The association between type 2 diabetes, non-alcoholic fatty liver disease, and liver fibrosis is well established, but it is unknown whether complications of type 2 diabetes influence fibrosis levels. We defined the complications of type 2 diabetes by the presence of diabetic nephropathy, retinopathy, or neuropathy and aimed to evaluate their association with the degree of liver fibrosis measured by the fibrosis-4 (FIB-4) index.
Materials and MethodsThis is a cross-sectional study evaluating the association of type 2 diabetes complications with liver fibrosis. A total of 2389 participants were evaluated from a primary care practice. FIB-4 was evaluated as a continuous and categorical measure using linear and ordinal logistic regression.
ResultsPatients with complications were older, had higher hemoglobin A1c, and a higher median FIB-4 score (1.34 vs. 1.12, P<0.001). On adjusted analysis, type 2 diabetes complications were associated with higher fibrosis by continuous FIB-4 score (Beta-coefficient: 0.23, 95% confidence interval [CI]: 0.004-1.65) and demonstrated increased odds of fibrosis by categorical FIB-4 score (odds ratio [OR]: 4.48, 95% CI: 1.7-11.8, P=0.003), independent of hemoglobin A1c level.
ConclusionsThe presence of type 2 diabetes complications is associated with the degree of liver fibrosis, independent of hemoglobin A1c level.
The incidence of non-alcoholic fatty liver disease (NAFLD) and its more severe form, non-alcoholic steatohepatitis (NASH), is rising [1,2]. Patients with advanced fibrosis or cirrhosis from NASH are at increased risk of complications, including the development of hepatocellular carcinoma (HCC) [3,4]. Additionally, NAFLD leads to poorer quality of life and significantly increases healthcare costs and resource utilization [5–7].
NAFLD is prevalent but under-recognized in the primary care setting [8,9]. Certain high-risk populations, such as those with type 2 diabetes, are significantly more affected by NAFLD [10], but large-scale screening strategies are challenging to implement. Patients with type 2 diabetes have an increased risk of NAFLD, advanced fibrosis, and HCC compared to those without type 2 diabetes [11–13]. The development of NAFLD is thought to be multifactorial, of which metabolic syndrome and insulin resistance, as seen in type 2 diabetes, remain the cornerstone of its pathophysiologic mechanism [14–16]. In fact, the American Diabetes Association (ADA) recommends evaluating NAFLD in all patients with type 2 diabetes [17].
Screening all patients with type 2 diabetes is challenging. Targeting screening protocols to those with complications from diabetes may be the way forward. We know those with elevated hemoglobin A1c (HbA1c) levels, for example, have an associated increase in liver fibrosis [18]. However, whether the presence of type 2 diabetes-related complications influences the degree of fibrosis, independent of HbA1c, is yet to be determined. More clearly defining which patients with type 2 diabetes are at higher risk of fibrosis from NAFLD can help narrow targets for screening and improve screening uptake in primary care practices.
The fibrosis-4 (FIB-4) index, a non-invasive serologic test shown to predict advanced fibrosis, cirrhosis, cardiovascular events, and mortality in those with NAFLD [19–21], while also risk stratifying type 2 diabetes patients in the primary care setting [22–25]. We sought to evaluate whether the presence of type 2 diabetes complications is associated with higher degrees of liver fibrosis, measured by FIB-4.
2Materials and MethodsIn this cross-sectional cohort study, we collected information on all patients with type 2 diabetes from a primary care practice at Beth Israel Deaconess Medical Center (BIDMC) who had available FIB-4 score data (age, aspartate aminotransferase [AST], alanine aminotransferase [ALT], and platelet count). We collected patient demographics, laboratory variables, and clinical data from May 2019 to May 2021. Complications of type 2 diabetes were defined as a composite measure, including nephropathy, retinopathy, and neuropathy, identified by using the International Classification of Diseases (ICD)-10 coding. As previously proposed by Vallet-Pichard et al.[26], the categorical FIB-4 score was separated into three stages: limited fibrosis (<1.45), indeterminate fibrosis (1.45-3.25), and advanced fibrosis (>3.25). We excluded patients with known liver disease from primary biliary cholangitis (N=6), viral hepatitis A and B (N=88), autoimmune hepatitis (N=8) and alcohol-associated liver disease (ALD)(N=33) identified by ICD-10 codes. Problem lists were examined to identify alcohol and tobacco history. Medications were captured from patient medication lists within the record. Institutional Review Board approval was obtained to perform this study.
2.1Statistical methodsNormally distributed variables were reported as mean and standard deviation (SD) and compared using Student's T-test. Variables that were not normally distributed were reported as the median and interquartile range (IQR) and compared using the Wilcoxon ranksum test. The chi-square test was used to compare categorical variables, which were reported as percentages. We evaluated the association of type 2 diabetes complications with continuous and categorical FIB-4 score using univariable and multivariable linear and ordinal logistic regression models, respectively. To validate the FIB-4 scores with better non-invasive fibrosis metrics, using transient elastography (TE), we correlated continuous FIB-4 score with median liver stiffness measurement (LSM) scores using the Pearson correlation coefficient. An alpha level of 0.05 was used to determine statistical significance. Stata version 16.1 (StataCorp LLC, College Station, TX) was used for all analyses.
2.2Ethical statementWritten informed consent was not required for the conduction of this study as it did not involve the recruitment of human subjects. However, Institutional Review Board (IRB) approval at BIDMC (2019P000886) was obtained to conduct this retrospective chart review.
3ResultsOf those with type 2 diabetes (N=3855) in the BIDMC primary care database, 2389 (62%) had labs available to calculate a FIB4 score. After excluding patients with another known liver disease, 2254 subjects were included in the study. Forty-six percent of the study population had complications of type 2 diabetes (N=1026). Of these patients, 444 (20%) had retinopathy, 695 (31%) had neuropathy, and 422 (19%) had nephropathy. Table 1 demonstrates the characteristics of the study population. Those with complications from type 2 diabetes were older (68±12 vs. 63±13 years) and had higher HbA1c levels (7.9% [63 mmol/mol] vs. 7.3% [56 mmol/mol], P=0.005) compared to those without complications. However, there was no difference in the proportion of females between the two groups. However, patients with complicated type 2 diabetes had higher international normalized ratio (INR) and creatinine levels but lower low-density lipoprotein (LDL). Prescription of aspirin, statin, and insulin was more frequent among those with complications. Patients with type 2 diabetes complications had a higher median FIB-4 score (1.34 vs. 1.12, P<0.001). Once FIB-4 was categorized, those with complications had greater estimated fibrosis (low: 55% vs. 69%, indeterminate: 39% vs. 27%, advanced: 5.2% vs. 3.7% for complicated vs. uncomplicated type 2 diabetes, respectively).
Baseline characteristics of type 2 diabetes study population.
SD: standard deviation, IQR: interquartile range, BMI: body-mass index, HbA1c: hemoglobin A1c, ALT: alanine aminotransferase, AST: aspartate aminotransferase, INR: international normalized ratio, LDL: low-density lipoprotein.
(Normally distributed variables are reported as mean (SD) whereas median (IQR) is reported for variables that are not normally distributed.)
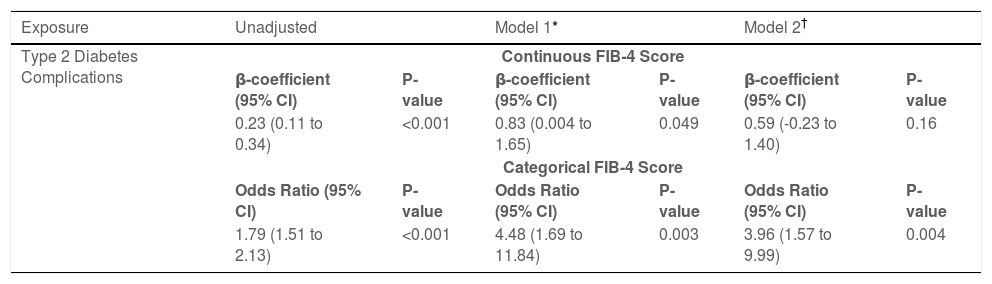
The presence of type 2 diabetes complications was associated with continuous and categorical FIB-4 scores in unadjusted and adjusted regression models (Table 2). On adjusted analysis accounting for a priori selected covariates including body-mass index (BMI), sex, HbA1c, and alcohol use, type 2 diabetes complications were significantly associated with fibrosis by continuous FIB-4 score (Beta-coefficient 0.23, 95% confidence interval [CI]: 0.004-1.65, P=0.049) and demonstrated increased odds of fibrosis by the categorical FIB-4 (odds ratio [OR]: 4.48, 95% CI: 1.7-11.8, P=0.003). Additionally, in another model adjusting for covariates chosen through stepwise selection, type 2 diabetes complications remain significantly associated with increased odds of fibrosis by categorical FIB-4 score (OR: 3.96, 95% CI: 1.57-9.9, P=0.004).
Association of type 2 diabetes complications with Fibrosis-4 index.
| Exposure | Unadjusted | Model 1* | Model 2† | |||
|---|---|---|---|---|---|---|
| Type 2 Diabetes Complications | Continuous FIB-4 Score | |||||
| β-coefficient (95% CI) | P-value | β-coefficient (95% CI) | P-value | β-coefficient (95% CI) | P-value | |
| 0.23 (0.11 to 0.34) | <0.001 | 0.83 (0.004 to 1.65) | 0.049 | 0.59 (-0.23 to 1.40) | 0.16 | |
| Categorical FIB-4 Score | ||||||
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | |
| 1.79 (1.51 to 2.13) | <0.001 | 4.48 (1.69 to 11.84) | 0.003 | 3.96 (1.57 to 9.99) | 0.004 | |
FIB-4: fibrosis-4 index, CI: confidence interval, HbA1c: hemoglobin A1c.
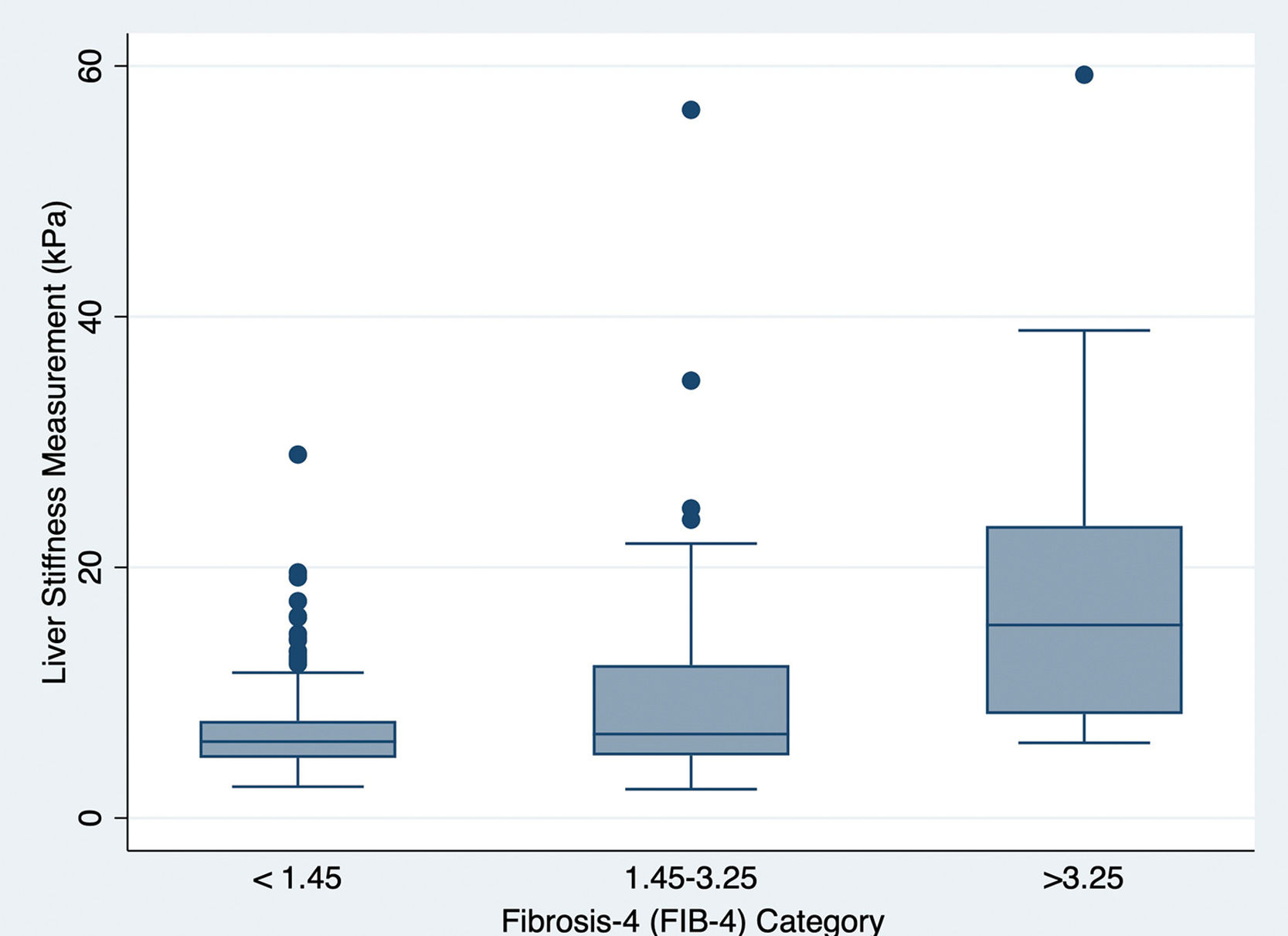
Of the study population, 276 (12%) patients also had LSM from TE. A Pearson correlation coefficient between continuous FIB-4 score and LSM from TE was 0.51 (P<0.0001), indicating a moderate correlation between the two metrics in this study population of those with type 2 diabetes within the primary care setting. Fig. 1 graphically demonstrates the correlation of LSM with the three FIB-4 categories.
4DiscussionType 2 diabetes is an important associated factor of NAFLD and its complications [11,13], but screening strategies are suboptimal [8]. Targeted liver fibrosis evaluation for those with type 2 diabetes who are at the highest risk of NAFLD may help narrow screening strategies. In this study, we demonstrate patients with type 2 diabetes complications, namely nephropathy, retinopathy, and neuropathy, are at increased risk of hepatic fibrosis and may represent a suitable screening target in primary care settings.
Optimal screening strategies for fibrosis from NAFLD in the community are under study. However, FIB-4 is an easily accessible and reliable tool comprising simple laboratory parameters, which predicts advanced fibrosis and its related complications in those with type 2 diabetes [22]. In two recent studies by Vieira Barbosa et al., an elevated FIB-4 score was independently associated with HCC, liver transplantation, cardiovascular events, and all-cause mortality [19,20]. FIB-4 can therefore be used to screen and risk stratify patients within the community who may be at risk of fibrosis from NAFLD.
In this study, we demonstrate a significantly higher degree of fibrosis, measured by FIB-4, in the presence of type 2 diabetes complications. In this primary care cohort, patients with complications of type 2 diabetes had 4.5 times greater odds of fibrosis independent of HbA1c and other important covariates. When measuring the FIB-4 score as a continuous outcome, those with complications had higher degrees of fibrosis (Beta-coefficient 0.83, P=0.049). Additionally, in those who had available TE measurements, we demonstrated a significant correlation of FIB-4 scores with LSM by TE (P<0.0001), reinforcing the value of FIB-4 for the estimation of hepatic fibrosis in this population.
These results suggest type 2 diabetes complications, defined by the presence of diabetic neuropathy, retinopathy, or nephropathy, are an important consideration when evaluating patients for NAFLD-related risk, particularly in resource-limited settings where more advanced diagnostic tools, such as TE or liver biopsy, may not be available and where widespread implementation of screening may be challenging. Although non-invasive assessment of fibrosis in those with type 2 diabetes is limited in accuracy [27], the FIB-4 score reliably serves as an initial screening method to inform the need for subsequent fibrosis assessment and/or specialist referral. Validated non-invasive measures, such as the Hepamet fibrosis scoring system, are more accurate [28] but require specialized parameters (i.e., homeostatic model assessment score) not readily available in resource-limited settings. Evaluating those with type 2 diabetes complications using the FIB-4 score may help primary providers or healthcare systems identify those patients most at risk for liver-related complications from NAFLD and focus resources accordingly (e.g., sub-specialty consultation). Fig. 2 illustrates a proposed algorithm for screening high-risk patients with type 2 diabetes in resource-limited settings.
Although the results are revealing, limitations of this study exist. First off, a more granular alcohol use history was not reliably captured in the record. Although those with known ALD were excluded, individuals with high-risk drinking behaviors may have been inadvertently included in the study population leading to misclassification. Additionally, patients who did not have available FIB-4 labs were not evaluated in this study, which may lead to selection bias. FIB-4 is also less reliable in those above 65 years of age and should be interpreted with caution in this subgroup or be used with different cut-off values, as suggested by McPherson et al. [29]. Additional factors, such as gamma-glutamyl transferase levels, glycemic/metabolic variability, or the presence of diabetic foot ulcer, are associated with type 2 diabetes, NAFLD, or related conditions but were not captured reliably in our record and may serve as future targets for study. Lastly, the retrospective nature of the study does not permit inquiry into the indication for laboratory testing. We adjusted for measured covariates; however, unmeasured covariates may still exist. Estimating fibrosis levels with the gold standard diagnostic method of liver biopsy is challenging when studying patients in the primary care community, but a preponderance of evidence now suggests FIB-4 as a reliable screening tool.
5ConclusionsDespite these limitations, the results from this study support the use of FIB-4 as a straightforward and low-cost method for the triage of diabetic patients at greatest risk for liver-related complications. In settings where evaluation with advanced non-invasive modalities for fibrosis staging and/or hepatology consultation is not available for all patients at risk of chronic liver disease, the FIB-4 score can serve as an effective method for identifying the patients at greatest risk. In conclusion, patients with type 2 diabetes, and particularly those with complications, should have non-invasive liver fibrosis evaluation as a component of their systematic type 2 diabetes screening protocols.
FundingHDT is supported by AASLD Transplant Hepatology Award. ZF is supported by the AASLD Foundation. MPC receives research funding from Sonic Incytes, Gilead, and Mallinckrodt, as well as consulting fees from Gilead, Sonic Incytes, Mallinckrodt and Alexion. This work was completed independently of the above funding sources.
Declaration of interestNone.
Author contributionsHirsh D. Trivedi: Conceptualization, Formal analysis, Data curation, Writing – review & editing. Qua Tran: Formal analysis, Data curation. Zachary Fricker: Writing – review & editing, Supervision, Visualization. Michael P. Curry: Supervision, Visualization. Jonathan X. Li: Conceptualization, Supervision, Visualization. Michelle Lai: Conceptualization, Supervision, Visualization.