Intestinal permeability is getting more and more attention in gastrointestinal research. Although well recognized, its exact role in health and disease is yet to be defined. There are many methods of quantifying intestinal permeability, but most of them fail to deliver tangible information about the morphological integrity of the intestinal barrier. In this review we aim to describe imaging options for the assessment of intestinal barrier integrity and their potential relevance for clinical practice. Our focus is on confocal laser endomicroscopy, which is at this time the only method for visualizing not only functional but also morphological aspects of the gut barrier in vivo.
According to current data, the intestinal barrier's integrity might play a key role in heath and disease. The assessment of the intestinal permeability is insufficiently standardized in clinical practice, and its clinical relevance for specific disease states remains to be delineated further. In this review, we summarize current knowledge on functional imaging tools for the assessment of intestinal permeability and barrier integrity. The latter contribute not only to the protection of the human body against invasion of pathogenic microorganisms and toxins, but also to the absorption of nutrients vital for life. Factors that influence intestinal permeability and barrier integrity are intestinal perfusion, systemic and/or gastrointestinal infections, diet, drugs as well as life-style and environmental factors [1]. Certain disease states are associated with increased intestinal permeability favoring mucosal inflammation, resulting in the translocation of intraluminal components. Not only primary gastrointestinal diseases such as inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS) are linked to impaired barrier function, but also a wide array of pathologies originating from other organs. There are data demonstrating an axis between chronic liver diseases and the gut barrier, thus highlighting the critical interplay between systemic and intestinal disease [2].
The measurable intestinal permeability as function of paracellular intestinal transport capacities reflects the intestinal barrier function, which, in turn, is closely related to intestinal health. The architecture of the gut barrier comprises various physical and cellular components (mucus, epithelial and vascular cells, stroma). In addition, chemical aspects of the barrier function likewise have to be taken into account, such as cellular products, antimicrobial peptides, and gastrointestinal secretions. Last but not least, the critical contribution of intestinal microbiota, the local and the systemic immune system as well as gastrointestinal motility to gut integrity has to be taken into account [3].
There are various tools and methods to assess intestinal permeability, which measure distinct components of this complex system. Most of the current instruments for (semi-)quantitative assessment, e.g. lactulose and mannitol permeability or blood zonulin measurements, provide information about the increase in permeability and, potentially, the degree of tissue alteration. However, these parameters are not capable of distinguishing functional changes from structural intestinal damage. Likewise, they provide little information about the exact mechanism and/or localization of disrupted gut permeability. In this review, we highlight less well-established imaging tools for intestinal barrier function assessment with emphasis on confocal laser endomicroscopy (CLE). CLE is a novel tool that integrates in vivo microscopic assessment into standard endoscopy [4,5]. Furthermore, available options for the quantitation of disturbed permeability as well as causes and localization of these functional changes are described.
2Imaging ToolsThere is an increasing number of studies looking at the functional aspect of intestinal permeability, both in vitro and in vivo, in animal models or patients. As we will present further on, most studies visualizing intestinal permeability in vivo are using the technique of confocal endomicroscopy, with inflammatory bowel disease being the most investigated pathology. Other conditions influencing the intestinal permeability studied with CLE to this date in humans are irritable bowel syndrome and allergies. Other in vivo imaging methods revealed by our PubMed-research were magnetic resonance tomography (MRI) and positron emission tomography (PET)
2.1Magnetic resonance imaging (MRI)In a study published in 2015, Towner et al. [6] demonstrated altered permeability in the urine bladder and bowel in an animal model by means of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). In their preliminary study, they used a concentration of contrast media that readily allowed for direct and quantifiable observation of leakage out of the bladder wall. They also observed a secondary contrast-enhancement in the colon, indicating a breakdown of the colonic permeability barrier in histologically undamaged mucosa after exposure of the bladder to protamine sulfate [7] In a second publication [8], the same research group looked at the crosstalk between colon and bladder in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis as established method of inducing transmural and segmental colonic inflammation similar to Crohn disease (CD).
Contrast enhanced MRI (CE-MRI) is in routine clinical use to determine sites and degree of intestinal inflammation in IBD. Assessing gastrointestinal, hepatobiliary or urinary tract with this method may be useful in providing information about how intestinal inflammation influences the permeability in histological healthy tissue of other organs in IBD patients.
2.2Positron emission tomography (PET)The authors of this study [9], published 2011, used PET-technology to assess intestinal permeability and absorption of orally administrated 18F-Fluordesoxyglukose (18F-FDG) in rats, concluding that this would be a powerful tool for in vivo investigation of the gastrointestinal drug absorption.
2.3HistologyAnother method of assessing gut barrier integrity is, among others, observing the shedding of the intestinal epithelial cells. There are various histological methods of describing a disrupted barrier, like increased intercellular space as a reflection of damaged tight junctions, quantifying the extracellular tight junction (TJ) protein occludin compared to the intracellular Zonula occludens-1 (ZO-1) protein by polymerase chain reaction (PCR), a decrease suggesting a disturbance in the cellular make-up of tight junctions [10].
As they are held together by tight junctions, any disruption of the equilibrium between epithelial cell shedding at the villus tip and generation of new cells in the crypt can affect the homeostasis and permeability of the gut. It can be assumed that shedding of intestinal cells from the epithelial monolayer, which can be seen under the microscope, causes transient gaps in the barrier with a corresponding impact on intestinal permeability [11].
The mechanism underlying this phenomenon of pathological cell shedding, observed in animal models of inflammation and human intestinal conditions like IBD remains poorly understood, but there is a sound rationale to believe that it might stem from systemic and intestinal inflammatory disease and a dysfunctional gut barrier [12].
Studies investigating the light microscopic aspect of the intestinal mucosa in liver cirrhosis have shown signs of physical injury like epithelial cell shortening, nuclear disarray and degenerative changes of the cytoplasm predominantly in cells located at the tip of the villi, as well as pronounced extrusion of dead cells. Furthermore, at a submicroscopic level liver cirrhosis was linked to a widened tight junction complex as well as disruption of the actin cytoskeleton, mitochondrial dysfunction with consecutive swelling and changes in permeability [13,14] as well as changes in sugar composition at the brush border [15]. At the immunohistochemical level, the expression of TJ proteins occludin and claudin-1 was repressed in cirrhosis. Of note, these findings were even more pronounced in patients with decompensated cirrhosis [16].
2.4Confocal endomicroscopy (CLE)This imaging method is based on tissue illumination after systemic application of fluorescein, which highlights the extracellular matrix [17]. This allows CLE to assess the internal microstructure of tissues within anatomical tracts, i.e. gastrointestinal (but also urinary or respiratory), through an endoscope. Currently, to our knowledge, the only device on the market providing this technology has the trade name Cellvizio is a fibered confocal microscopic system. It uses the so-called probe based CLE (pCLE) where a probe is passed through the working channel of a standard gastroscope or colonoscope [18].
A 488-nm wavelength laser system is used. There are different probes for different indications available and the confocal images are steamed at a frame rate of 12 frames per second, obtaining real-time videos of the mucosa [19].
Confocal miniprobes are made of a distal tip, a fiber with a protective sheath and a connector. When connected to the Cellvizio system through their connector, confocal miniprobes transport the scanned laser beam through the sheathed fiber to the site of observation in contact with the distal tip and capture the fluorescent light emitted back from the tissue, in order to provide in-vivo fluorescence imaging of tissues. The parts of the confocal miniprobes that get in contact with the patient consists of biocompatible glass, plastic and metal derivatives.
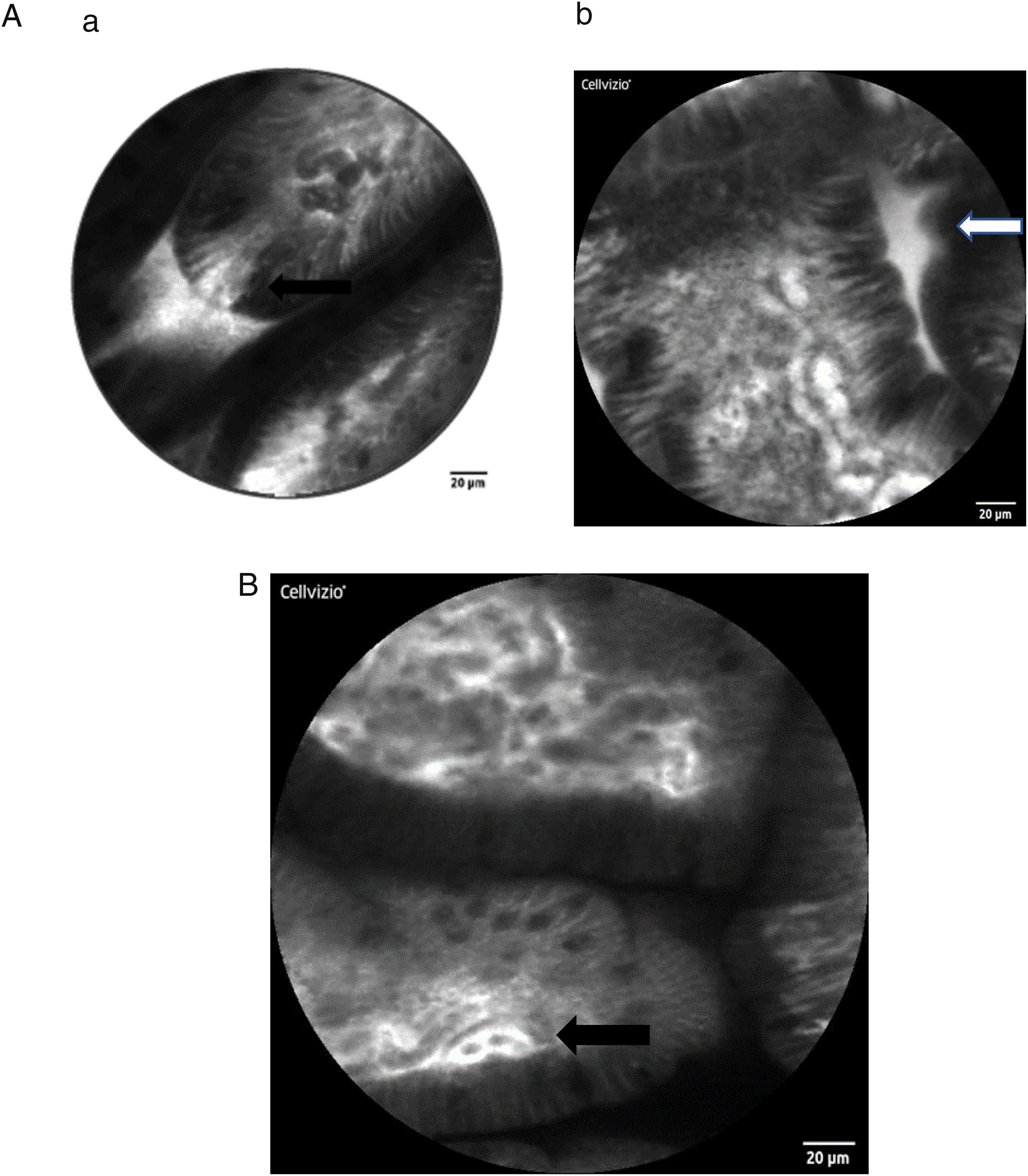
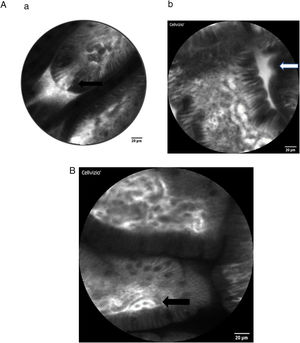
Here some examples of intestinal images obtained with the Cellvizio pCLE system:
The method permits in vivo microscopy of the human gastrointestinal mucosa during endoscopy, providing optical "virtual biopsies". Endomicroscopy has been applied for the study of several diseases, with most studies focusing on inflammation and neoplasia, such as Barrett esophagus, gastric cancer, celiac disease, IBD or colorectal neoplasia. CLE facilitates the study of pathophysiological events in their natural environment (functional imaging) [20–24]. To our knowledge, the role of endomicroscopy in patients with hepatobiliary diseases has been confined to imaging the biliary tree [25] but has not been used so far to evaluate the intestinal mucosa in patients with chronic liver diseases (CLD). Of note, sugar absorption tests (lactulose and mannitol) have been used to assess intestinal permeability and mucosal barrier function in CLD, but there are no data at this point that demonstrate signs of intestinal barrier dysfunction in vivo in human liver disease by CLE.
As stated above, intestinal epithelial cells and tight junctions play a crucial role in maintaining the intact gut barrier. On the one hand, they assure the absorptive character of the intestine, on the other they are necessary to prevent potentially harmful components from entering the systemic circulation [26,27]. Representing a high cellular turnover tissue, the intestinal epithelium is constantly regenerated from stem cells originating at the crypt base. They migrate to the top of the villi as they mature and from where they are shed in time [27]. The cell shedding is responsible for epithelial gaps, which can be directly observed in real-time by CLE in humans [28,29]. Several studies confirmed that increased intestinal permeability can be visualized by CLE, especially in patients with IBD, but also in IBS and food allergy.
The epithelial gap density visualized by CLE has been suggested as quantitative parameter for intestinal permeability. It was shown that the epithelial gap density of the terminal ileum is significantly higher that that of controls without IBD, in both CD and ulcerative colitis (UC) patients. Interestingly, gap density did not correlate with disease activity, hence further studies are needed to better understand the potential significance of these obervations in IBD [30]. Since cell shedding is a physiological regenerative phenomenon, the qualitative observation of epithelial gaps may not be sufficient to distinguish physiological versus pathological gut permeability. In 2011 Liu et al. [31] described a method of defining gap density by counting the epithelial gaps per 1,000 cells in adequately imaged villi in the terminal ileum of IBD patients. This definition was also used by Turcotte et al. [32] who described increased gap density in IBS patients compared with controls in a small sample of six versus 32 individuals. Thereby, they were able to achieve a diagnostic accuracy for the diagnosis of IBS with 62% sensitivity, 89% specificity, 83% positive predictive value, and 73% negative predictive value.
Fritscher-Ravens et al. [33] used CLE technology in IBS patients to assess their response to specific food components by analyzing intraepithelial lymphocytes and fluid extravasation through epithelial leaks. Using this method, they established a diagnosis of (immune-mediated) food allergy in patients with negative immunoglobulin E (IgE) and skin-prick tests. In this study, several CLE criteria for the detection of barrier disruption were suggested: density of intraepithelial lymphocytes, epithelial breaks, luminal fluorescein leakage, presence of fluorescein between enterocytes and widening of the intervillous space.
3DiscussionAlthough an emerging field, imaging methods for intestinal permeability assessment remain immature and in evolution. Radiology methods might provide in vivo information about the functional integrity of the intestinal barrier but fail to characterize morphological changes that lead to permeability change. As of now, CLE represents the only imaging method that may provide detailed cellular and anatomic information as well as functional alterations of the intestinal barrier in real time.
This has been pioneered in a potential clinical application by Fritscher-Ravens for diagnosis and monitoring of distinct types of food allergies [33]. Further potential clinical utility of this innovative technology may lie in prognostication of treatment responses and its fine-tuning in the IBD field [34].
Functional imaging with the CLE method also allows for testing of emerging therapies in IBS contributing to better understanding the pathophysiologic mechanisms underlying this condition.
Extraintestinal conditions can be influenced by the intestinal permeability and integrity of the gut barrier. It is well known that in individuals with liver cirrhosis and ascites, disturbed intestinal permeability favours the development of spontaneous bacterial peritonitis (SBP) as a potentially deleterious clinical complication. An early diagnosis and risk stratification by quantification of impaired gut barrier may allow for targeted intervention and preventive measures, such as pre-emptive antibiotic prophylaxis.
Several studies confirmed increased intestinal permeability in liver cirrhosis, however, these disturbances have not yet been addressed thoroughly by in vivo functional imaging such as CLE. Among others, increased gut permeability is considered an essential and early factor in the pathophysiology of SBP as well as other infectious cirrhosis complications related to bacterial translocation [35,36].
All these changes could not, to our knowledge, be visualized in real time with in vivo imaging methods.