.
Bile acids (BAs), one of the main components of bile, are amphipathic molecules with detergent properties known mainly for their participation in the absorption of lipids and fat-soluble vitamins in the gut, as well as cholesterol solubilization in the bile and induction of bile flow.1
In recent studies, it has been reported that BAs have pleiotropic effects, also functioning as hormones or signaling molecules that act as metabolic regulators.2 This regulatory function is accomplished when bile acids bind to cytoplasmic G protein-coupled receptor TGR5 (TGR5/ M-BAR) and nuclear farnesoid X receptor (FXR) activating different signaling pathways in distinct organs and tissues that have important roles in the metabolism of lipid, glucose, and energy expenditure.3,4,5
In this review, we focused on the importance of BAs in metabolic regulation mechanisms of glucose and their signaling pathways as therapeutic targets for diabetes. In order to understand this topic, first of all, we will give an explanation about the metabolism and synthesis of BAs.
Bile Acids Synthesis and MetabolismPrimary bile acids, cholic acid and chenodeoxycholic acid, are synthetized from cholesterol in the hepatocytes of the liver by two different pathways, the classic or neutral pathway and the alternative or acidic pathway.5-8 The classic pathway is controlled by the first and rate-limiting step; in which hydroxylation of cholesterol is catalyzed by the cholesterol-7α-hydroxylase (CYP7A1) and then other enzymes (sterol-12α-hydroxylase or CYP8B1, 25-hydroxy-cholesterol-7α-hydroxylase or CYP7B1 and sterol-27α-hydroxylase or CYP27A1) convert bile acid intermediates to bile acids.5-8 CYP8B1 controls the production of cholic acid and determines the amount of cholic acid versus chenodeoxycholic acid in bile acid pool.9 This pathway is more important because the CYP7A1 is also responsible for controlling the synthesis of bile acids.9 In the alternative pathway, which only contributes to less than 10% of bile acid synthesis, oxys-terols are converted to bile acids by the sterol-27α-hy-droxylase (CYP27A1).5-8 Primary BAs are conjugated with glycine or taurine in the liver and subsequently are secreted by the bile salt export bump into the bile and stored in the gallbladder.5,7 After ingestion of a meal, the duodenum secretes cholecystokinin, which stimulate gallbladder contraction and release of bile acids into the intestine. Primary BAs in the gut interact with intestinal bacteria resulting in the formation of secondary bile acids (deoxycholic acid and lithocholic acid) and facilitate the absorption of dietary lipids and fat-soluble vitamins.5-7 Afterwards, the enterohepatic circulation allows 95% of bile acids to be efficiently absorbed in the terminal ileum and transported to the liver via the portal cir-culation.5,7,8 The daily fecal loss of bile acids is only 5% and is replenished via the novo synthesis in the liver.5,7,8 In the liver, active transport systems facilitate the uptake by hepatocytes.5,7,8
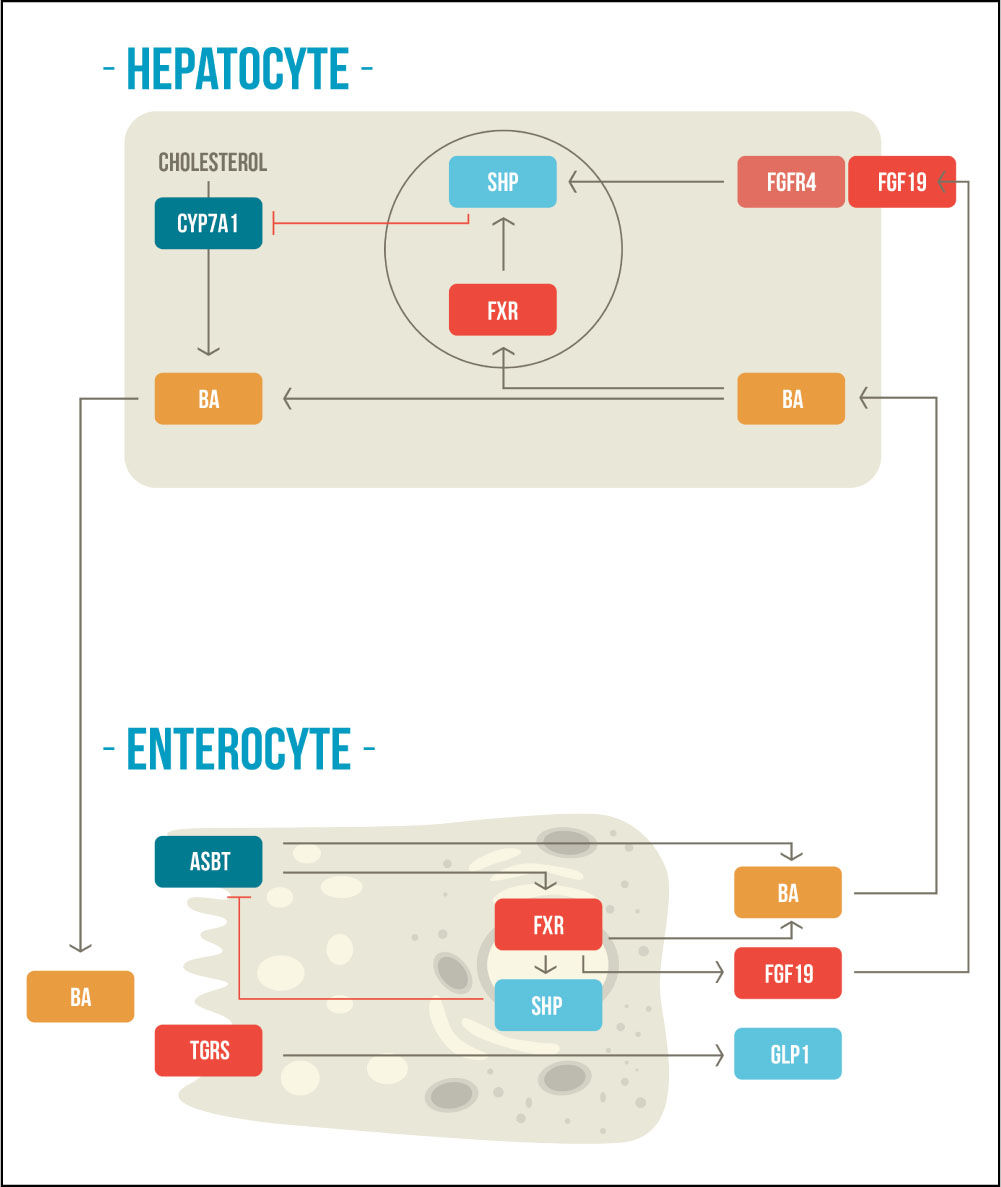
BAs also regulate their own homeostasis by regulated bile acid mediated negative feedback mechanisms within the liver and intestine in response to changes in bile acid levels, through FXR and TGR5/M-BAR.10-12 These signaling pathways maintain the expression of the rate-limiting enzyme CYP7A1 that is crucial for de novo bile acids synthesis and to preserve the bile acid pool.12 In the liver, FXR upregulates the inhibitory nuclear receptor small heterodimer partner (SHP), which acts as a corepressor with liver-related homolog-1 (LRH-1) and hepatocyte nuclear factor-4α (HNF-4α), repressing the gene transcription of CYP7A1.13,14 Additionally, in the intestine, FXR activation induces the expression of fibroblast growth fac-tor-19 (FGF-19), which binds to fibroblast growth factor receptor-4 (FGFR4) to repress the expression CYP7A1 by a c-Jun N-terminal kinase (JNK)-dependent pathway.15,16 The second receptor, TGR5/M-BAR, is less well studied; but it have been demonstrated that TGR5 knockout mice have smaller BAs pool size with altered composition and plays a role in the suppression of CYP7A1.17,18 In this way, these two receptors help to control bile acid synthesis (Figure 1).
Enterohepatic negative feedback mechanisms of bile acid synthesis. BAs are synthetized from cholesterol molecules in the hepatocyte and then are stored in the gallbladder. After meal ingestion, they are released into the intestine where they activate FXR and TGR5. BAs are reabsorbed through the ASBT, activating FXR at the enterocyte, which produces a negative feedback mechanism in the synthesis of bile acids. FXR activation also stimulates FGF-19, which concomitantly participates in the negative feedback mechanism at the hepatocyte. The activation of TGR5 increases secretion of GLP-1. CYP7A1: cholesterol-7α-hydroxylase. BA: bile acid. SHP: small heterodimer partner. FXR: farnesoid X receptor. FGFR4: fibroblast growth factor receptor-4. FGF19: fibroblast growth factor-19. ASBT: apical sodium-dependent bile salt transporter. TGR5: G protein-coupled receptor TGR5. GLP1: glucagon like peptide 1. Adapted from Sonne, et al. 2014.49
Previous studies have clarified a relation between BAs and glucose metabolism. It has been demonstrated that diabetic rats had decreased FXR expression and that FXR direct activation has been showed to significantly lowered serum glucose and upgrade insulin sensitivity.19,20 Although, more recent data suggests that long-term direct activation of FXR also reduces bile acid pool size, which consequently causes a decrease in energy expenditure and augments insulin resistance.21 Conversely, glucose and insulin have been associated as major postprandial factors that increased BAs synthesis, but it have been determined that it is in an FXR independent manner.22 Nevertheless, bile acid mediated FXR-activation could expand bile acid pool size, diminish gluconeogenesis, and increment glyco-genesis; making it an interesting therapeutic target for diabetes.
Studies have focus on the possibility that FXR regulates hepatic glucose production and reduce serum glucose levels. It has been proved that BAs and FXR via small het-erodimer partner (SHP) cascade suppress the activity of phosphoenolpyruvate carboxykinase (PEPCK), glucose 6-phosphatase (G6Pase) and fructose 1,6-bisphosphatase 1 (FBPase), which are enzymes that participate in hepatic gluconeogenesis pathway.23,24 The effect of FXR in ameliorating glucose homeostasis may not only be fulfilled by inhibiting gluconeogenesis.
Several studies had revealed that FXR plays a role in regulating peripheral insulin sensitivity.24,25 Other papers, have addressed that FXR induces FGF-19 in the intestine during postprandial period, thereby repressing gluconeo-genesis, and increasing glycogen synthesis and energy ex-penditure.26-29 Also recent evidence suggests that FXR is expressed in human pancreatic β-cells and stimulates insulin gene transcription producing a positive control on glucose dependent insulin secretion.30
The implications of BAs on glucose metabolism include complicated regulation between FXR-dependent and FXR-independent pathways.
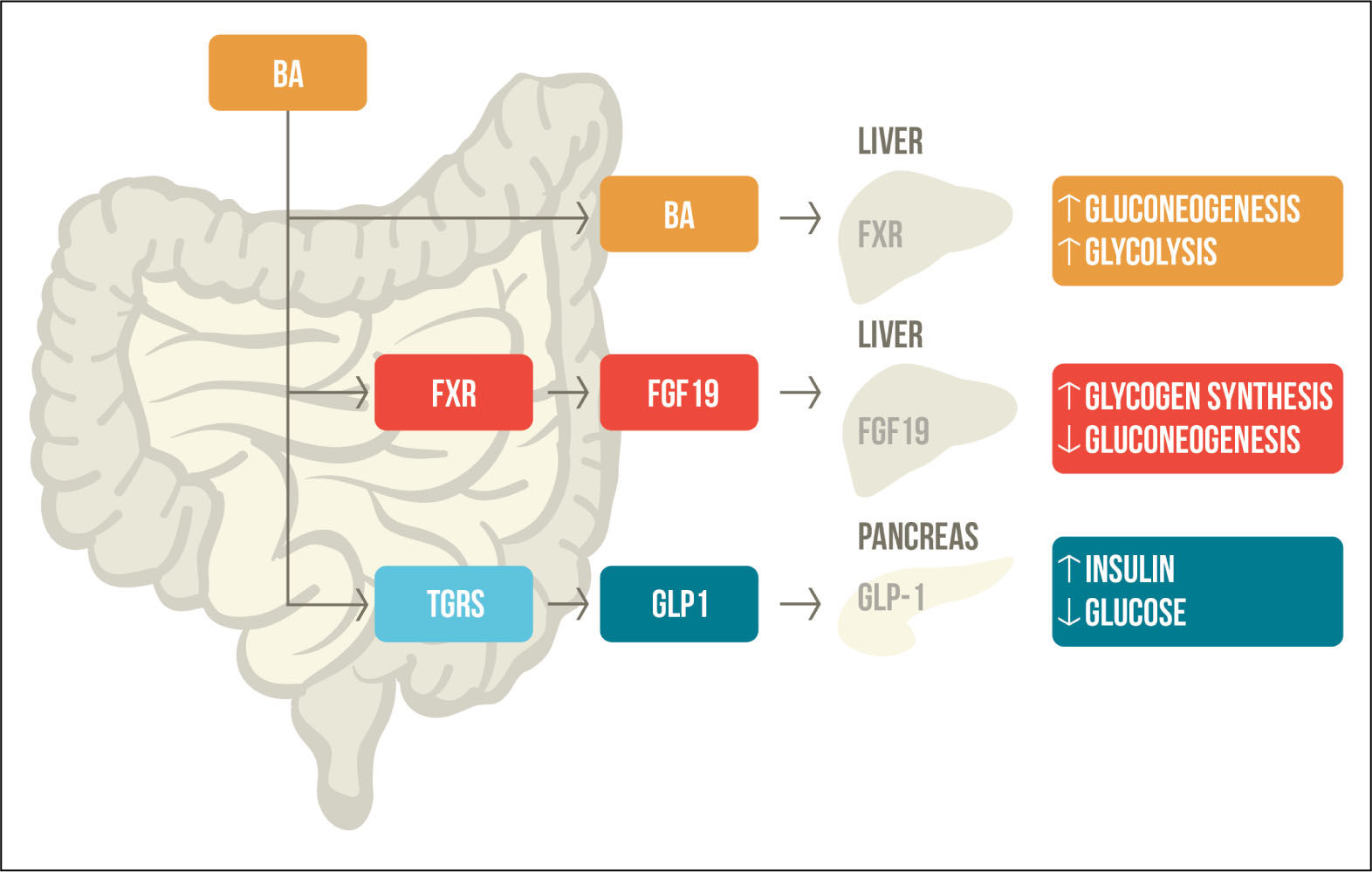
G protein-coupled receptor TGR5 in glucose metabolismTGR5/M-BAR is highly expressed in the intestine and exposed to high levels of BAs, which activate and promotes the secretion of GLP-1 in the pancreatic β-cells.31 GLP-1 is a hormone secreted by intestinal L-cells in response to meal intake that promotes insulin secretion and inhibits glucagon secretion; therefore, regulating glucose homeostasis.32 It has been seen that many patients with diabetes have a combination of reduced GLP-1 secretion and resistance to its effect.33 TGR5/M-BAR agonist increases the levels of cAMP and the intracellular ATP/ADP ratio, which leads to calcium influx and induced GLP-1 re-lease.34 Evidence suggest the importance of TGR5/M-BAR in GLP-1 secretion, that is why bile acid-based TGR5 agonists may be an interesting therapeutic target for diabetes (Figure 2).
Bile acid mediated regulation of glucose metabolism. After meal ingestion, BAs are released into the intestine where they activate FXR and TGR5. FXR activation stimulates FGF-19, which participates in glycogen synthesis and gluconeogenesis. TGR5 activation increases levels of GLP-1, promoting insulin secretion and decreased serum glucose levels. BAs that are reabsorbed through the enterohe-patic circulation activate FXR in the liver, which also participates in gluconeogenesis and glycolysis. BA: Bile acid. FXR: farnesoid X receptor. TGR5: G protein-coupled receptor TGR5. FGF19: fi-broblast growth factor-19. GLP1: glucagon like peptide 1. Adapted from Sonne, et al. 2014.49
Diabetes is a complex chronic illness characterized by an increase in serum glucose concentration associated with microvascular and macrovascular complications, as well as cardiovascular diseases.35 It has been reported that diabetic patients have a change in bile acid metabolism, which is characterized by an increase in bile acid pool size and fecal excretion, as well as changes in their composi-tion.36,37
Numerous antidiabetic drugs are used in the treatment of diabetes, but research on BAs and their application in this disease have gained importance in recent years. Despite the aforementioned, the role of BAs in the treatment of diabetes has so far showed to be useful through the use of bile acid chelating agents in patients with type 2 diabe-tes.38 Also bariatric surgery has demonstrated its contribution related to bile acid metabolism as part of the benefits that helps in glucose control in diabetic patients.39 However, with the previously exposed knowledge regarding bile acid synthesis and its relation to glucose metabolism, new potential targets for the treatment of diabetes had been identified through FXR and TGR5/M-BAR signaling pathways.
Bile acid chelating agents are anionic exchange resins that form a nonabsorbable complex with BAs in the intestine, which prevents reabsorption and augments fecal excretion of BAs.40 Bile acid chelates have been used in the treatment of hypercholesterolemia since long ago and in 1994 it was first described that cholestyramine may also reduce serum glucose in patients with type 2 diabetes.41 Afterwards, several studies corroborated that bile acid chelating agents lower serum glucose in diabetic pa-tients.38,42-44 Today, colesevelam is approved by the Food and Drug Administration (FDA) and included in management algorithms for the treatment of type 2 diabetes. The mechanisms behind improvement of serum glucose levels with bile acid chelates are multiple and not fully elucidat-ed.45
Bariatric surgery is being used as an effective therapeutic option for obesity and type 2 diabetes. Some studies have revealed that bariatric surgery not only decreases body weight, it also improves serum glucose levels, β-cell function and insulin resistance.46,47 The mechanism linked to the improvement of glucose control with bariatric surgery are associated with increased BAs and FGF-19 secretion, which promotes bigger amounts of GLP-1.28,39 This finding towards improvement of glucose metabolism had been more effective in malabsortive surgeries such as gastric bypass than in restrictive surgeries.48
ConclusionBile acids, known mainly for their participation in the absorption of lipids and fat-soluble vitamins, have also an important role in the metabolism of lipid, glucose, and energy expenditure. FXR and TGR5/M-BAR, two signaling pathways, are important bile acid synthesis regulators. Also, it has been revealed that they are relevant metabolic regulators for maintaining glucose homeostasis and has converted them in possible new therapeutic targets for diabetes. Nowadays, the only approved therapeutic option for the treatment of diabetes, related to BAs, involves the use of bile acid chelates. However, it has been shown that some of the benefits of bariatric surgery on glucose control in diabetic patients are related to bile acid metabolism.
Abbreviations- •
BAs: bile acids.
- •
CYP27A1: sterol-27α-hydroxylase.
- •
CYP7A1: cholesterol-7α-hydroxylase.
- •
CYP7B1: 25-hydroxy-cholesterol-7α-hydroxylase.
- •
CYP8B1: sterol-12α-hydroxylase.
- •
FGF19: fibroblast growth factor-19.
- •
FGFR4: fibroblast growth factor receptor-4.
- •
FXR: nuclear farnesoid X receptor.
- •
GLP-1: glucagon like peptide 1.
- •
TGR5/M-BAR: cytoplasmic G protein-coupled receptor TGR5.
This work was support by the Clinic of Digestive Diseases and Obesity of Medica Sur Clinic & Foundation. We also want to thank Mariluz Regueiro assistance for the adaptation of the figures.
Grant and Other Financial SupportThe authors declare no grant and no other financial support.
Conflict of InterestThe authors declare no conflict of interest.
Copyright AssignmentIn consideration of the Fundación Clínica Médica Sur (FCMS) taking action to review and edit my submission, the undersigned authors, jointly and severally, hereby transfer, convey, and assign all right, title, and interest therein, including any and all copyrights in all forms and media now or hereafter known, to the FCMS. The authors retain the nonexclusive right to use all or part of the Article in future works of their own in a noncompeting way, provided proper copyright credit is given to the Foundation.
Should the FCMS not publish the aforesaid submission, the FCMS agrees to release its rights therein (Note: material prepared by employees of the federal government in their official duties may not be copyrightable). No guarantee is made that the Article will be published.
Authorship ResponsabilityI, the undersigned author, certify that I have participated sufficiently in the intellectual content, the analysis of data, if applicable, and the writing of the Article, to take public responsibility for it.
I have reviewed the final version of the Article, believe it represents valid work, and approve it for publication. As an author of this Article, I certify that none of the material in the manuscript has been published previously, is included in another manuscript, or is currently under consideration for publication elsewhere. I also certify that the Article has not been accepted for publication elsewhere, nor have I assigned any right or interest in the Article to any third party.
Financial DisclosureI, the undersigned author, certify that I have no commercial associations (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements) that might pose a conflict of interest in connection with the submitted Article, except as disclosed on a separate attachment. All funding sources supporting the work and all institutional or corporate affiliations of mine are acknowledged in a footnote.
Institutional Review Board/Animal Care Committee ApprovalNot applicable for this concise review.