Introduction and aim. 5-Fluorouracil (5-FU) is the most commonly used chemotherapeutic drug in the treatment of cholangiocarcinoma (CCA). Since development of drug resistance to 5-FU in CCA patients is the primary cause of treatment failure, a better understanding of the mechanism of drug resistance of this cancer is essential to improve the efficacy of 5-FU in CCA therapy.
Material and methods. A 5-FU resistant CCA cell line (M214-5FUR) for a comparative chemo-resistance study was established. Real time RT-PCR was used to determine gene expression levels. Cell cytotoxicity was measured by the MTT assay. Protein expression levels were detected by the immunofluorescene method.
Results. It was found that 5-FU resistance was associated with the overexpression of Tβ10 in CCA cell lines. 5-FU treatment at various concentrations induced the expressions of Tβ10 and ABC transporters (ABCB1, ABCG2 ABCA3) in two CCA cell lines, KKU-M055 and KKU-M214. M214-5FUR, a 5-FU-resistant cell line, exhibited a 5-FU resistant phenotype with a 16-fold extremely high expression of Tβ10 and ABC transporters, as compared to the parental cells, KKU-M214. siRNA targeted to Tβ10 significantly reduced expression of ABC transporters tested in the M214-5FUR cells (P < 0.05).
Conclusions. The present novel findingsof Tβ10 connected with drug resistance as shown in this study provides a new insight for the therapeutic value of Tβ10 as a predictive biomarker of 5-FU chemoresistance. Inhibiting Tβ10 may be a valuable adjunct for suppression of ABC transporters and sensitizing chemotherapy treatment, especially 5-FU in CCA patients.
Cholangiocarcinoma (CCA) is a major health problem in the northeast of Thailand. The cancer originates from the biliary epithelium of both the intra- and extra-hepatic bile ducts. Most cases are extremely invasive, develop rapidly without clinical symptoms, and thus are usually diagnosed at an advanced stage when complete removal of the tumor is very difficult. To date, surgery represents the main therapeutic option, however, it is only effective at an early stage of the disease1 and the outcome of surgery remains unsatisfactory due to the high recurrence rate. This complication impairs the survival rate after resection. Prognosis of CCA is extremely poor. Several attempts with multiple regimens of chemotherapy have been tried for CCA treatment; however, the results were not satisfactory [reviewed in 2]. Chemotherapy could reach a response rate of 30% with a median survival time less than one year.3–7 Cisplatin plus gemcitabine was shown to give a significant survival advantage to patients with biliary tract cancer than those given gemcitabine alone with median progression-free survival 8 months vs. 5 months.8 Fluoropyrimidine or gemcitabine-based chemotherapy, or fluoropyrimidine- based chemoradiotherapy are acceptable option for intra- and extra- hepatic CCA as the guideline recommended by the National Comprehensive Cancer Network.9
Low response of CCA to multiple anti-cancer drugs might be due to development of a multidrug resistant (MDR) phenotype in cancer cells. The ATP-binding cassette (ABC) transporter plays an important role in drug efflux and relates to poor prognosis in many cancers.10,11 To improve the treatment, identification of novel drug resistance markers and development of therapeutic strategies are of clinical importance. A predictive biomarker of the chemotherapeutic response could not only establish the survival benefits of chemotherapy but may also be expected to increase the curability of chemotherapy treatment.
5-Fluorouracil (5-FU) is the most common therapeutic agent used in the treatment of various types of cancers, including CCA.2,7 5-FU is anabolized into the active metabolite 5’-fluoro-2’-deoxyuridine 5’-monophosphate intracellularly and inhibits thymidylate synthaseleading to the inhibition of DNA synthesis. The ribonucleoside triphosphate of 5-FU, FUTP, also incorporates into RNA and interfereswith the transcription processes.12–14 Many studies showed the connection between 5-FU resistance status and the gene expression pattern. Thymosin β10 (Τβ10), an actin sequester protein, was identified as a 5-FU inducible target gene which may be involved in mediating resistance to 5-FU.14–16 The exact role of Tβ10 in drug resistance, however, is still unclear. In the present study, whether or not Tβ10 is involved in mediating 5-FU resistance of CCA cells was exploredusing in vitro molecular biological techniques and a 5-FU-resistant CCA cell line.
Experimental ProcedureCell lines and cell cultureCCA cell lines, KKU-M055 and KKU-M214 were established from primary tumors of Thai patients with mass forming intrahepatic CCA. The cell lines were obtained from the National Institute of Biomedical Innovation, JCRB Cell Bank, Osaka, Japan. All cell lines were cultured in a complete medium of Ham-F12 supplemented with 10% w/v fetal bovine serum, 100 U/mL penicillin and 100 µg/mL streptomycin at 37 °C and 5% CO2.
Establishment of a 5-FU-resistant CCA cell lineA 5-FU-resistant cell line was established as described by Namwat, et al.17 In brief, 1x105 cells of KKU-M214 were seeded in a 25-cm2 flasks and treated with 5 mL of the complete medium containing 2.5 5-FU, the concentration equivalent to its IC50, for 72 hours at 37 °C under 5% CO2. After incubation, culture medium was replaced with drug-free medium and maintained to allow cells to attain 70% confluence. The surviving cells were cloned and then exposed to 2 x IC50, 3 x IC50 and 4 x IC50. The final 5-FU-resistant cell line was designated as M214-5FUR. To ensure the continued resistance, the M214-5FUR was established through continuous culturing in DMEM containing 4 x IC50 5-FU. To eliminate the effects of 5-FU from the experimental outcomes, the resistant cells were cultured in a drug-free medium for at least 10 passages before experiments as per the previously tested protocol described by Wattanawongdon, et al.18
RNA extraction and Real time RT-PCRTotal RNA was extracted using the Ambion RNAqueous-4PCR kit (Ambion, Austin, TX) following the manufacturer’s instructions. Briefly, cells were lysed using lysis buffer, transferred to a mini-column and centrifuged at 10,000 x g for 1 min. The column was washed and eluted in 60 µL of elution buffer. RNA solution was treated with DNAse-I to remove any trace amounts of genomic DNA contamination. The mRNA levels were determined using real time reverse transcription (RT)-PCR. Briefly, mRNA was reverse-transcribed into cDNA using the iScriptcDNA synthesis kit and real time RT-PCR was performed using the iQ SYBR Green supermix kit (Bio-Rad, Hercules, CA). The PCR reaction of 100 nM of each primer, 20 ngcDNA templates and iQ SYBR Green supermix, ran for 40 cycles of 95 °C for 20 sec and 60 °C for 1 min. Each cDNA sample was run in duplicate. β2M was used as an internal loading control. The relative mRNA level was presented as unit values of 2(Ct(B2M)–Ct(gene)). The primers for human β2-microblobulin (B2M; AAGATGAGTATGCCTGCCG) and ABC transporters were as described previously.19,20
Transient silence of Tβ10 by siRNAExpression of Tβ10 in CCA cells was transiently suppressed as described by Sribenja, et al.19 Briefly, 2x104 CCA cells/well were seeded into a 6-well plate for 24 h before transfection. The siRNA specific sequence for targeting human Tβ10 (5’-GCGGAGUGAAAUUUCCUAA-3’), was obtained from Ambion (Austin, TX). Cells were transfected either with 50 pM siTβ10 or a control scrambled RNA. Transfections were carried out using LipofectAmineTM 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Cytotoxicity assaySensitivity of cells to the chemotherapeutic drug, 5-FU, was determined using the MTT assay. Briefly, CCA cells (3 x 103 cells/well) in the complete media with or without various concentrations of 5-FU were seeded in a 96 well culture plate and incubated at 37 °C, 5% CO2. Cell growth was assessed at 0, 24, 48, and 72 h. The MTT reagent (Invitrogen, Carlsbad, CA) (10 of MTT mixed with 100 µL of phosphate buffer saline, pH 7.4) was added to each well and incubated at 37 °C for 4 h. HCl (0.04 N, 100 µL in isopropanol) was added to dissolve the crystals, and the absorbance was measured at 570 nm with an automatic ELISA plate reader (Magellan, Tecan Trading AG, Switzerland).
Immuno-cytofluorescent stainingCCA cells were plated on a slide chamber and treated with si-Tβ10 as described above. After incubation, cells were fixed with 4% (w/v) paraformaldehyde and permeabilized with 0.2% (v/v) Triton X-100. Fixed cells were then incubated with antibodies specific for Tβ10 (Biodesign International, Dublin, OH)or ABCC3 (anti-multidrug resistance-associated protein 3, MRP3, Novocastra Laboratories, Newcastle, UK) followed by secondary antibodies conjugated with Alexa Fluor 488 or 568 (Invitrogen, Carlsbad, CA), for 1 h. Preparations were mounted on slides with Hoechst33342dye for nuclear staining. Cells were examined under a fluorescent microscope with a 10x objective lens.
Statistical analysisExperimental data were analyzed using SPSS 13.0 Windows Evaluation software (SPSS Inc., Chicago, IL). All quantitative data were expressed as mean or percentage ± SD. The two-tailed Student’s t-test was used for comparisons between two groups. Statistical significance was established at P< 0.05.
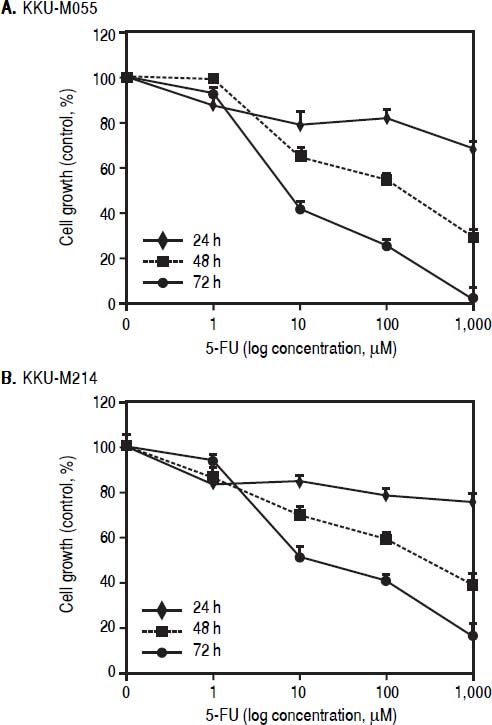
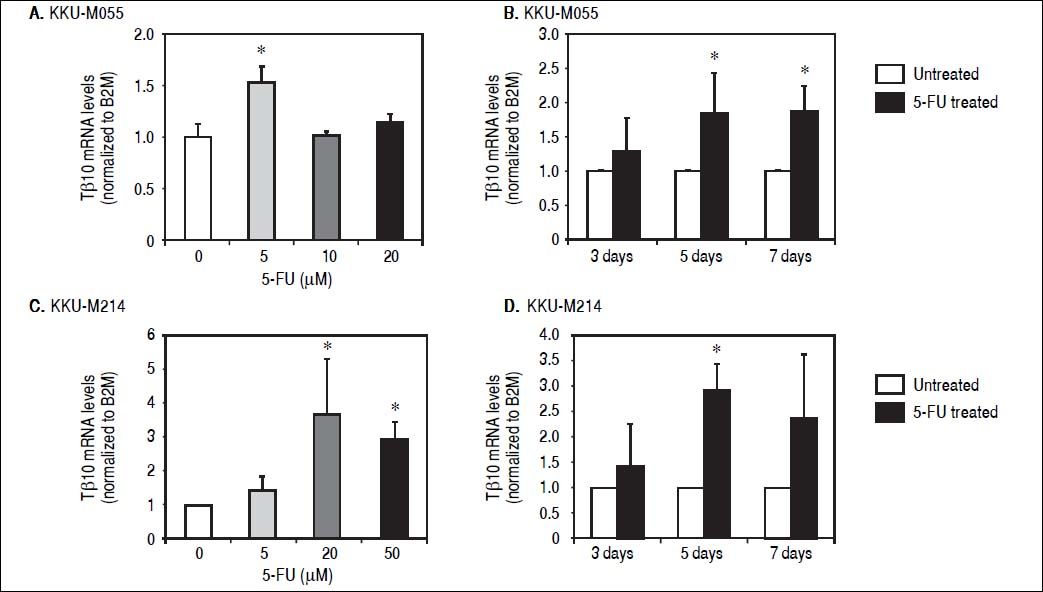
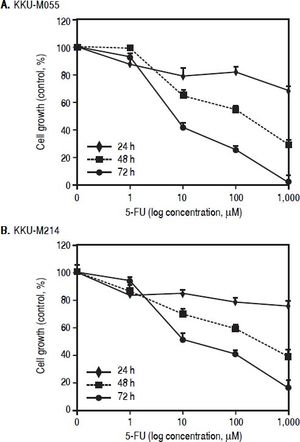
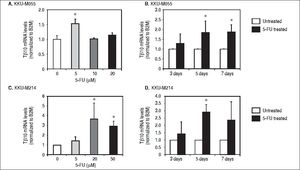
ResultsTβ10 and ABC transporters were up-regulated in CCA cell lines treated with 5-FUTo identify whether Tβ10 is a 5-FU responsive gene, two CCA cell lines, KKU-M055 and KKU-M214, were treated with different concentrations of 10-1000 μM of 5-FU. The cell survivals were determined by the MTT assay at 24, 48 and 72 h after 5-FU treatment. As shown in figures 1A and 1B, 5-FU exhibited cytotoxicity to KKU-M055 and KKU-M214 in dose and time dependent manners. To assess the association of 5-FU treatment on Tβ10 expression, CCA cells were treated with a low dose of 5-FU for a period of 5 days and the expression levels of Tβ10 were determined by real-time RT-PCR. The levels of Tβ10 expression were significantly increased in KKU-M055 treated with 5 μM of 5-FU compared with the untreated control cells (P <0.05; Figure 2A). The significant up-regulation of Tβ10 with 5 μM of 5-FU treatment wa sobserved at 5 days of treatment (Figure 2B). Similar observations were obtained for KKU-M214 treated with 5-FU. Increasing levels of Tβ10 expression were shown in KKU-M214 treated with 20 and 50 μM of 5-FU for 5 days (Figures 2C and 2D).
The growth inhhibitory effect of 5-FU on CCA ceii lines. (A) KKU-M055 and (B) KKU-M214 cells were treated with various concentration of 1-1,000 μM) of 5-FU for 24, 48 and 72 h. Untreated cells were used as controls. Viability of cells was determined using the MTT assay and the percentage of viable cells was calculated compared with the untreated group. The data represent mean ± S.D. of triplicate assays.
Tβ10 was upregulated in the 5-FU treated CCA cell lines. CCA cells were treated with different doses of 5-FU and the expression level of Tβ10 was determined using real time RT-PCR. Treatment of 5-FU increases the expression of Tβ10 at mRNA levels in KKU-M055 (A, B) and KKU-M214 (C, D). KKU-M055 treated with (A) 5-20 µM of 5-FU for 5 days and (B) with 5 µM of 5-FU for 3, 5 and 7 days; KKU-M214 treated with(C) 5-50 μΜ 5-FU for 5 days and (D) with 50 μΜ of 5-FU for 3, 5 and 7 days. The data represent mean ± S.D. of three independent experiments. * P < 0.05 vs. the untreated control group.
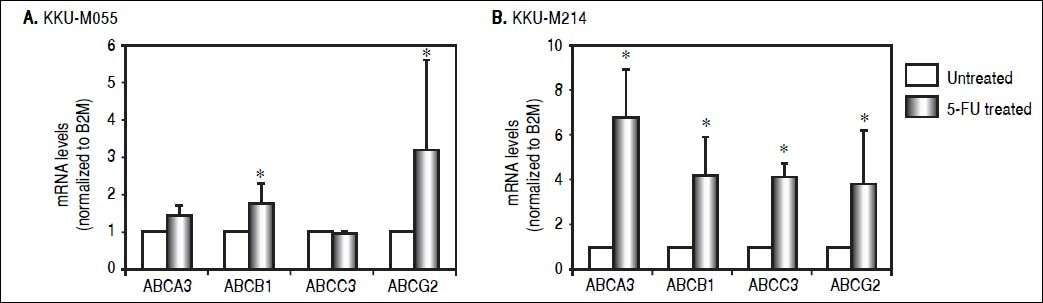
As ABC transporter is responsible for multiple drug resistance (MDR) against various drugs, it was questioned whether 5-FU treatment induced Tβ10 expression associated with ABC transporter. Expression levels of ABCB1, ABCG2 ABCA3 and ABCC3 were determined in CCA cells untreated or treated with 5-FU. Using real-time RT-PCR, expression of endogenous ABC transporters, ABCB1 and ABCG2, were significantly increased in KKU-M055 cells treated with 5 μM of 5-FU for 7 days compared with those of the untreated cells (P < 0.05; Figure 3A). In a similar experiment, the ABC transporters tested were up-regulated in KKU-M214 cells treated with 50 μM of 5-FU for 5 days (Figure 3B).
ABC transporters were upregulated in the 5-FU treated CCA cell lines. The expressions of ABC transporters in CCA cells were analyzed using real time RT-PCR. (A) ABC transporters; ABCB1 and ABCG2 were upregulated in M055 treated with 5 μΜ of 5-FU for 7 days. The upregulation of ABC transporters was confirmed in (B) KKU-M214 cells treated with 50 μΜ of 5-FU for 5 days. The data represent mean ± S.D. of three independent experiments. * P<0.05 vs. the untreated control group.
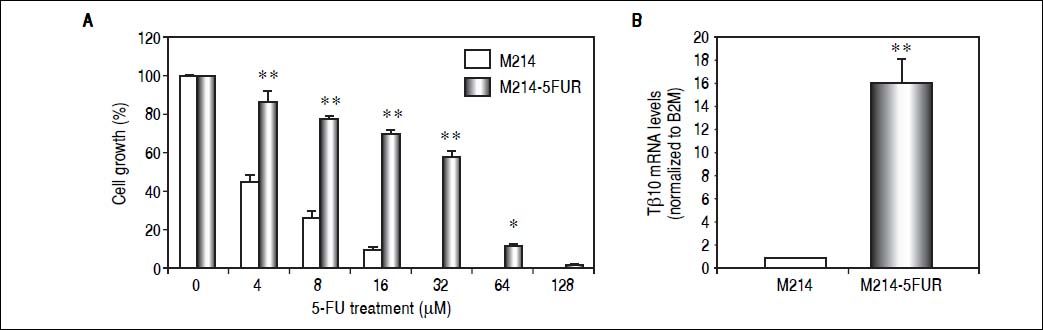
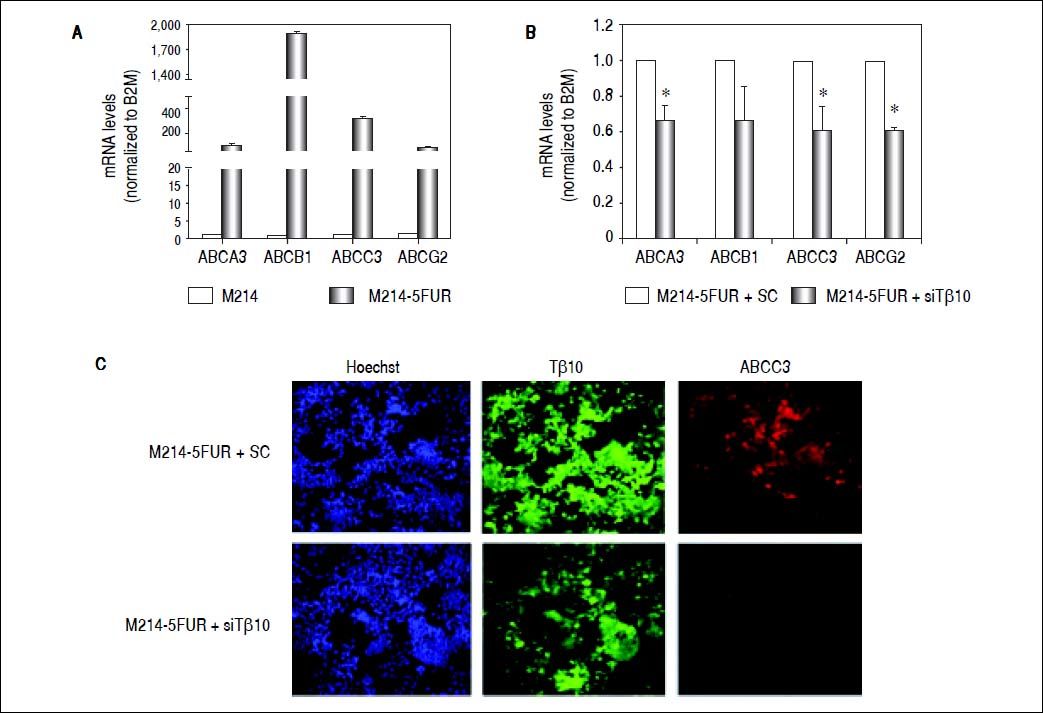
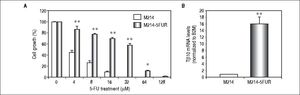
To assure the relationship between Tβ10 expression and 5-FU resistance in CCA cells, a 5-FU resistant cell line, designated as M214-5FURwas developed by continuous culturing KKU-M214 in a complete medium containing increasing concentrations of 5-FU from 1 x IC50 to 4 x IC50 of 5-FU. The effects of 5-FU against M214-5FUR and KKU-M214 were examined in cells cultured in various concentrations of 5-FU for 72 h (Figure 4A). As shown in figure 4B, M214-5FUR exhibited a 5-FU resistant phenotypewitha 16-fold higher expressionof Tβ10, as compared to the parental cells, KKU-M214. This indicates the involvement of Tβ10 in mediating resistance to 5-FU in CCA cells.
Knockdown of Tβ10 re-sensitizes chemosensitivity for 5-FU in M214-5FUR resistance cell line. (A) The sensitivity to 5-FU and Tβ10 expression of M214-5FUR cells were determined in comparison to M214 parental cells. CCA cells were incubated with various concentrations of 5-FU for 72 h and the cell viability was determined using the MTT assay. (B) Expression levels of Tβ10 in M214-5FUR and the parental cells were determined using real time RT-PCR. The data represent mean ± S.D. of three independent experiments. *P < 0.05 vs. the untreated control group.
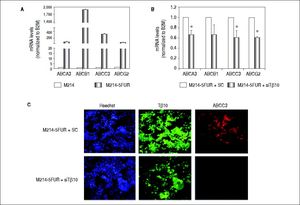
It is well known that ABC transporters are involved in development of resistance to multiple drugs in many cancers. To investigate whether Tβ10 mediated 5-FU resistance in 5-FU resistant cells associated with ABC transporters, the expression levels of ABC transporters were determined in M214-5FUR and the parental cells. Expressions of ABC transporters were extremely high in the 5-FU resistant clone compared to those of the parental cells (P < 0.05; Figure 5A). To ascertain the association observed, the expression of Tβ10 in M214-5FUR cells was suppressed and the expression levels of ABC transporters were determined. As shown in figure5B, siRNA targeted to Tβ10 significantly reduced expression of ABC transporters tested in the M214-5FUR cells (P < 0.05). The observation was confirmed using immuno-cytofluorescent staining for Tβ10 and ABCC3 expression. As compared with the control cells, suppression of Tβ10 expression using si-Tβ10 significantly reduced the expression of Tβ10 and ABCC3 in the si-Tβ10 treated M214-5FUR cells (Figure 5C). These results suggest that Tβ10 mediates expression of ABC transporters and that the RNAi of Tβ10 decreases ABC transporter expression.
Expression of ABC transporters are up-regulated in 5FU-resistant CCA cell line. (A) The mRNA levels of ABC transporters (ABCA3, ABCB1, ABCC3, ABCG2) in the 5-FU resistant cell line, M214-5FUR, were compared with those of the parental cells, KKU-M214. (B) Suppression of Tβ10 expression using si-Tβ10 significantly decreased the expression of ABC transporters in M214-5FUR cells compared with the scrambled controls (SC). The data represent mean ± S.D. of three independent experiments. * P < 0.05 vs. the SC control group. (C) Immunocytochemistry of Tβ10 (green) and ABCC3 proteins (red) in M214-5FUR cells treated with siTβ 10 (+siTβ10) or scrambled controls (+SC). Cell nuclei were demonstrated by Hoechst staining (blue). Silencing of Tβ 10 in 5-FU resistance cell line revealed reduced expression of ABCC3 when compared with the scrambled control group. Origina magnification x200.
The findings of the present study provide the first evidence of the clinical significance of Tβ10 as a predictive factor for 5-FU resistance in CCA. Increased expression of Tβ10 was observed in CCA cell lines (KKU-M055 and KKU-M214), treated with 5-FU and in a 5-FU resistant CCA cell line (KKU-M214-5FUR). The underlying mechanism of Tβ10 related 5-FU resistance in CCA was shown to be via the expression of ABC transporters.
5-FU is widely used in the treatment of a variety of cancers including CCA. 5-FU alone or 5-FU-based formulas are the commonly administered regimens for CCA patients.21,22 The outcome of the 5-FU therapy, however, may be unfavorable and overall efficiency following 5-FU treatment in patients presenting with advanced disease is still unsatisfactory.23–25 The main therapeutic complications which limit the successful outcomes of 5-FU treatment in most cases is drug resistance, the mechanismof which, to date remains largely unknown.
In the present study, it was shown for the first time that expression of Tβ10 corresponded to the competency of 5-FU resistance in CCA cells. CCA cells responded to the 5-FU cytotoxicity in dose and time dependent manners. Increasing levels of Tβ10 expression were significantly revealed in CCA cells treated with 5 μM 5-FU in KKU-M055 and 10-20 μM 5-FU in KKU-M214 cells for 5 days. The increasing levels of Tβ10 expression in cells treated with high concentrations of 5-FU was not observed, as in these conditions 5-FU dramatically induced cell death. The association of Tβ10 expression and 5-FU resistant phenotype was confirmed from the finding that the 5FU resistant clone, M214-5FUR cells, exhibited much higher levels of Tβ10 expression than the parental clone.
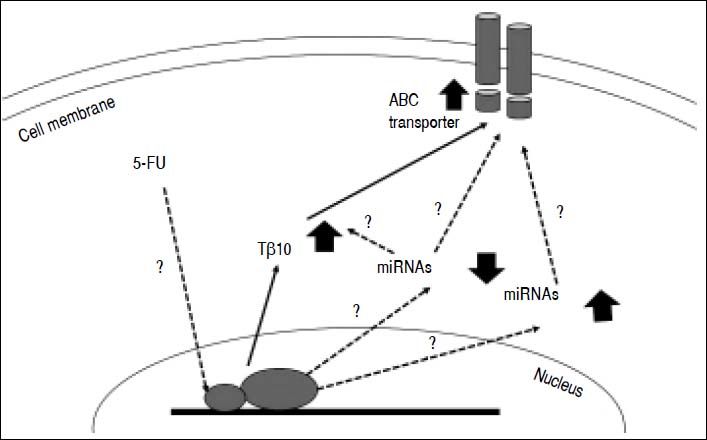
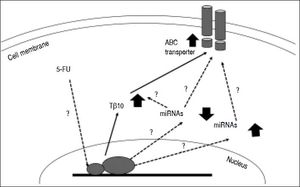
Untilthis research, there are only a few studies related to the mechanisms underlying chemotherapeutic drug resistance in CCA. Little is known about the connection between thymosin-β and the drug resistance mechanism. Over expression of membrane transport proteinsis suggested for chemotherapeutic drug resistance in CCA. These include ABC transporters which act as efflux pumps for anticancer drugs18,20,26 oralter drug targets or enzymes in the metabolism of 5-FU.17,27,28 Recently, there is increasing evidence suggesting a role for miRNAs in the cholangiopathies [reviewed in29-31] and chemoresistance. For instance, miR-29b, miR-205, and miR-221 were shown to modulate the sensitivity of CCA cell line, HuH28, to Gemcitabine,32 while MiR-200b was reported to contribute to CCA chemoresistance by modulating the chemotherapy-induced apoptosis.33miRNAs also play role in cellular drug disposition and chemosensitivity through regulation of ABC transporters.34–36 hsa-miR-1291 modulates chemosensitivity of pancreatic carcinoma PANC-1 cells through regulation of ABCC1 expression,37 where as underexpression of miR-328 contributed to tumor resistance to chemotherapy in glioblastomamultiforme by regulating expression of ABCG2.38 miRNAs may directly or indirectly involve in 5-FU resistance of CCA by modulating the expressions of Tβ10 and/or ABC transporters (Figure 6). How miRNAs modulate the 5-FU resistance in CCA cells and regulate Tβ10 and ABC transporters is challenging and is, however, beyond the scope of this study.
Postulated mechanism of Tβ10 mediated 5-FU resistance of CCA cells. Expression of Tβ10 is induced by 5-FU and persuades 5-FU resistance of CCA cells via increased expression of ABC transporters. miRNA regulatory network is possibly contribute to this process. Solid arrows = defined in the present study; dotted arrows with ? = theoretical proposed with undefined mechanism.
CCAs are heterogenous cancers with a very poor prognosis. Adjuvant therapy is widely recommended for postoperative patients who have positive margins or those who have had a complete resection but with node-positives [reviewed in 2]. Prediction of chemotherapy resistant status may enhance the benefit of the treatment. Using PCR based technology, the expression levels of Tβ10, ABC transporters and chemoresistant related miRNAs may be used as predictive biomarkers for chemoresistance in CCA patients who have undergone surgical resection. The similar strategy can also be offered for unresectable CCA patients, as these markers can be investigated from circulating tumor cells39–41 or circulating miRNAs in blood or bile.30
Tβ10 is a small actin-binding protein that induces depolymerization of the intracellular F-actin networks. Alteration of Tβ10 expression has been shown to affect the balance of cell homeostasis, e.g., cell growth, cell death, cell attachment and cell migration.42–44Accumulated reports indicate the association of Tβ10 and cancer, however, the function of Tβ10 appears to be different in different cancer cells. It has been shown to be involved in cell proliferation, apoptosis and vascularization of cancer cells as well as cell metastasis. Even though the study of Tβ10 and cancer is of limited, the association of Tβ10 expression and poor prognosis of cancer patients has been collectively reported. Recently, Tβ10 was shown to inhibit the apoptosis and promote cell proliferation in non-small cell lung cancer cell lines.45 Contribution of Tβ10 to the malignant progression was shown in the patients with hepatocellular carcinoma46 and papillary thyroid carcinoma.47 High expression of Tβ10 expression in hepatocellular carcinoma tissues was significantly related to advancingthe TNM stage, shorter overall survival and disease-free survival of the patients. In addition, high expression levels of Tβ10 correlated with lymph node metastasis in papillary thyroid carcinoma, especially in the central neck region.
High expressions of ABC transporters were observed in CCA cells treated with 5-FU and in the 5FU-resistant cells. Decreasing Tβ10 expression using si-RNA also reduced the expression levels of ABC transporters as demonstrated using real-time RT-PCR and immunocytochemical staining. Hence, Tβ10 developed 5-FU resistance in CCA cells may be, in part, via enhanced expression of ABC transporters (Figure 6). A comprehensive understanding of the possible molecular mechanisms and signal pathways involved Tβ10 and multidrug resistance of 5-FU in CCA, however, is warranted to be explored. There may be complicated regulatory mechanisms of Tβ10, multidrug resistance-related genes and miRNAs regulatory networksthat remain undefined and require further elucidation.
To predict chemotherapy resistant status is important to achieve a satisfactory treatment for patients. The present novel finding in Tβ10 connected with drug resistance may provide a new insight therapeutically valuable for Tβ10 as a predictive biomarker of 5-FU chemoresistance and may probably be the targetfor sensitizing chemotherapy treatment, especially with 5-FU in CCA patients. Further study is required for a better understanding of the involvement of Tβ10 and multidrug resistance in chemoresistance in CCA.
Abbreviations- •
5-FU: 5-Fluorouracil.
- •
ABC: adenosine triphosphate-binding cassette.
- •
CCA: cholangiocarcinoma.
- •
FUTP: 5-Fluorouracil-ribonucleoside triphosphate.
- •
M214-5FUR: M214-5FU-resistant.
- •
MDR: multiple drug resistance.
- •
miRNA: microRNA.
- •
MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
- •
Tβ10: thymosin β10.
This study was supported by the TRF Senior Research Scholar Grant to S. Wongkham, Thailand Research Fund and KhonKaen University (RTA5780012) and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Center of Excellence in Specific Health Problems in Greater Mekong Sub-region cluster (SHeP-GMS) to C. Wongkham (NUR-582013).
AcknowledgementsWe would like to acknowledge Prof. James A Will, University of Wisconsin, for editing the manuscript via the Faculty of Medicine Publication Clinic, KhonKaen University, Thailand. S Sribenja was supported by the research assistantship from the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, SHeP-GMS (PD PD55210) through S Wongkham.