The Baveno VI criteria to rule out varices needing treatment (VNT) was introduced in 2015. Soon after, the expanded Baveno VI and stepwise platelet-MELD criteria were proposed to be equal/more accurate in ruling out VNT; however, neither has been widely validated. We aimed to validate all 3 criteria in compensated cirrhosis from assorted causes.
Materials and methodsWe conducted a cross-sectional study including all adult compensated cirrhotic patients who underwent endoscopic surveillance at our center from 2014 to 2018 and had transient elastography (TE), and laboratory data for criteria calculation within 6 months of endoscopies. Exclusion criteria were previous decompensation, unreliable/invalid TE results, and liver cancer. The diagnostic performances of all criteria were evaluated.
ResultsA total of 128 patients were included. The major cirrhosis etiologies were hepatitis C and B (37.5% and 32.8%, respectively). VNT was observed in 7.8%. All criteria yielded high negative predictive values (NPVs)>95%, missed VNT was observed in 2%, 2.7%, and 2.8% in the original, expanded Baveno VI, and platelet-MELD criteria, respectively. The expanded Baveno VI and the platelet-MELD criteria yielded significantly better specificities and could spare more endoscopies than the original Baveno VI criteria.
ConclusionsAll 3 criteria showed satisfactorily high NPVs in ruling out VNT in compensated cirrhosis from various causes. The expanded Baveno VI and the platelet-MELD criteria could spare more endoscopies than the original Baveno VI criteria. From a public health standpoint, the platelet-MELD criteria might be useful in a resource-limited setting where TE is not widely available.
Cirrhosis, late-stage fibrosis caused by chronic inflammation of the liver, is a common problem in general practice. It can be diagnosed by clinical suspicion plus imaging findings compatible with cirrhosis [1–3], or liver biopsy [3]. The significant and fatal complications of cirrhosis are portal hypertension, end-stage liver failure, and hepatocellular carcinoma. A recent study showed that cirrhosis is the 13th leading cause of death globally [4]. The risk of variceal bleeding (esophageal varices (EV) and/or gastric varices (GV)) is 5–15% per year in cirrhotic patients [5], and the mortality rate is at least 20% at 6 weeks after a bleeding episode [6]. Nonetheless, EV can be screened and early intervention can be provided to patients with varices needing treatment (VNT) before a bleeding episode occurs. The gold standard procedure for the diagnosis of VNT is esophagogastroduodenoscopy (EGD). Traditionally, it is recommended that all cirrhotic patients should undergo EGD surveillance for varices at the time of diagnosis [7], in which 6–12% of VNT is expected to be seen in compensated cirrhotic patients.
Despite it being the standard recommendation, EGD is an invasive procedure which carries the risk of uncommon but significant complications, such as pain, bleeding, and perforation. Moreover, it needs to be performed by well-trained healthcare providers in well-equipped centers.
In 2015, the international consensus meeting on portal hypertension published the “BAVENO VI recommendation” [8]. The non-invasive screening method that uses the liver stiffness measurement (LSM) combined with platelet count was introduced with this recommendation in order to avoid unnecessary EGDs. The Baveno VI criteria were validated in subsequent studies showing <5% missed VNT and thus sparing 11–39% EGD [9–12]. Later in 2017, the expanded Baveno VI and stepwise platelet-MELD criteria were proposed in order to spare more EGDs while balancing the risk of missed VNT [13,14]. This study aims to validate these 3 criteria (the Baveno VI, the expanded Baveno VI, and the stepwise platelet-MELD criteria) in compensated cirrhotic patients from various etiologies.
2Materials and methodsWe conducted a single center, cross-sectional study at our institute, which is a tertiary care center. We included all adult (age≥18 years old) compensated cirrhotic patients who underwent routine EGD for EV surveillance at our endoscopic centers between January 2014 and October 2018 and had transient elastography (TE) performed within 6 months of EGD. Exclusion criteria were: (1) patients with a history of decompensation episode(s) e.g. Child–Pugh C cirrhosis, or Child–Pugh B cirrhosis with ascites/variceal hemorrhage/hepatic encephalopathy, (2) coexisting liver tumor during the study period, (3) patients who had limitations to undergo and/or interpret the results of TE i.e. congestive heart failure, ascites, abdominal wound at the TE examination site, aminotransferase levels≥5 times of upper normal limit [15–17], total bilirubin ≥2mg/dL, narrow intercostal spaces, and patients with a body mass index (BMI)≥25mg/m2 in whom size M probe was used in TE [18], (4) patients whose TE validity criteria [19,20] were not met (success rate >60%, and an IQR <30%), and (5) no available laboratory data for calculating all 3 criteria within 6 months of endoscopy. All transient elastography was performed with a Fibroscan® (Echosens, France). TE was performed by trained doctors at the right lobe of the liver after patients had fasted for at least 2h.
The criteria validated in this study were the Baveno VI (original Baveno VI), the expanded Baveno VI, and the stepwise platelet-MELD criteria, which defined patients with a low risk of having VNT as follows:
- -
Original Baveno VI[8]: liver stiffness measurement (LSM)<20kPa (determined by TE) and a platelet count >150,000/mm3
- -
Expanded Baveno VI[13]: liver stiffness measurement (LSM) <25kPa (determined by TE) and a platelet count >110,000/mm3
- -
Stepwise platelet-MELD[14]: platelet>150,000/mm3 or platelet≤150,000/mm3 but MELD=6.
Varices needing treatment (VNT) is defined as the presence of EV≥F2, or EV of any size with high-risk stigmata, or any size of GV on EGD. Cirrhosis was diagnosed by imaging (ultrasonography, computed tomography, magnetic resonance imaging) or liver biopsy.
The study protocol was approved by the Institutional Human Research Ethics Committee (HREC). The study protocol was approved in 2017, and we collected patients' data from 2014 to 2018. Hence, for patients who underwent EGD for EV surveillance in 2014–2016, we retrospectively collected the endoscopic reports, transient elastography, and laboratory data and the informed consent was waived; for the patients who underwent EGD in 2017–2018, all the data were prospectively collected, and all the patients who were enrolled in 2017–2018 (after the study protocol was approved by the HREC) provided informed consent before participating in the study.
2.1Statistical analysisSample size calculation was based on the sample size estimation in diagnostic test studies of biomedical informatics [21]. A total of 106 patients were calculated with a significance level of 0.05, yielding an 80% power to detect 99% sensitivity in the Baveno VI criteria for ruling out VNT with a precision of 0.06. Statistical analysis was performed using program R version 3.4.4. Descriptive statistics were used for baseline demographic data. Quantitative measurements were shown as mean±SD or median with IQR according to the distribution of observed values. Categorical variables were expressed using numbers and percentages. The diagnostic performance of the Baveno VI, the expanded Baveno VI, and the stepwise platelet-MELD criteria, including sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV), were reported as percentages. Using the Baveno VI criteria as a reference, Sn and Sp of the remaining 2 criteria were compared with the original Baveno VI using McNemar chi-square test, and the relative predictive value method [22] was used in the comparisons of PPVs and NPVs. A p-Value <0.05 was considered statistically significant.
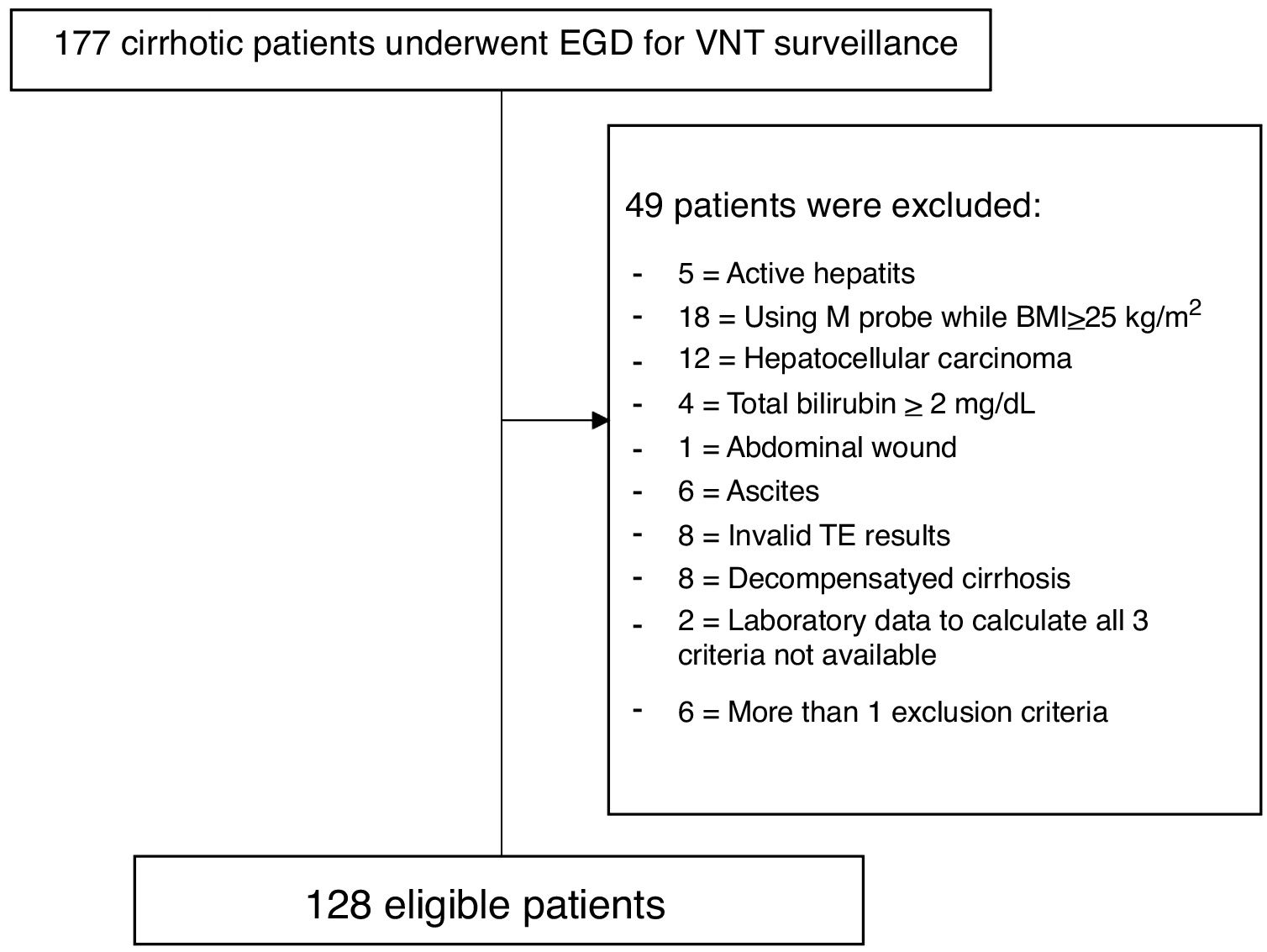
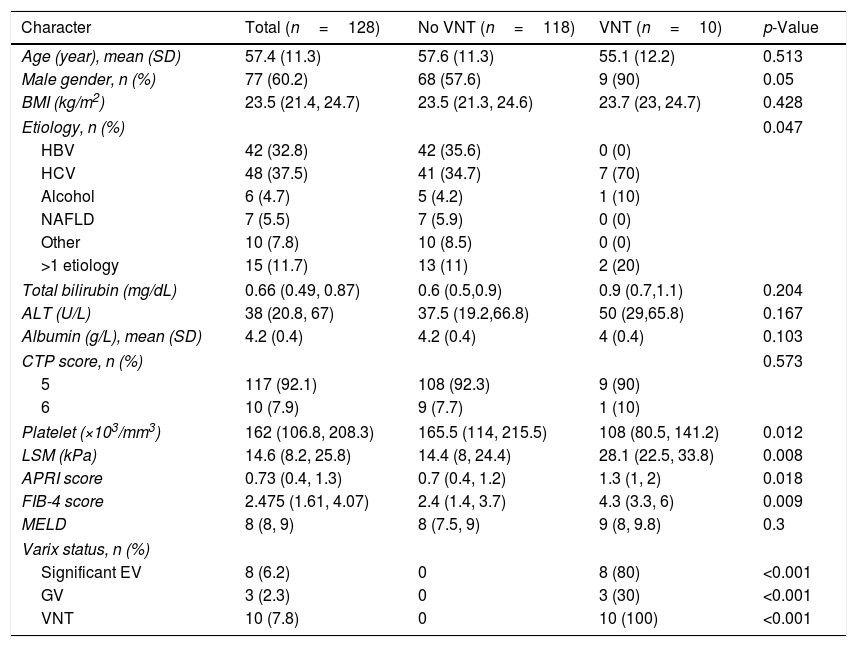
3Results3.1Baseline characteristics and prevalence of VNTDuring the study period, 177 cirrhotic patients were assessed for eligibility; 49 patients were excluded according to the exclusion criteria (Fig. 1). A total of 128 patients were included in this study. Baseline characteristics of eligible patients are shown in Table 1. Sixty percent were male, with a mean age of 57.4 years old, and all were in Child–Pugh A. The major etiologies of cirrhosis were chronic viral hepatitis C (HCV) and B (HBV) infection (37.5% and 32.8%, respectively). The other etiologies from Table 1 comprise Wilson disease (n=1), autoimmune hepatitis (AIH) (n=7), and cryptogenic (n=2). For the patients with >1 etiology, most had viral hepatitis as follows: HBV-HCV co-infection (n=3), HBV+alcohol (n=3), HCV+alcohol (n=3), HBV+non-alcoholic fatty liver disease (NAFLD) (n=2), HCV+NAFLD (n=2), HBV+AIH in 1 patient, and NAFLD+AIH in 1 patient. VNT was identified in 10 patients (7.8%).
Baseline characteristics of the study population with data presented in median (IQR) unless stated otherwise.
| Character | Total (n=128) | No VNT (n=118) | VNT (n=10) | p-Value |
|---|---|---|---|---|
| Age (year), mean (SD) | 57.4 (11.3) | 57.6 (11.3) | 55.1 (12.2) | 0.513 |
| Male gender, n (%) | 77 (60.2) | 68 (57.6) | 9 (90) | 0.05 |
| BMI (kg/m2) | 23.5 (21.4, 24.7) | 23.5 (21.3, 24.6) | 23.7 (23, 24.7) | 0.428 |
| Etiology, n (%) | 0.047 | |||
| HBV | 42 (32.8) | 42 (35.6) | 0 (0) | |
| HCV | 48 (37.5) | 41 (34.7) | 7 (70) | |
| Alcohol | 6 (4.7) | 5 (4.2) | 1 (10) | |
| NAFLD | 7 (5.5) | 7 (5.9) | 0 (0) | |
| Other | 10 (7.8) | 10 (8.5) | 0 (0) | |
| >1 etiology | 15 (11.7) | 13 (11) | 2 (20) | |
| Total bilirubin (mg/dL) | 0.66 (0.49, 0.87) | 0.6 (0.5,0.9) | 0.9 (0.7,1.1) | 0.204 |
| ALT (U/L) | 38 (20.8, 67) | 37.5 (19.2,66.8) | 50 (29,65.8) | 0.167 |
| Albumin (g/L), mean (SD) | 4.2 (0.4) | 4.2 (0.4) | 4 (0.4) | 0.103 |
| CTP score, n (%) | 0.573 | |||
| 5 | 117 (92.1) | 108 (92.3) | 9 (90) | |
| 6 | 10 (7.9) | 9 (7.7) | 1 (10) | |
| Platelet (×103/mm3) | 162 (106.8, 208.3) | 165.5 (114, 215.5) | 108 (80.5, 141.2) | 0.012 |
| LSM (kPa) | 14.6 (8.2, 25.8) | 14.4 (8, 24.4) | 28.1 (22.5, 33.8) | 0.008 |
| APRI score | 0.73 (0.4, 1.3) | 0.7 (0.4, 1.2) | 1.3 (1, 2) | 0.018 |
| FIB-4 score | 2.475 (1.61, 4.07) | 2.4 (1.4, 3.7) | 4.3 (3.3, 6) | 0.009 |
| MELD | 8 (8, 9) | 8 (7.5, 9) | 9 (8, 9.8) | 0.3 |
| Varix status, n (%) | ||||
| Significant EV | 8 (6.2) | 0 | 8 (80) | <0.001 |
| GV | 3 (2.3) | 0 | 3 (30) | <0.001 |
| VNT | 10 (7.8) | 0 | 10 (100) | <0.001 |
BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; ALT, alanine aminotransferase; CTP, Child-Turcott-Pugh; LSM, liver stiffness measurement; kPa, kilopascal; APRI, AST to platelet ratio; FIB-4, Fibrosis-4; EV, esophageal varix; GV, gastric varix; VNT, varices needing treatment
In comparison, patients with VNT included a higher number of the male gender, chronic hepatitis C as the etiology, a higher liver stiffness, aspartate aminotransferase (AST) to Platelet Ratio (APRI), and Fibrosis-4 (FIB-4) scores, and a lower platelet count (Table 1).
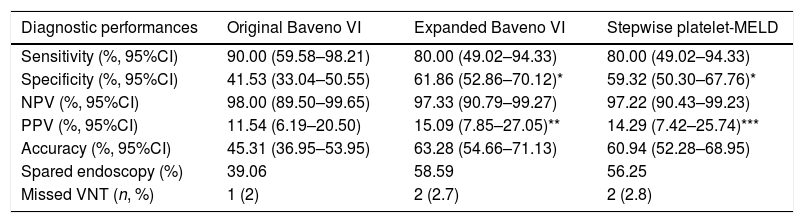
3.2Diagnostic performances of the original Baveno VI, expanded Baveno VI, and stepwise platelet-MELD criteriaSensitivity, specificity, PPV, NPV, accuracy, number of endoscopies that could be avoided, and missed VNT of all 3 criteria are shown in Table 2. The NPVs to exclude VNT were higher than 95% in all criteria.
Diagnostic performances for VNT of all 3 criteria in the study.
| Diagnostic performances | Original Baveno VI | Expanded Baveno VI | Stepwise platelet-MELD |
|---|---|---|---|
| Sensitivity (%, 95%CI) | 90.00 (59.58–98.21) | 80.00 (49.02–94.33) | 80.00 (49.02–94.33) |
| Specificity (%, 95%CI) | 41.53 (33.04–50.55) | 61.86 (52.86–70.12)* | 59.32 (50.30–67.76)* |
| NPV (%, 95%CI) | 98.00 (89.50–99.65) | 97.33 (90.79–99.27) | 97.22 (90.43–99.23) |
| PPV (%, 95%CI) | 11.54 (6.19–20.50) | 15.09 (7.85–27.05)** | 14.29 (7.42–25.74)*** |
| Accuracy (%, 95%CI) | 45.31 (36.95–53.95) | 63.28 (54.66–71.13) | 60.94 (52.28–68.95) |
| Spared endoscopy (%) | 39.06 | 58.59 | 56.25 |
| Missed VNT (n, %) | 1 (2) | 2 (2.7) | 2 (2.8) |
NPV, negative predictive value; PPV, positive predictive value; VNT, varices needing treatment. *p-Value<0.001, **p-value<0.05, ***p-value=0.08 compared with the original Baveno VI criteria.
In comparison with the original Baveno VI criteria, both the expanded Baveno VI and stepwise platelet-MELD criteria provided a significantly better specificity (61.86% and 59.32% vs 41.53%, p<0.001 for both comparisons), the expanded Baveno VI yielded a significantly better PPV (p=0.03), and the PPV in the stepwise platelet-MELD criteria were numerically higher as well, but a statistically significant level was not reached (p=0.08). The sensitivities and NPVs were comparable. The expanded Baveno VI and the stepwise platelet-MELD criteria could spare more endoscopies than the original criteria (58.59%, 56.25%, and 39.06%, respectively), and the number of missed VNT were low in all criteria.
4DiscussionIn the management of cirrhotic patients, the criteria for ruling out VNT are of interest from the aspect of non-invasive techniques, and it is possible to provide a basic screening step for general practitioners before referring patients to endoscopic centers. There are many non-invasive tests for the detection of VNT in the literature, including blood tests, liver and/or spleen stiffness measurement, and imaging characteristics, or a combination of the aforementioned parameters [23–25]. In this study, we focused on the original Baveno VI, the expanded Baveno VI, and stepwise platelet-MELD criteria.
In the present study, we found that the original Baveno VI criteria could be used to rule out VNT in compensated cirrhotic patients with a sensitivity of 90% and a satisfactorily high NPV of 98%. Endoscopic surveillance could have been avoided in 39% of the patients if the original Baveno VI criteria were used to categorize patients prior to sending them for an EGD surveillance. The result of this approach is concordant with other published studies [9–14,26–28] in which missed VNTs in the Baveno VI criteria were consistently lower than 5%, and spared endoscopies were observed in 11.3–39%.
However, it has come to our attention that whether we can spare more endoscopies without increasing the number of missed VNTs. Augustin et al. [13] proposed the expanded Baveno VI in late 2017 with different cut-off levels for LSM and platelet count as mentioned earlier. This approach could double the number of endoscopies spared from 21.5 to 40%, while missed VNTs were almost identical. In our study, the expanded Baveno VI criteria provided a significantly better specificity and PPV compared with the original Baveno VI criteria, while the NPVs were comparable (97.33% vs 98%, p=0.65), and the number of endoscopies that could have been spared was almost 60%. Other studies to validate the expanded criteria in some specific groups of patients were recently published. Petta et al. [28] validated this approach in only NAFLD patients. Missed VNTs were observed in 3.8–4.4%, and the endoscopies that could be spared in 58%, while in the study of Moctezuma-Velazquez et al. [27], the false negative rate was 6% in 147 primary biliary cholangitis (PBC) patients. Bae et al. [26] found that in their Asian cohort, the missed VNT using the expanded Baveno VI criteria were high in HBV, alcoholic, and NAFLD cirrhotic patients (7.5%, 8%, and 6.7%, respectively). Nevertheless, it is important to note that the VNT in compensated advanced chronic liver disease in the study by Bae et al. was 19.5%, which exceeded the number of expected VNT 6–12% in compensated cirrhotic patients [7]. Our study results are concordant with other studies in the fact that more endoscopies could be spared by using the expanded Baveno VI criteria. However, larger studies are required in order to aid in the decision of whether we should use this approach to rule out VNT in terms of missed VNT rates.
In contrast, both the original and expanded Baveno VI criteria necessitate LSM to categorize patients into low-risk or high-risk of having VNT. Despite its advantages of being non-invasive, easy to perform and interpret, LSM requires a specific device, which the cost might be outrageous to many centers, and failure to attain the measurements was reported in some groups of patients. In early 2017, Jangouk et al. published a study validating the Baveno VI criteria, as well as the proposed stepwise platelet-MELD criteria [11], in which LSM was not required, showing that the stepwise platelet-MELD criteria performed as well as the Baveno VI in NPVs, sensitivity, and endoscopies spared. We are interested in this approach as it might be a simple and useful way for general practitioners to screen for cirrhotic patients with a low risk of having VNT. In our study, the stepwise platelet-MELD criteria yielded not only comparably high NPVs compared with the original Baveno VI criteria (97.22% vs 98%, p=0.60), but also a higher specificity (59.32% vs 41.53%, p<0.001), a larger number of endoscopies that could have been spared (56% vs 39%). To date, the stepwise platelet-MELD criteria have been validated in only one study prior to our study [27], which demonstrated false negative rates of 0% and 5%, and that endoscopies could be avoided in 31% and 26% in PBC and primary sclerosing cholangitis patients, respectively.
The highlights of our study were the comparison of the 3 criteria, including the elastography-based and stepwise platelet-MELD criteria, for the detection of only VNT, which is clinically more meaningful than any size of esophageal varices. We enrolled only the patients with compensated cirrhosis who would have been most likely to benefit from non-invasive approaches, as they had a low prevalence of VNT, while most of other studies included both compensated and decompensated cirrhotic patients.
There are some limitations in our study. First, despite including various cirrhosis etiologies, the distribution might not reflect the true order of the etiologies in Thailand, as HCV was accounted for the most common cause in this study, in contrast to the earlier established data in which HBV was more prevalent than HCV in Thailand [29,30]. Also, the previously published study based on the Nationwide Hospital Admission Data in 2010 [31] reported that alcoholic liver disease is the most common cause of cirrhosis in Thai patients. This could be due to the fact that our study was conducted in a tertiary care center in which some biases in patients’ referral exist, because in our country, uncomplicated HBV and alcoholic liver disease patients can be treated by general practitioners, while the treatment of HCV needs to be managed by a gastroenterologist or hepatologist. Second, different endoscopists performed EGD and different doctors performed TE, resulting in an inevitable interobserver variation; nonetheless, it might reflect the real-world situation in clinical practice. Third, we collected the data from both retrospective and prospective part in patients whose data were retrospectively collected, the endoscopist and the doctor who performed TE might be the same person or the EGD results might not been blinded to the doctor who performed TE, however, they were unaware of the study aims at that time, making the misclassification of the outcome bias unlikely. And for the prospective part, all the doctors who performed TE were blinded to the endoscopic results. Lastly, although we calculated the sample size in order to evaluate the diagnostic performances of the criteria, and enrolled the patients according to that sample size, the number of patients may not have been large enough to identify the significant differences in missed VNT rates among the 3 criteria, as the missed VNTs were numerically higher in the expanded Baveno VI and stepwise platelet-MELD criteria than in the original Baveno VI. Thus, a larger study should be conducted.
In conclusion, the original Baveno VI criteria have now been validated in many studies and endoscopies could be avoided safely using this approach. From our study, the stepwise platelet-MELD approach might be useful as an alternative method to the Baveno VI criteria in a resource-limited setting where LSM is not widely available, or in patients for whom a measurement cannot be obtained. However, a strong recommendation cannot be made at this time. Future studies should be undertaken from a public health standpoint. Concerning the expanded Baveno VI criteria, although the number of spared endoscopies is consistently high across numerous studies, but missed VNT rates are yet to be validated in a larger study.AbbreviationsEV esophageal varices gastric varices varices needing treatment esophagogastroduodenoscopy liver stiffness measurement platelet-MELD transient elastography body mass index human research ethics committee sensitivity specificity positive predictive value negative predictive value hepatitis B virus hepatitis C virus autoimmune hepatitis non-alcoholic fatty liver disease aspartate aminotransferase aspartate aminotransferase to platelet ratio fibrosis-4 Child-Turcott-Pugh
Study concept and design: S. Nawalerspanya and P. Sripongpun; data acquisition: S. Nawalerspanya; data analysis and interpretation: S. Nawalerspanya, and P. Sripongpun; drafting of the manuscript: S. Nawalerspanya; critical revision of the manuscript for important intellectual content: P. Sripongpun, N. Chamroonkul, and T. Piratvisuth; statistical analysis: P. Sripongpun and C. Kongkamol; study supervision: P. Sripongpun and T. Piratvisuth
Statement of financial supportThis study was funded by Faculty of Medicine, Prince of Songkla University, Thailand.
Conflict of interestThe authors have no conflicts of interest to declare.
The study was granted by faculty of Medicine, Prince of Songkla University, Thailand.