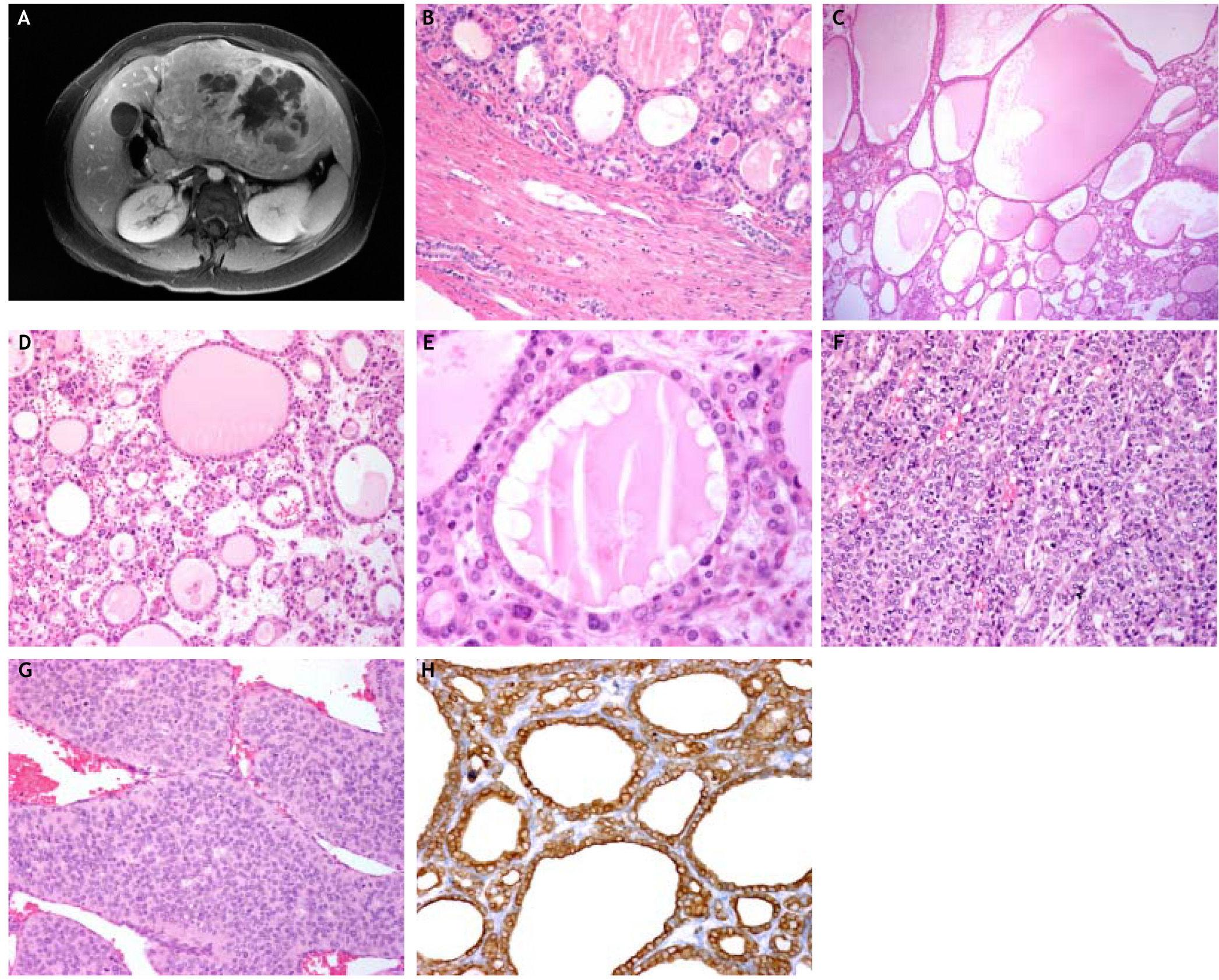
We report the case of a 26-year-old woman with a 19 cm malignant hepatic neoplasm with morphological features that closely resembled a follicular thyroid carcinoma. Despite this, it was interpreted as a cholangiocarcinoma due to the absence of a primary thyroid tumor and the lack of thyroglobulin and TTF-1 immunoreactivity by the hepatic tumor. The left hepatic lobectomy specimen showed an encapsulated and multinodular gray-white mass with cystic and hemorrhagic areas. Microscopically, it displayed predominant macro and microfolicullar patterns with focal solid, trabecular and insular areas. The small and distended follicles contained a colloid-like secretion and were lined by low cuboidal cells with scant cytoplasm, round or oval hyperchromatic nuclei with fine chromatin. The solid areas, trabecular and insular structures were similar to those of follicular or papillary thyroid carcinomas. In addition, some of the neoplastic cells had clear nuclei with occasional grooves. The tumor was positive for cytokeratin (CK) 7, CK 19 and CD138, and negative for TTF-1, thyroglobulin, Hepar-1, Glypican-3, alpha-fetoprotein and neuroendocrine markers. A thyroid neoplasm was excluded clinically and by ultrasound and computed tomography. Although, the residual hepatic parenchyma was initially not cirrhotic, the patient eventually developed cryptogenic cirrhosis. The patient received adjuvant chemotherapy and died of metastatic disease 18 months after surgery. The thyroid-like pattern broadens the morphologic spectrum of cholangiocarcinoma.
Cholangiocarcinoma (CHC) is a malignant epithelial tumor arising in the intrahepatic bile ducts accounts for 8 to 10% of all malignant hepatic tumors. Cholangiocarcinomas are more frequent in men than in women (60-70%) and their mean age at presentation is in the seventh decade.1 A variety of microscopic patterns have been described in CHC.2
The purpose of this report is to describe the second case of a new morphological variant of CHC that resembles a thyroid carcinoma. In addition, we compare the incidence of hepatocellular carcinoma and cholangiocarcinoma recorded in the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute from 1992 through 2008.
Clinical HistoryA 26-year-old woman presented with abdominal distention and pain associated with occasional vomiting of one year duration. During this period, she did not loose weight. The thyroid gland and lymph nodes were not enlarged. An abdominal ultrasound and a computed tomography (CT) showed a left hepatic mass that measured 16 cm. The magnetic resonance showed a heterogeneous neoplasm with solid and cystic areas as well as a nodule in the right adrenal gland thought to be metastatic (Figure 1A). The residual hepatic parenchyma was not cirrhotic. The transaminases, alkaline-phosphatase, bilirrubines, viral hepatitis antibodies and auto-antibodies were normal or negative. The alpha-fetoprotein (AFP) serum levels were normal. Because of the age of the patient the neoplasm was clinically diagnosed as a probable hepatoblastoma. A left hepatic lobectomy was performed.
A.The magnetic resonance shows a large mass with solid and cystic areas. The residual hepatic parenchyma is not cirrhotic. B. An encapsulated tumor with follicular pattern replaced the hepatic parenchyma. Residual intrahepatic bile ducts are seen within the capsule (H&E stain, × 150). C. The distended follicles contained a colloid-like substance and resemble a goiter or the macrofollicular variant of papillarythyroid carcinoma (H&E stain, × 150). D. The varying sized neoplastic follicles contained abundant colloid-like secretion, some of which showed a few foamy macrophages (H&E stain, × 150). E. The follicles are lined by low cuboidal cells some with clear nuclei. The intrafollicular colloid-like secretion showed peripheral vacuolation mimicking a follicle of goiter (H&E stain, × 250). F. The trabecular areas showed cells with clear nuclei, some with grooves similar to those of papillary thyroid carcinoma (H&E stain, × 150). G. Insular areas with mitotic figures similar to those of papillary and follicular thyroid carcinomas (H&E stain, × 250). H. The neoplastic cells are diffusely and strongly positive for cytokeratin 7 (Immunostain, × 150).
The hepatic lobectomy specimen showed an encapsulated and multinodular gray-white mass with cystic and hemorrhagic areas measuring 19 cm in greatest dimension. Microscopically, it had a thickened capsule and was composed of a predominant macro and microfolicullar pattern (70%) with solid, trabecular and insular areas (Figures 1B-1C). The follicles were lined by low cuboidal cells with scant cytoplasm, round or oval hyperchromatic nuclei with fine chromatin, resembling a thyroid neoplasm with follicular phenotype (Figure 1D). The vast majority of neoplastic follicles contained an abundant colloidlike secretion, some of which showed peripheral vacuolation and foamy macrophages that mimicked a hyperplastic goiter (Figure 1E). The interfollicular stroma had numerous microcalcifications but no psammoma bodies. The solid areas were composed of cords, trabecular and insular structures similar to those that occur in follicular or papillary thyroid carcinomas (Figures 1F-1G). Moreover, some of the cells had clear nuclei with occasional grooves similar to those of papillary carcinoma. However, no nuclear pseudoinclusions were present. Mitotic figures were seen only in the insular areas. Lymphovascular invasion was also seen. The neoplasm extended to the surgical margin and the portal vein. The residual hepatic parenchyma was not cirrhotic. However, portal spaces showed moderate fibrosis and a chronic inflammatory infiltrate composed predominantly of lymphocytes. Moreover, non-alcoholic steatohepatitis was not seen.
Material and MethodsMultiple sections of the hepatic tumor were available for review. From representative paraffin blocks additional sections were obtained for immunohisto-chemical analysis. The following antibodies were used:
- •
Hepar-1 (Biocare, Concord CA, 1:200).
- •
Glypican-3 (BioSB, Santa Barbara CA, 1:500).
- •
AFP (Dako, Carpinteria CA, 1:500).
- •
CK 7 (Biocare, Concord CA, 1:100).
- •
CK 19 (Biocare, Concord CA, 1:200).
- •
CD138 (Syndecan-1) (Biocare, Concord CA, 1:100).
- •
Chromogranin (Biocare, Concord CA, 1:300).
- •
Synaptophysin (Biocare, Concord CA, 1:100).
- •
CD56 (Biocare, Concord CA, 1:50).
- •
Thyroid Transcription Factor 1 (TTF-1). (Cell marque, Rocklin CA, 1:200), thyroglobulin (Biogenex, San Ramon CA, 1:500).
- •
HBME-1 (Dako, Carpinteria CA, 1:100).
Data were obtained from the SEER Program, which is a population-based registry that collects demographic, anatomic, morphologic, extent of disease, and survival information on patients with cancer. For this study data from de SEER program on cholangiocarcinoma and hepatocellular carcinoma from 1992 through 2008 were analyzed.
Immunohistochemical profileThe neoplastic cells expressed CK 7 (Figure 1H), CK 19 and CD138, and were negative for TTF-1 and thyroglobulin. The tumor also lacked reactivity for Hepar-1, Glypican-3, AFP, chromogranin, synaptophysin and CD56. This immunophenotype was consistent with cholangiocarcinoma and excluded a thyroid carcinoma.
Follow-upA metastatic thyroid carcinoma was excluded by physical examination of the neck, ultrasound and CT of the thyroid gland. The patient developed CHILD C stage cirrhosis and additional serological tests for viral hepatitis and autoimmune hepatic disease were performed, but were all negative. The diagnosis of cryptogenic cirrhosis was established.
The patient received adjuvant chemotherapy for 14 months, but she developed local recurrence. A repeat determination of serum AFP was normal. She died with massive hepatic recurrence and metastasis in the right adrenal gland and regional lymph nodes, 18 months after surgery. The clinical stage was considered as IVB (pT2a, N1, M1). An autopsy was not performed.
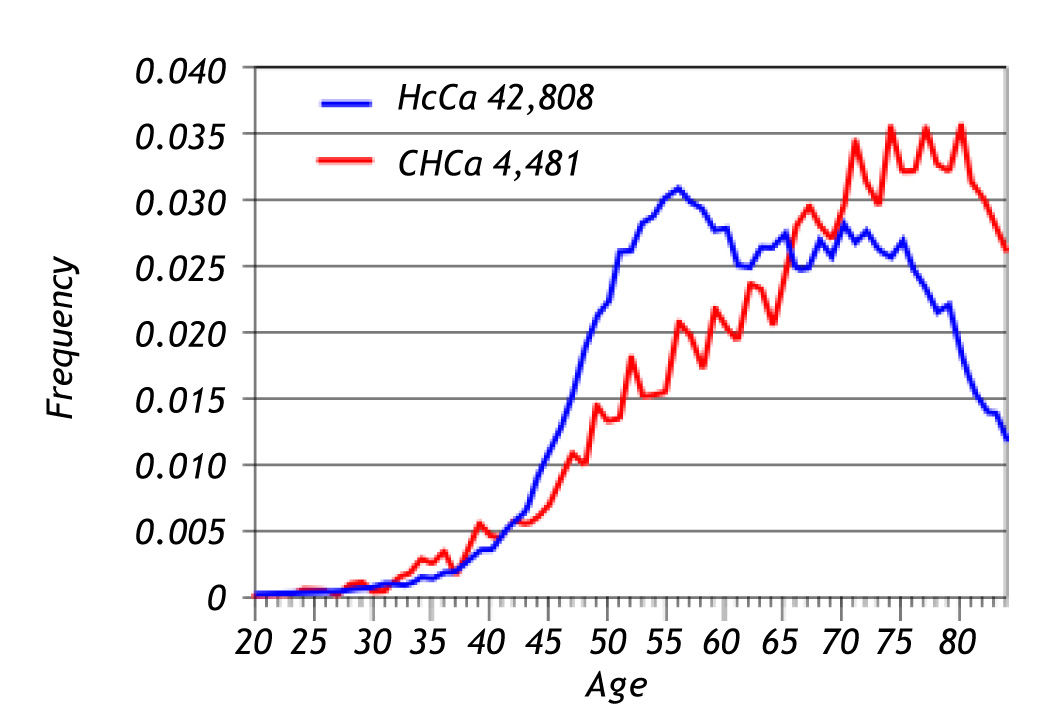
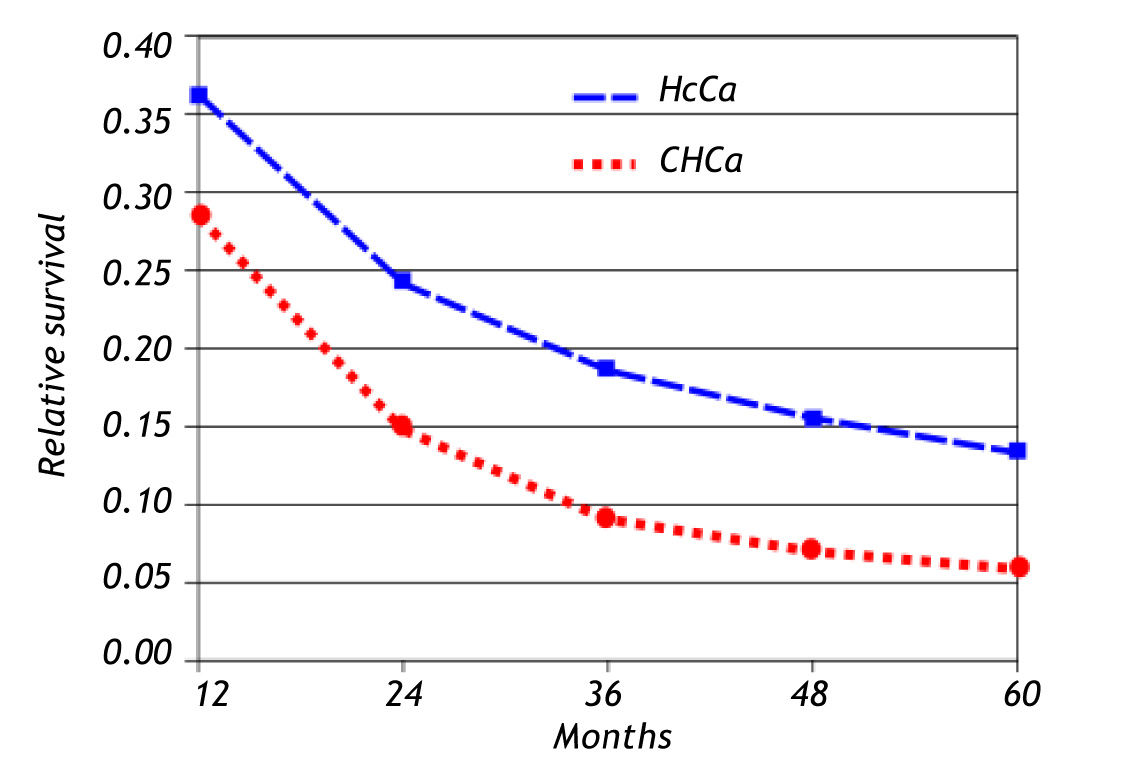
Seer dataFrom 1992 through 2008, there were 47,289 cases of carcinoma of the liver of which 42,808 (90.5%) were hepatocellular carcinoma and only 4,481 were cholangiocarcinoma (9.5%). The ratio of hepato-cellular carcinoma to cholangiocarcinoma was 10 to 1. The age at presentation was 54 years-old for hepatocellular carcinoma and 74 years-old for cholangio-carcinoma. Figure 2 summarizes these findings. The 5-year relative survival rate of patients with hepato-cellular carcinoma was 13% and for patients with cholangiocarcinoma it was 6% (Figure 3).
Cholangiocarcinoma is the second most common malignant neoplasm of the liver. The SEER data shows that the hepatocellular carcinoma/CHC ratio is 10:1. Both tumors are associated with a poor prognosis. The 5-year survival rate of patients with hepatocellular carcinoma is 13% and 6% for patients with CHC (Figures 2 and 3). The data reveal that the incidence of hepatocellular carcinoma has been increasing since 2000. This increase in incidence of hepatocellular carcinoma is attributed to a rise in the incidence of viral hepatitis C and related cirrhosis, whereas the incidence of CHC has remained relatively stable.
In contrast to hepatocellular carcinoma, CHC is usually not associated with cirrhosis. Only 5% of CHC arise in a background of chronic hepatitis C.1,2 Moreover, carcinomas of the extrahepatic bile duct are not associated with chronic hepatitis.3 A number of histologic patterns have been described in CHC including tubular, mucinous,2 adenosquamous,4 with clear cells,5 spindle-sarcomatoid6 and lymphoepithe-lioma-like.7,8 However, only one case reported previously had a thyroid-like growth pattern.9
The microscopic features of this hepatic tumor closely resemble a thyroid carcinoma. It had a predominant follicular pattern (70%) with trabecular and insular areas as described in papillary and follicular thyroid carcinomas.10,11 In some areas the distended follicles resembled those of the macrofollicullar variant of papillary carcinoma.10,12,13 In other areas, the tumor had features of papillary thyroid carcinoma with small follicles lined by low columnar cells with clear nuclei and occasional nuclear grooves but without nuclear pseudoinclusions. Moreover, the interfollicular tissue showed numerous microcalcifications but no psammoma bodies. Initially, the morphologic diagnosis of a metastatic well-differentiated thyroid carcinoma was strongly considered, but the absence of a primary tumor and the lack of thyroglobulin and TTF-1 immunoreactivity excluded such a diagnosis and favored the interpretation of cholangiocarcinoma. Thyroglobulin is a specific marker for benign and malignant tumors with follicular cell phenotype while TTF-1 is a homeodomain transcription factor normally expressed by thyroid follicular cells and pneumocytes and a sensitive but not specific marker for benign and malignant thyroid neoplasms with follicular phenotype. Moreover, the diagnosis of a metastatic thyroid carcinoma was excluded by ultrasographic and tomographic studies. Few cases of hepatic metastasis from malignant thyroid neoplasms had been reported.14-16 The differential diagnosis includes intrahepatic thyroid ectopic tissue, but in these cases the ectopic thyroid tissue is normal.14,17,18 Both metastastic tumors and ectopic thyroid tissue expressed TTF-1 and thyroglobulin.14-18
Other tumors included in the differential diagnosis were hepatocellular carcinoma with acinar or pseudoglandular pattern and neuroendocrine carcinoma.2 However, the lack of reactivity for Hepar-1, AFP and Glypican-3 ruled out a hepatocellular carcinoma.2,19 The lack of neuroendocrine markers (chromogranin, synaptophysin and CD56) excluded the possibility of neuroendocrine carcinoma.20,21
Cholangiocarcinoma is not the only neoplasm that shows a thyroid-like pattern. Tumors with thyroidlike morphology had been described in the breast and kidneys. In fact, some carcinomas of the breast superficially resemble a metastatic tall-cell variant of papillary thyroid carcinoma.22,23 Likewise, some renal cell carcinomas are similar to well-differentiated follicular thyroid carcinomas.24-26 In these organs, thyroid carcinoma metastases were excluded by the lack of reactivity for TTF-1 and thyroglobulin.22-27 Finally, a case of plasmacytoma with thyroid-like features has been reported, but morphologically this tumor was not similar to ours.28
Previously, Fornelli, et al.9 had described a primary intrahepatic thyroid-like CHC but without microcalcifications, trabecular or insular patterns. They reported a 52-year-old male with an 18 cm hepatic tumor with a pure follicular pattern that lacked thyroid follicular markers and expressed CK 7, CK 19, CAM 5.2 and CK AE1&AE. In addition, the thyroid gland did not harbor a malignant primary tumor.28
In conclusion, CHC represents the second most common malignant hepatic tumor, usually not associated with cirrhosis or hepatitis C, with variable morphological patterns and associated with poor prognosis. We report a CHC with microscopic features closely resembling a metastatic well-differentiated thyroid carcinoma with follicular, solid, trabecular and insular areas. The absence of a primary thyroid tumor, knowledge of the morphological features and the lack of thyroid markers in the liver tumor are useful in the diagnosis of this rare and new morphological variant of cholangiocarcinoma.
AcknowledgmentsThis paper was supported by La Fundación Clínica Médica Sur.