Introduction and Objectives: We aimed to compare the usefulness of the ultra-high-sensitivity hepatitis B surface antigen (iTACT-HBsAg), high-sensitivity hepatitis B core-related antigen (iTACT-HBcrAg), and anti-HBs assays in determination of cessation of nucleot(s)ide analogue (NA) treatment to prevent against hepatitis B virus (HBV) reactivation.
Patients and Methods: Twenty-two patients who developed HBV reactivation under immunosuppressive therapy or chemotherapy and had been administered NA and subsequently discontinued were enrolled. The stored serum samples taken at NA cessation were applied to iTACT-HBsAg (lower limit of detection; 0.0005 IU/mL), iTACT-HBcrAg (lower limit of detection; 2.1 log U/mL), and anti-HBs assays. Detection of serum HBV DNA level ≥1.3 log IU/mL after NA cessation was defined as virological relapse (VR).
Results: Two patients were excluded due to re-introduction of NA despite a negligible level of HBV DNA (<1.3 log IU/mL). Of the remaining 20 patients, 11 (55 %) had HBcrAg <2.1 log U/mL at the cessation of NA, and 7 of the 11 patients (64 %) had no VR thereafter. On the other hand, 15 patients (75 %) had HBsAg <0.0005 IU/mL at the cessation of NA, and 13 of the 15 patients (87 %) subsequently lacked VR. Further, 12 patients (60 %) had anti-HBs ≥10 mIU/mL at the cessation of NA, and 10 of the 12 patients (83 %) had no VR thereafter. The iTACT-HBsAg assay had the highest positive predictive value and the best overall diagnostic performance for predicting non-VR after cessation of NA.
Conclusions: The iTACT-HBsAg assay was useful to determine the cessation of NA treatment to prevent against HBV reactivation.
Hepatitis B virus (HBV) reactivation during and after systemic chemotherapy is a serious problem, and occasionally leads to fulminant hepatitis [1,2]. HBV reactivation may appear not only in hepatitis B surface antigen (HBsAg)-positive patients, but also in patients who are HBsAg-negative, as well as seropositive for hepatitis B core antibody (anti-HBc) and/or hepatitis B surface antibody (anti-HBs), namely “resolved HBV infection” cases [3–6]. To prevent HBV reactivation-related hepatitis, the Japanese Society of Hepatology (JSH) Guidelines for Management of HBV recommend that nucleot(s)ide analogue (NA) treatment should be administered after HBV DNA has been detected or positivity for HBsAg before systemic chemotherapy and/or immunosuppressive therapy [7].
Once NA has been administered, the JSH guidelines state that NA therapy should be continued for at least 12 months after completion of immunosuppressive therapy or chemotherapy for patients with resolved HBV infection at baseline, and NA cessation may be considered after this period if alanine aminotransferase (ALT) level remains normal and HBV DNA remains undetectable [7]. However, safety after discontinuation of NA is not assured and established.
Recently, we revealed the usefulness of the iTACT-HBcrAg assay (Fujirebio Inc., Tokyo, Japan) for the monitor of HBV reactivation, and now this assay is clinically applied [8,9]. The ultra-high-sensitive iTACT-HBsAg (Fujirebio Inc., Tokyo, Japan) assay was also developed, which uses the “immunoassay for total antigen including complex via pretreatment (iTACT)” technology. This assay can detect total HBsAg with high sensitivity, the lower limit of detection is 0.0005 IU/mL even in the presence of anti-HBs, and may be useful in identifying subclinical or occult HBV carriers [10]. In addition, we showed that the iTACT-HBsAg assay was also useful for monitoring HBV reactivation [9], and a recent study showed that detection of a high level of HBsAg by the iTACT-HBsAg at NA cessation was associated with virological relapse (VR) in chronic hepatitis B patients treated with NA [11].
As an indicator for cessation of NA therapy, we reported that the recurrence of HBV reactivation after NA cessation was not observed in most of the patients who had an undetectable level of HBcrAg (<2.1 log U/mL) by the iTACT-HBcrAg assay or were seropositive for ani-HBs (≥10 mIU/mL) during follow-up [12]. Another report also suggested the usefulness of anti-HBs as an indicator for discontinuing NA in HBV patients [13]. However, the number of enrolled patients in these studies was limited. On the other hand, there are no reports to date on the usefulness of the iTACT-HBsAg assay in determination of cessation of NA treatment.
Thus, in this study, we compared the usefulness of the iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs assays for determining the cessation of NA therapy to prevent against HBV reactivation in a multicenter setting with a reasonable number of cases.
2Patients and methods2.1Study populationThis study included patients who were treated at Nagoya City University Hospital (Nagoya, Japan), and Osaka Metropolitan University Hospital (Osaka, Japan) from December 2007 to February 2021. A total of 22 patients were enrolled. All the patients were in a state of resolved HBV infection, HBsAg-negative, but seropositive for anti-HBc and/or anti-HBs, and HBV DNA was not detected before immunosuppressive therapy or chemotherapy. During and after immunosuppressive therapy or chemotherapy, HBV DNA was measured at intervals of 1 to 3 months to monitor HBV reactivation according to the JSH Guidelines for Management of HBV [7]. When they were positive for HBsAg or HBV DNA was quantifiable, NA treatment was introduced according to the JSH Guidelines for Management of HBV [7]. The NA treatment was subsequently discontinued based on the decision of their attending physician. After the cessation of NA, HBV DNA levels were measured at intervals of 1 to 3 months for monitoring VR. Serum specimens were obtained from these patients upon termination of NA. In addition, serial serum specimens after the cessation of NA were obtained in several patients. These samples were stored at −80 °C.
2.2Definition of virological relapseDetection of serum HBV DNA level of 1.3 log IU/ mL or more after the cessation of NA was defined as VR [7].
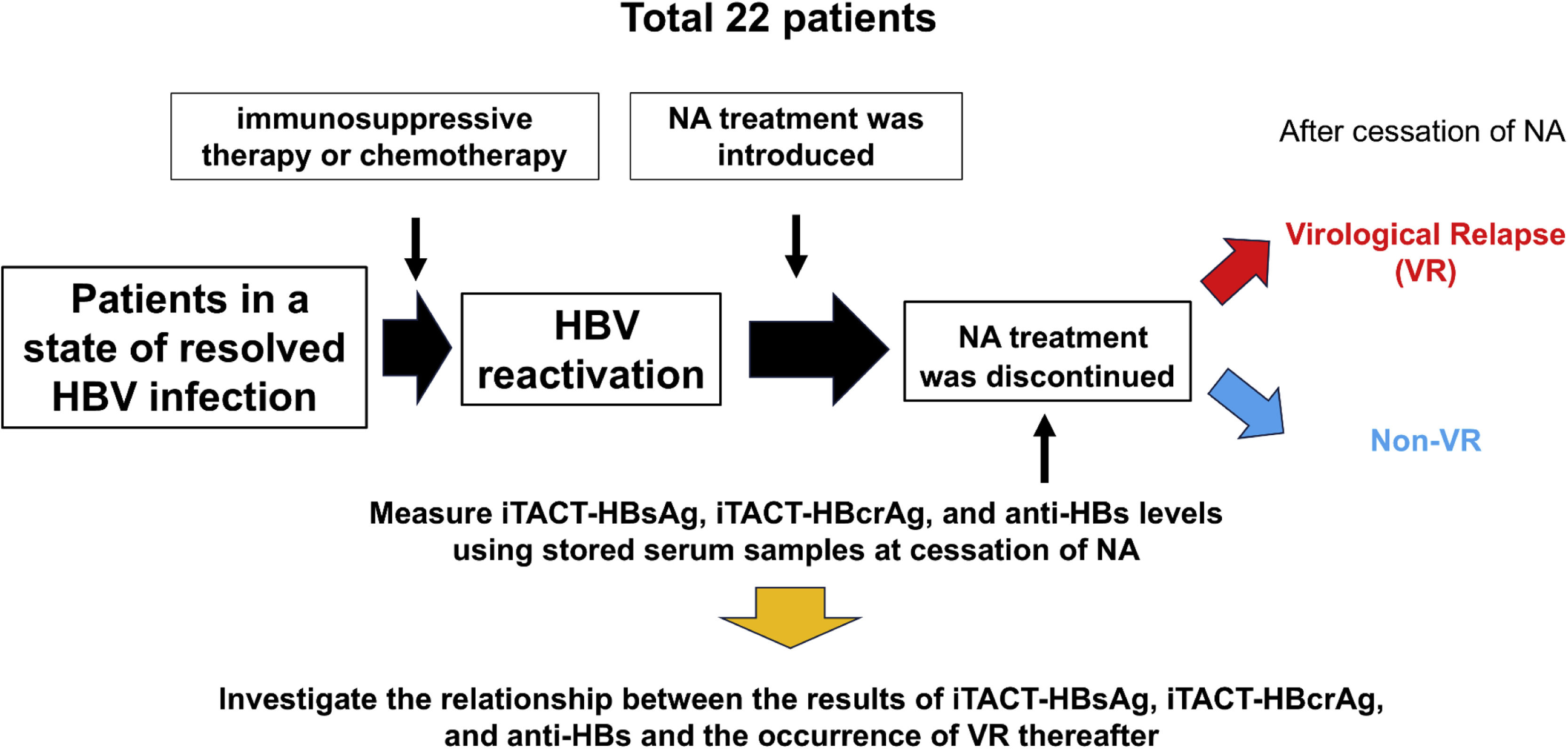
2.3Study designAt and/or after cessation of NA, iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs assays were used to measure HBsAg, HBcrAg, and anti-HBs levels, respectively, in the stored serum samples, and we investigated the relationship between these results and the occurrence of VR thereafter. The flowchart of this study is shown in Fig. 1.
Flowchart of this study.
Abbreviations: HBV, hepatitis B virus; NA, nucleos(t)ide analogues; VR, virological relapse; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody.
Serum HBcrAg was assessed by the iTACT-HBcrAg assay, and the lower limit of detection was 2.1 log U/mL [8]. Serum HBsAg was assessed by the iTACT-HBsAg assay, and the lower limit of detection was 0.0005 IU/mL [10]. Serum anti-HBs level was measured by a fully automated chemiluminescence enzyme immunoassay kit, HISCL HBsAb (Sysmex Corporation, Kobe, Japan), and positivity of anti-HBs was defined as ≥10 mIU/mL. Plasma or serum HBV DNA level was measured by the COBAS AmpliPrep/COBAS TaqMan HBV v2.0 assay (Roche Diagnostics K.K., Tokyo, Japan), and the lower and upper limits of quantitation were 2.1 log copies/mL and 9.0 log copies/mL, respectively. After 2017, the lower and upper limits of quantitation were 1.3 log IU/mL and 8.2 log IU/mL. The unit change formula is “log IU/mL = log copies/mL −0.76” according to the manufacturer's instructions.
The other hematologic and blood chemistry tests were carried out using standard assays.
2.5Statistical analysisAll patients who met the eligibility criteria were included in the analyses. The detection rates of the iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs assays and the association of the levels of these markers at NA cessation with clinical background were compared using the chi-square test for categorical variables. Receiver operating characteristic curve (ROC) analyses were used to determine the area under the curve (AUC) for potential predictors of VR after the cessation of NA during follow-up. Statistical analyses were two-sided, and a P-value of <0.05 was considered significant. EZR (Easy R, Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a modified version of R commander (version 4.0.3), was used for the statistical analyses [14].
2.6Ethical statementWritten informed consent was obtained from each patient. The study protocol was approved by the Institutional Review Board of Nagoya City University (acceptance number: 60-21-0063), Osaka Metropolitan University Hospital (acceptance number: 2023-0047), and was implemented according to the Declaration of Helsinki.
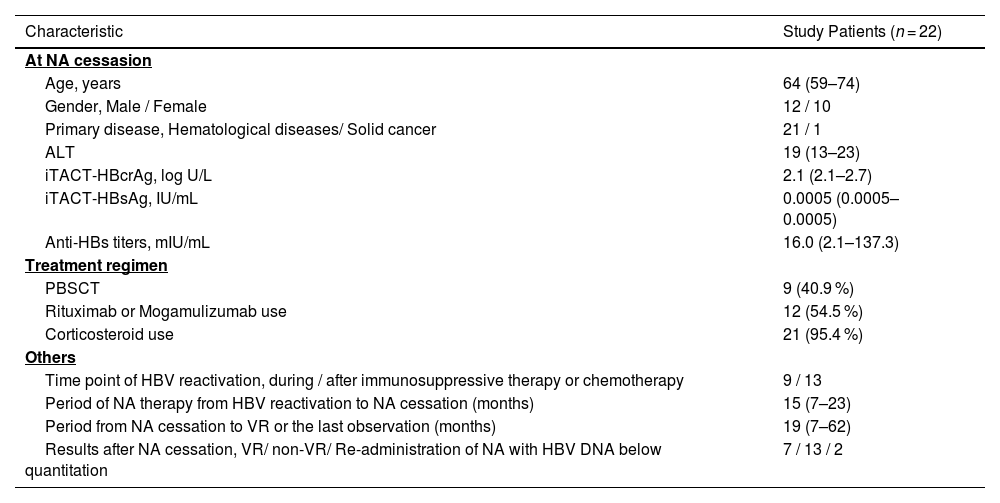
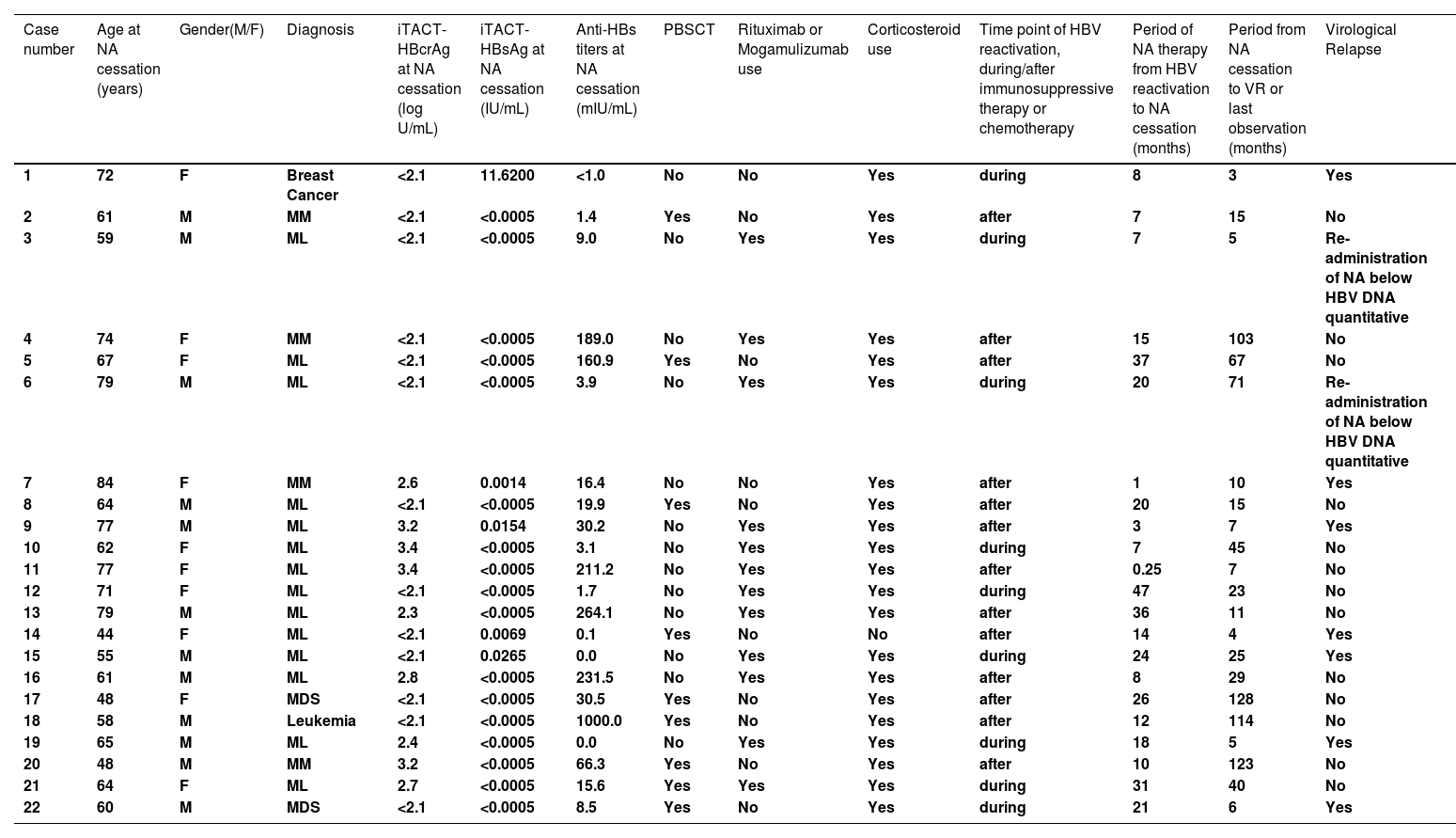
3Results3.1Clinical characteristics of the study patientsThe clinical characteristics of the 22 patients are summarized in Table 1. The median age was 64 at the cessation of NA. Twelve were male. Regarding primary diseases, 21 had hematological diseases, and 1 had breast cancer. The median levels of ALT, HBcrAg, HBsAg, and anti-HBs at the cessation of NA were 19 U/L, 2.1 logU/mL, 0.0005 IU/mL, and 16.0 mIU/mL, respectively. Of the 22 study patients, 9 patients (40.9 %) were treated with peripheral blood stem cell transplantation (PBSCT), 12 patients (54.5%) with rituximab and/or mogamulizumab, and 21 patients (95.4 %) with corticosteroid. HBV reactivation occurred during immunosuppressive therapy or chemotherapy in 9 patients, and after immunosuppressive therapy or chemotherapy in 13 patients. The median period of NA therapy from HBV reactivation to NA cessation was 15 (IQR: 7 - 23) months, and the median period from the cessation of NA to VR or the last observation was 19 (IQR: 7 - 62) months. After the cessation of NA, 7 patients had VR and NA treatment was re-introduced, 13 had no VR, and 2 underwent re-introduction of NA therapy despite a negligible level of HBV DNA (<1.3 log IU/mL) based on the decision of their attending physician.
Clinical characteristics of the enrolled patients.
Data from all patient are expressed as numbers for categorical data and medians (first–third quartiles) for noncategorical data.
Abbreviations; NA, nucleos(t)ide analogues; ALT, alanine aminotransferase; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody; PBSCT, peripheral blood stem cell transplantation; VR, virological relapse.
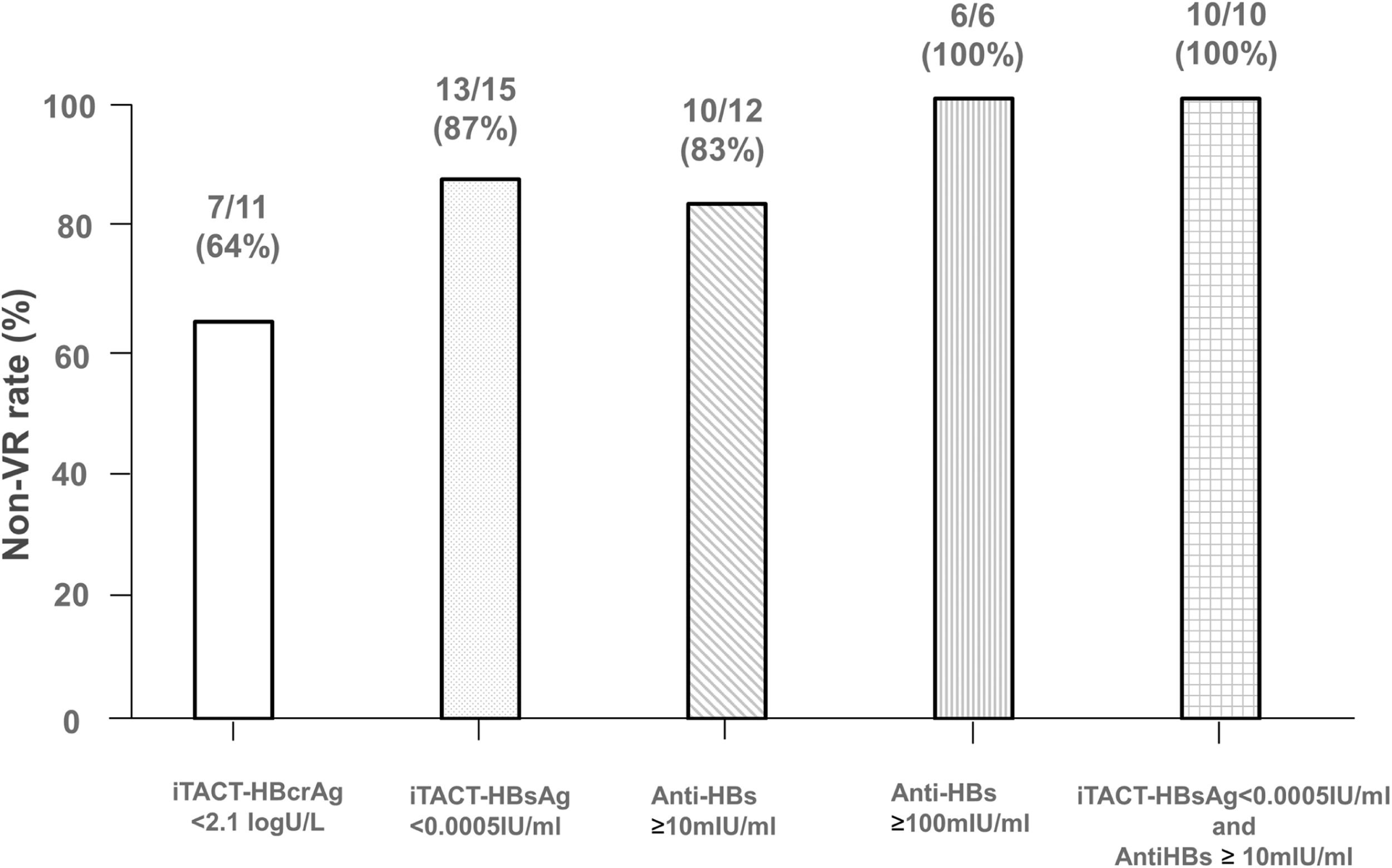
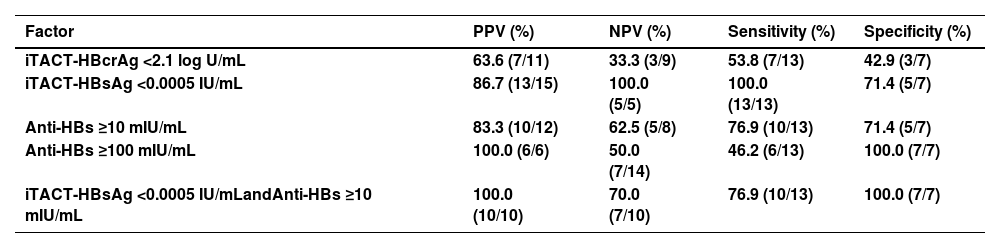
The details of the various markers and background of the 22 study patients are described in Table 2 We excluded 2 patients (Case 3 and 6 in Table 2) who underwent re-introduction of NA therapy despite having a negligible level of HBV DNA (<1.3 log IU/mL) based on the decision of their attending physician. Of the remaining 20 patients, 11 (55 %) had HBcrAg level below the quantification limit (<2.1 log U/mL) at the cessation of NA, and 7 of the 11 patients (64 %) had no VR thereafter. In comparison, 15 patients (75 %) had HBsAg level below the quantification limit (<0.0005 IU/mL) at the cessation of NA, and 13 of the 15 patients (87 %) had no VR afterwards. Further, 12 patients (60 %) had anti-HBs ≥10 mIU/mL at the cessation of NA, and 10 of the 12 patients (83 %) had no VR thereafter (Fig. 2). Comparison of the efficacy of the iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs assays showed that the iTACT-HBsAg assay had the highest positive predictive value (PPV) for predicting non-VR after the cessation of NA. In addition, 10 patients (45 %) had HBsAg <0.0005 IU/mL and anti-HBs ≥10 mIU/mL at the cessation of NA, and all of these patients had no VR thereafter. Meanwhile, 6 patients had anti-HBs ≥100 mIU/mL at the cessation of NA, and all of these patients also had no VR thereafter (Fig. 2). We next examined the negative predictive value (NPV), the sensitivity, and the specificity for predicting non-VR after the cessation of NA using the above-mentioned cut-off values of the assays. The overall diagnostic performance of the cut-off level of iTACT-HBsAg (<0.0005 IU/mL) was better than that of iTACT-HBcrAg (<2.1 log U/mL) and that of anti-HBs (≥10 mIU/mL); the NPV and sensitivity of the cut-off level of iTACT-HBsAg were both 100 %, and the specificity was 71.4 % (Table 3). The ROC curve analyses to predict VR after the cessation of NA showed that the AUC was 0.857 for iTACT-HBsAg, 0.445 for iTACT-HBcrAg, and 0.742 for anti-HBs, respectively (Fig. S1A, B, C).
Clinical characteristics of the enrolled patients who were administered and subsequently terminated NA therapy which was introduced as a preventative measure against HBV reactivation.
Abbreviations: NA, nucleos(t)ide analogues; HBV, hepatitis B virus; M, male; F, female; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; PBSCT, peripheral blood stem cell transplantation; VR, virological relapse; MM, multiple myeloma; ML, malignant lymphoma; MDS, myelodysplastic syndromes.
Comparison of the non-VR rates according to the levels of HBcrAg, HBsAg (as determined by the iTACT-HBcrAg and iTACT-HBsAg assays, respectively), and anti-HBs in sera at cessation of NA treatment to prevent against HBV reactivation.
The vertical bar represents the non-VR rates in those with HBcrAg <2.1 log U/mL, HBsAg <0.0005 IU/mL, anti-HBs ≥10 mIU/mL, anti-HBs ≥100 mIU/mL, and the combination of HBsAg <0.0005 IU/mL and anti-HBs ≥10 mIU/mL at NA cessation.
Abbreviations: VR, virological relapse; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody; NA, nucleos(t)ide analogues.
Diagnostic performance of factors predicting non-VR after the cessation of NA.
Abbreviations:VR, virological relapse; NA, nucleos(t)ide analogues; PPV, positive predictive value; NPV, negative predictive value; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody.
In addition, of the 13 non-VR patients, two had detection of HBV DNA less than quantitative after NA cessation, but HBV DNA subsequently became less than detection without administration of NA (Case 10 and 13 in Table S2).
Next, we examined whether the duration of NA administration was related to VR. Of the 12 patients treated with NA for ≥12 months, 4 had VR, meanwhile on the 8 patients treated with NA for <12 months, 3 had VR (P = 1.000). Thus, the duration of NA administration was not related to VR. The other parameters such as primary disease, treatment regimen, and time point of HBV reactivation were not significantly related to VR.
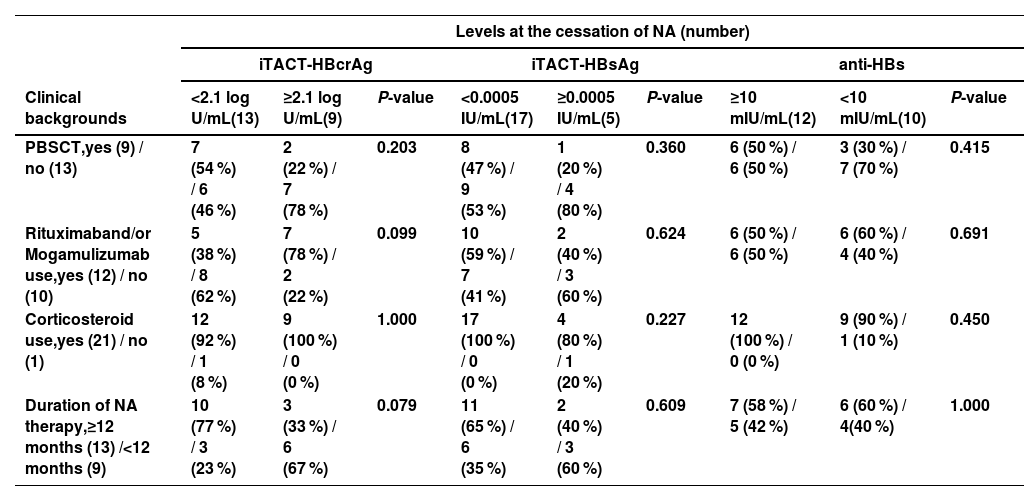
3.3Association of HBcrAg, HBsAg, and anti-HBs levels at the cessation of NA with clinical backgroundWe next compared the clinical backgrounds of the two groups, which were distinguished by their respective cutoff values of iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs. The duration of NA administration was examined separated by 12 months, because the JSH guidelines state that NA therapy should be continued for at least 12 months after completion of immunosuppressive therapy or chemotherapy [7]. The patients with HBcrAg <2.1 log U/mL at the cessation of NA tended to include less cases with use of rituximab and/or mogamulizumab (P = 0.099) and more cases with duration of NA therapy ≥12 months (P = 0.079). However, there were no differences in the association of HBsAg and anti-HBs levels at the cessation of NA with clinical background (Table 4).
Association of iTACT-HBcrAg, iTACT-HBsAg, and anti-HBs levels at NA cessation with clinical background.
Categorical variables were compared between groups using the chi-square test.
Abbreviations: iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody; NA, nucleos(t)ide analogues; PBSCT, peripheral blood stem cell transplantation.
Of the 20 study patients, we were able to obtain serial serum specimens after the cessation of NA over time in several patients, and we measured HBsAg, HBcrAg, and anti-HBs levels in these samples. Two of these cases are shown in detail:
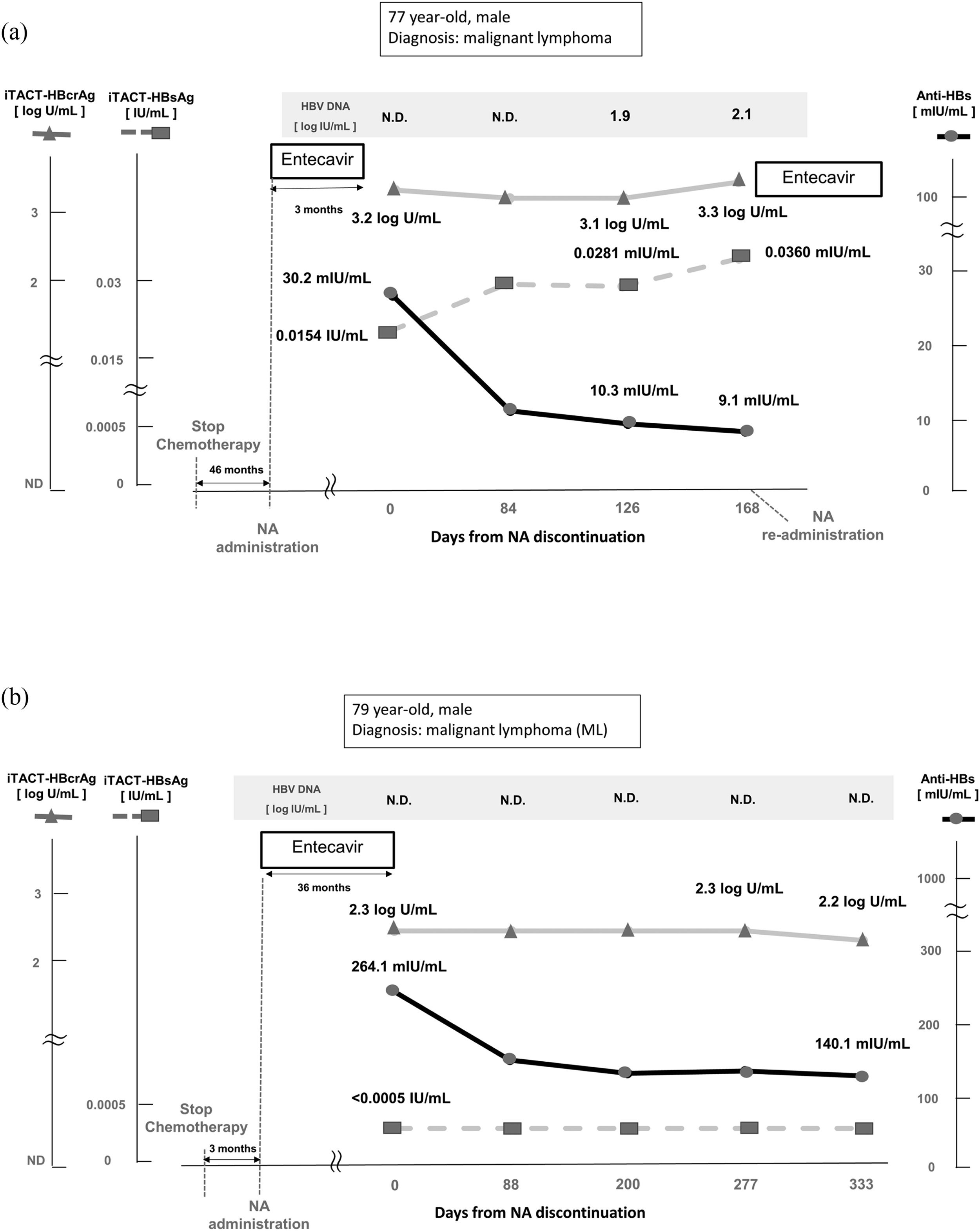
1) Case No 9 in Table 2 was a 77-year-old male at the cessation of NA. He was diagnosed with malignant lymphoma (ML) and received CHOP therapy (cyclophosphamide plus doxorubicin plus oncovin plus prednisolone), followed by mogamulizumab therapy. Entecavir (ETV) treatment to prevent against HBV reactivation had been administered for 3 months and was subsequently discontinued. At the cessation of NA, HBV DNA was not detected, the level of HBcrAg was 3.2 log U/mL, HBsAg was 0.0154 IU/mL, and anti-HBs was 30.2 mIU/mL. After NA cessation, the level of HBcrAg remained unchanged, but the level of HBsAg increased gradually, whereas the level of anti-HBs decreased gradually. Finally, at 126 days after the cessation of NA, HBV DNA was detected (1.9 log IU/mL), HBcrAg level was 3.1 log U/mL, HBsAg level was 0.0281 IU/mL, and anti-HBs titer was 10.3 mIU/mL. At 168 days after cessation of ETV, HBV DNA level increased to 2.1 log IU/mL, HBcrAg level was 3.3 log U/mL, level of HBsAg was 0.0360 IU/mL, and anti-HBs titer was 9.1 mIU/mL, after which NA therapy was re-introduced (Fig. 3A).
Clinical course of two representative cases who were administered and subsequently terminated NA therapy for prevention of HBV reactivation.
This figure shows the clinical course, including the evolution of HBV DNA, iTACT-HBcrAg, iTACT-HBsAg, and anti-HBs in two representative cases who were administered and subsequently terminated NA therapy.
Abbreviations: NA, nucleos(t)ide analogues; iTACT-HBcrAg, high-sensitivity hepatitis B core-related antigen assay; iTACT-HBsAg, ultra-high-sensitivity hepatitis B surface antigen assay; anti-HBs, hepatitis B surface antibody; HBV, hepatitis B virus; N.D., not detected.
2) Case No 13 in Table 2 was a 79-year-old male at the cessation of NA. He was also diagnosed with ML and received modified LSG15 therapy (prednisolone, vincristine, doxorubicin, cyclophosphamide, ranimustine, vindesine, etoposide, carboplatin), followed by mogamulizumab therapy. Administration of ETV was administered for 36 months and was subsequently discontinued. At the cessation of NA, HBV DNA was not detected, the level of HBcrAg was 2.3 log U/mL, HBsAg was <0.0005 IU/mL, and anti-HBs was 264.1 mIU/ml. After cessation of NA, the levels of HBcrAg and HBsAg were unchanged, but the level of anti-HBs was slightly decreased. At 333 days after cessation of NA, HBV DNA was not detected, the level of HBcrAg was 2.2 log U/mL, HBsAg remained <0.0005 IU/mL, and anti-HBs was 140.1 mIU/mL (Fig. 3B).
4DiscussionIn this study, we showed that the ultra-high-sensitivity HBsAg assay, iTACT-HBsAg, was the most useful for determining the cessation of NA treatment which was introduced to combat against HBV reactivation. In addition, it was more even more useful to combine the results of the iTACT-HBsAg and anti-HBs assays (Fig. 2).
Up to the present, very few reports showed that the levels of HBcrAg and anti-HBs were surrogate markers for determining the cessation of NA therapy [12,13]. In these reports, the recurrence of HBV reactivation after NA cessation was not observed in most of the patients who had an undetectable level of HBcrAg (<2.1 log U/mL) by the iTACT-HBcrAg assay or were seropositive for ani-HBs (≥10 mIU/mL) during follow-up [12]. Further, the recurrence of HBV reactivation after NA cessation was also not observed in patients positive for anti-HBs (>10mIU/mL) at cessation of NA [13]. In this study, we compared the usefulness of the iTACT-HBsAg, iTACT-HBcrAg, and anti-HBs assays, and showed that the iTACT-HBsAg assay had the highest PPV and the best overall diagnostic performance for predicting non-VR after cessation of NA.
Resolved HBV infection is characterized by HBsAg seroclearance with anti-HBc and/or anti-HBs. However, even if HBsAg seroclearance is achieved in HBV-infected patients, the HBV genome is known to be present in the liver as transcriptionally inactive covalently closed circular DNA (cccDNA) and/or integrated HBV DNA [15]. HBV reactivation could occur under immunosuppressive conditions from the cccDNA in the liver. Previous reports revealed that the levels of HBsAg and HBcrAg in peripheral blood correlated with the amount of cccDNA in the liver [16–18]. From these findings and our results, HBsAg and HBcrAg levels detected by the iTACT assays may reflect the amount of cccDNA in the liver more precisely than conventional assays, and detection of these markers could be a risk factor for VR after NA discontinuation. Therefore, they could be useful for determination of cessation of NA therapy which is introduced to prevent against HBV reactivation.
This study also revealed that a high titer of anti-HBs (≥100 mIU/mL) at the cessation of NA was useful for prediction of a non-VR outcome, but only a small number of cases met this criterion in the present study (6/22, 27 %). Previous reports showed that amplification of anti-HBs by HB vaccination could possibly prevent HBV reactivation in hematopoietic stem cell transplantation recipients [19,20]. However, the problem with anti-HBs is that HBV with a surface antigen escape mutation may appear in patients with high titers of anti-HBs [21,22]. As previously reported, among patients who were administered and subsequently terminated NA treatment, those with anti-HBs and an escape mutant had a higher probability of VR after cessation of NA [21,23]. The iTACT-HBsAg assay has been verified to detect various HBsAg mutations (Table S1). Thus, the advantages of the iTACT-HBsAg assay are that it can detect HBsAg with high sensitivity and also detect HBsAg escape mutants. Therefore, a combination of the iTACT-HBsAg and anti-HBs assays may be effective in determination of NA cessation. In fact, 2 of 15 patients with HBsAg <0.0005 IU/mL at the cessation of NA developed VR, but 10 patients with HBsAg <0.0005 IU/mL and anti-HBs ≥10 mIU/mL at the cessation of NA had no VR thereafter (Fig. 2). Further studies are warranted to confirm this point.
Various guidelines for hepatitis B indicate that NA for preventing HBV reactivation may be discontinued if administration of NA is introduced for a long enough period after completion of immunosuppressive treatment or chemotherapy [7,15,24]. Our data showed that the patients with HBcrAg <2.1 log U/mL by the iTACT-HBcrAg assay at NA cessation tended to include more cases with duration of NA therapy ≥12 months (P = 0.079) (Table 4), but the period of NA administration did not affect VR (data not shown). Therefore, we suggest that it may be better to determine cessation of NA by the levels of HBV markers, HBsAg, HBcrAg, and anti-HBs, rather than the period of NA administration. Further studies are also warranted to confirm this point.
Several limitations of this study are listed as follows. First, this is an observational study, and the findings might include selection bias. In fact, this study included one patient with breast cancer, unlike hematological diseases. Thus, there is the lack of uniformity in the study patients. Second, cessation of NA therapy was based on each physician's judgement and not by the same protocol. Therefore, several patients had taken NA for short periods (< 12 months). Third, we measured the levels of HBsAg, HBcrAg, and anti-HBs after cessation of NA in only a few cases, since the examinations were carried out retrospectively using stored serum samples. As a result, we showed the kinetics of HBcrAg, HBsAg, and anti-HBs levels in only two cases (Fig. 3), therefore extrapolating these findings to similar populations should be approached with caution. Fourth, despite the multi-center setting, the number of cases in this study was small, because the majority of patients who were introduced to NAs after HBV reactivation often remain on NA, and the number of cases with stored serum samples for measuring iTACT-HBsAg and iTACT-HBcrAg at the time of NA cessation was limited. Therefore, we could not show statistically significant differences of the values of various markers. Further prospective studies are required. Finally, VR after NA discontinuation was noted in this study, but the observation periods after NA discontinuation might not be long enough (median was 19 months). Most cases of VR become positive for HBV DNA in the quantitative range within one year after NA cessation (Table 2). In contrast, the median observation period after NA cessation in non-VR cases was 40 (IQR: 15-103) months. We therefore consider to be relatively sufficient observation periods after NA cessation. However, we could not investigate the relationship between the time to VR after NA discontinuation and HBV markers adequately, due to the small number of cases. Further investigations in longer-term follow-up are needed.
5ConclusionsBased on a multicenter setting, we confirmed that the iTACT-HBsAg assay was useful for determination of cessation of NA therapy which was introduced as a preventative measure against HBV reactivation. Further studies should be conducted to evaluate the usefulness of HBsAg as well as HBcrAg as biomarkers for the clinical management of patients with HBV infection.
Short title: New HBsAg assay for HBV-R