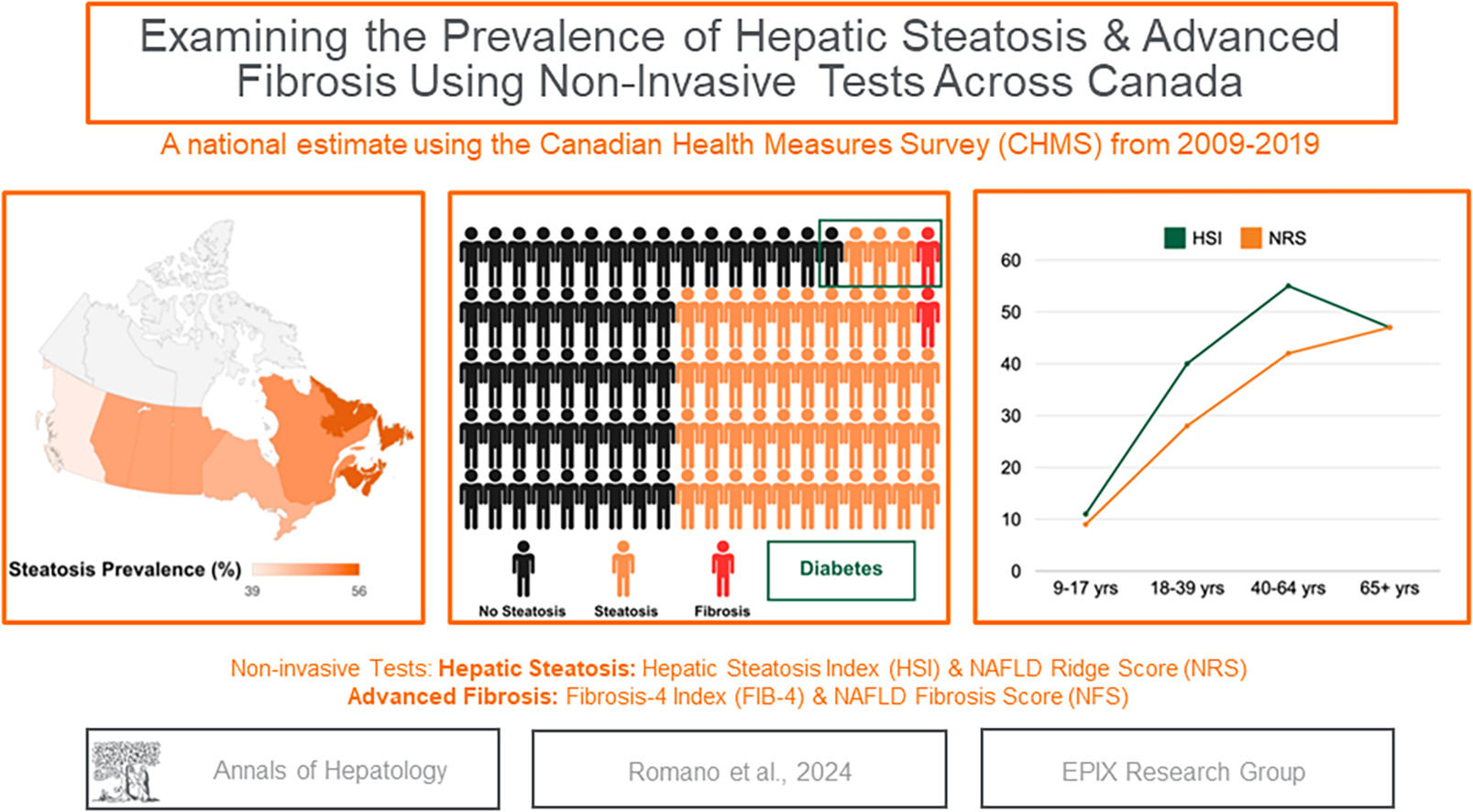
The global prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) is estimated to be 38.0 % (95 % confidence interval (CI): 33.7 % to 42.5 %), with projections indicating incidence is likely to continue to rise [1–3]. The MASLD spectrum begins with hepatic steatosis, which involves fat accumulation within hepatocytes [4]. Over time, these hepatocytes can become injured and inflamed, leading to the development of metabolic dysfunction-associated steatohepatitis (MASH). Approximately 20 % of individuals with MASH will progress to hepatic fibrosis [5]. Advanced liver fibrosis is an important prognostic indicator in people with MASH as it places individuals at heightened risk for developing cirrhosis, liver failure, portal hypertension and hepatocellular carcinoma (HCC) [6–8]. The rise in MASLD prevalence is primarily fueled by the twin obesity and type-2 diabetes mellitus (T2DM) epidemics [9]. Co-morbid metabolic conditions not only increase the likelihood of MASLD but also intensify the risk of a person advancing to severe liver disease and mortality [10]. Data from the United Network of Organ Sharing reveals that MASLD is now the second most common etiology for liver transplantation in the United States [11].
Despite MASLD being a highly prevalent chronic liver disease (CLD) globally, epidemiological data on the sequelae of MASLD is limited in Canada. A recent study evaluating global preparedness to address MASLD as a public health threat found that most countries were unprepared [12]. Canada was no exception, highlighting the need for policies, guidelines, epidemiological data, and enhanced surveillance of MASLD [12]. Except for one modelling study by Swain et al. in 2020, which used obesity/T2DM rates as a proxy to estimate the prevalence of MASLD, there have not been any generalizable prevalence studies of hepatic steatosis and fibrosis in Canada [9].
Canada is a high-income country with an ethnically diverse population. While Canada has a universal health care system, it is segmented between provinces, resulting in differences in services across the country. Estimating the burden of disease is crucial for understanding and improving preparedness to prevent and manage liver disease. In the absence of systematic screening, nationally representative surveys have been used to estimate the prevalence of chronic conditions in the USA and other countries [13,14]. However, unlike other countries, in Canada national surveys do not include routine liver ultrasounds, transient elastography or other imaging technologies, leaving serum-based non-invasive tests (NITs) as the only option to assess liver disease stages. In this study, we leveraged data from the Canadian Health Measures Survey (CHMS), a nationally representative survey across Canada, to estimate the prevalence of hepatic steatosis and advanced fibrosis overall and by sociodemographic subgroups, using validated NITs between 2009 and 2019.
2Materials and methods2.1Data sourceWe used the Canadian Health Measures Survey (CHMS), a nationally representative direct health measures survey – the largest of its kind in Canada. The CMHS employs a stratified sampling approach to gather comprehensive information across sexes, age groups, geographical areas, and socioeconomic backgrounds. The objective of this survey is to acquire data for enhancing disease prevention, diagnosis, and treatment while also contributing to the overall improvement of the health and well-being of Canadians [15]. Cross-sectional data on infectious, chronic, and environmental diseases, along with detailed health information, including height, weight, panel blood chemistry, blood pressure, and other metrics, have been collected since 2007 through a combination of physical examinations and interviews [15].
2.2Study populationData from cycles 2, 4, and 6 of the CHMS were pooled and analyzed, representing survey collection between 2009 and 2019. Participants aged 9–79 with completedata necessary to calculate the chosen NITs were included in the study. We excluded participants who tested positive for hepatitis B or C virus and human immunodeficiency virus (HIV)/ acquired immunodeficiency syndrome (AIDS) to allow for direct comparison with similar studies in the United States.
2.3Demographic characteristicsChildren were defined by age as 9–17 years old. Adults sociodemographic and clinical characteristics obtained during the household survey collection include - age groups (18 to 39, 40 to 64, ≥65), sex, province of residence (Alberta, Atlantic [New Brunswick, Newfoundland and Labrador, Nova Scotia, and P.E.I,], British Columbia, Prairies [Manitoba and Saskatchewan], Ontario, Quebec), race (White, Black, Asian [Filipino, Japanese, Chinese, Korean, South Asian, Southeast Asian], other [Arab, West Asian, Latin American, other racial or cultural origin], not stated), income (<$10,000, $10,001 - $30,000, $30,001 - $65,000, $65,001 -$100,000, $100,001+, not stated), and weekly alcohol intake (0 drinks/week, 1–3 drinks/week, 4–15 drinks/week, 15+ drinks/week). Body mass index (BMI) [weight (kg)/ height (m2)] and waist circumference (cm) were recorded during physical examination. Self-reported diagnoses of chronic conditions, including T2DM [16], were also recorded. Results from blood chemistry included aspartate aminotransferase (AST, U/L), alanine aminotransferase (ALT, U/L), albumin (g/L), gamma-glutamyltransferase (GGT, U/L), triglycerides (mmol/L), platelet count (109/L), white blood cell count (WBC, 109/L), hemoglobin A1c (HbA1c, %), and high-density lipoprotein cholesterol (HDL-C, mmol/l).
2.4Non-invasive tests to measure the likelihood of hepatic steatosis and advanced fibrosisThe presence of hepatic steatosis and advanced liver fibrosis were each determined using two non-invasive tests. The following NITs were selected based on comparability to similar studies in other countries as well as those that can be used in future Canadian studies based on availability of the components to calculate the NIT score.
2.4.1Hepatic steatosis measuresThe Hepatic Steatosis Index (HSI) utilizes measurements of ALT, AST, BMI, as well as the presence of T2DM and sex: an HSI value of >36 is associated with the presence of hepatic steatosis and <30 can be used to rule out steatosis. Values that fall between 30 and 36 are considered indeterminate [17]. This non-invasive marker has been used in numerous epidemiological studies[18,19] based on the following formula: [17]
The NAFLD Ridge Score (NRS) is a newer NIT that was developed using machine learning methods to identify steatosis with routinely collected serum results as opposed to anthropometric measures. The NRS uses measurements of ALT, HDL-C, TG, HbA1c, WBC, and a binary indicator of hypertension [20]. The NRS employs a dual threshold: individuals with a score <0.24 are considered free from hepatic steatosis, those with a score between 0.24 and 0.44 are categorized as indeterminate, and a score >0.44 signifies the presence of hepatic steatosis [20]. In previous longitudinal and cross-sectional studies, the NRS has been used to detect hepatic steatosis [19,21] based on the following formula:
2.4.2Advanced fibrosis measuresThe FIB-4 index combines age, AST, ALT, and platelet count. A FIB-4 index < 1.3 is categorized as low risk of fibrosis (sensitivity 74 %, specificity 71 %), while a FIB-4 index > 2.67 is categorized as high risk (sensitivity 33 %, specificity 98 %) [22]. Scores between 1.3 and 2.67 are considered indeterminate for advanced fibrosis. The FIB-4 index is currently recommended by most international liver associations as a screening tool for liver fibrosis [23,24]. The FIB-4 is based on the following formula [22]:
The NAFLD Fibrosis Score (NFS) identifies individuals at risk for significant liver fibrosis related to steatosis by utilizing age, BMI, diabetes, AST/ALT ratio, platelet count, and albumin based on the below formula. Scores > 0.676 indicate a high probability of advanced fibrosis (sensitivity 51 %, specificity 98 %), values between –1.455 and 0.676 indicate an indeterminate probability of advanced fibrosis, and < –1.455 suggest a low probability of advanced fibrosis (sensitivity 82 %, specificity 77 %) [25]. The NFS has also been used in epidemiological studies [13,26].
2.5Statistical analysisWe performed a complete case analysis; therefore, participants missing BMI or panel chemistry data necessary to calculate the non-invasive tests, were excluded. Descriptive statistics for categorical variables were reported as a proportion with 95 % confidence intervals (CI) calculated using the logit method. Continuous variables are described as median with 25th and 75th percentiles representing interquartile range (IQR) or mean with 95 % CI. We report the overall prevalence of steatosis in children. Fibrosis status was assessed amongst those who had steatosis based on the HSI. All other predefined subgroups of steatosis and fibrosis prevalence for steatosis and advanced fibrosis were among adults. Survey weights were used to account for the complex survey design, such as oversampling, survey nonresponse, and post-stratification, to ensure that calculated estimates are representative of the Canadian population. We examined the agreement between the NITs using Cohen Kappa statistics by comparing those with and without steatosis (indeterminate excluded) and those with and without fibrosis (excluding indeterminate results). All statistical analyses were conducted using Stata version 17 (Stata Corporation, College Station, TX, USA).
2.6Ethical statementsThe information obtained through the CHMS is subject to the protection provided by the federal Statistics Act. Participation in this survey was voluntary, allowing participants to withdraw from any section of the survey at their discretion. Participants in the survey provided informed written consent [27]. The Health Canada's Research Ethics Board of Canada approved the CHMS in addition to the Office of the Privacy Commissioner of Canada [27]. A thorough security check was required to obtain access to the CHMS and the commitment to signing The Statistics Act Oath or Solemn Affirmation of Office and Secrecy[27]. The study only presents summarized results to maintain privacy and confidentiality, avoiding small cell counts (<15 observations) to protect individual or personal anonymity.
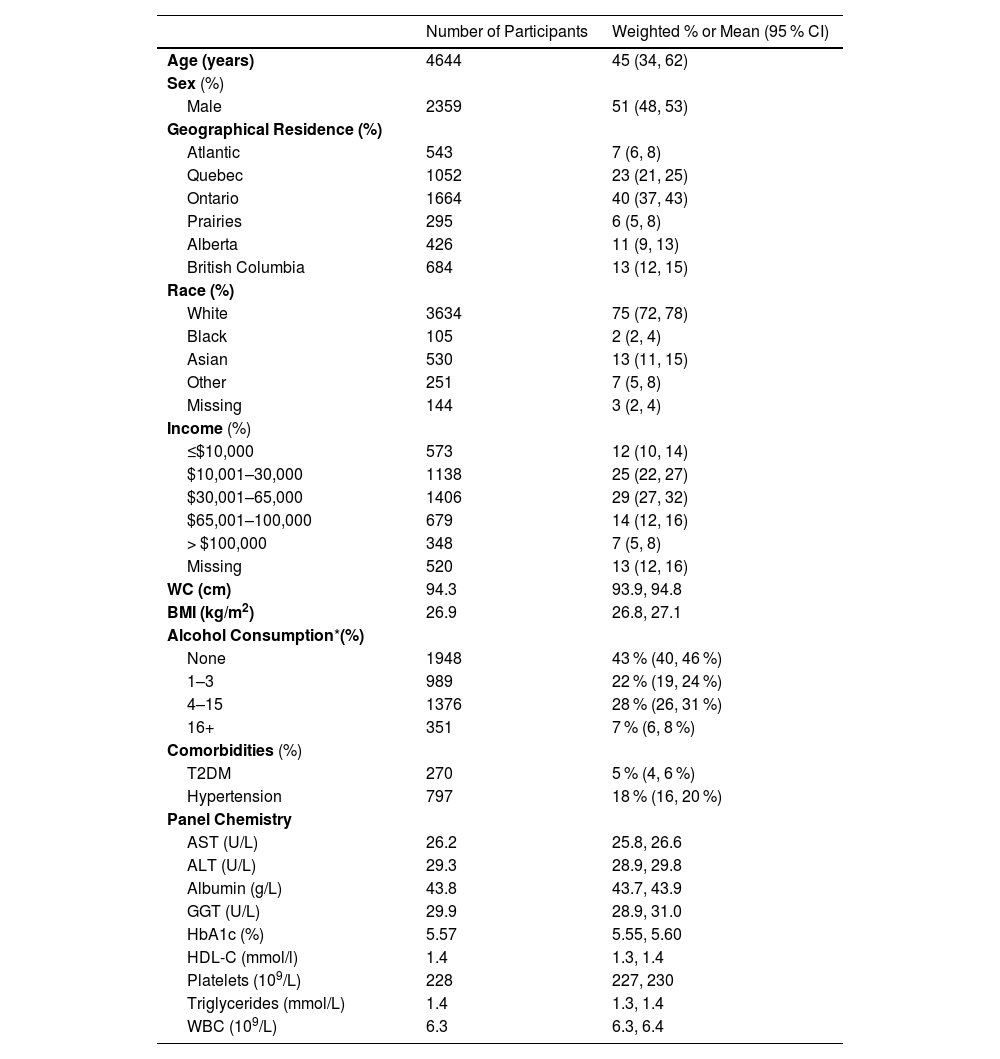
3Results3.1Survey demographicsA total of 17,986 people aged 9 to 79 from cycle 2 (n = 6395), cycle 4 (n = 5794), and cycle 6 (n = 5797) of the CHMS completed the survey. Between 2009 and 2019, after exclusion criteria, 1365 children and 4664 adults were included in this study, representing a national estimate of 37 million people in Canada. Among the children, the median age was 13 (IQR: 10–15), and 55 % were male (95 % CI: 50–59 %). The median age of the adults was 45 (IQR: 34, 62); 51 % were male; 75 % self-identified as White, 29 % had a household income of $30,000–65,000/year; 57 % consumed alcohol; 5 % had T2DM; 18 % had hypertension, and there was representation from all the provinces in Canada (Table 1).
Characteristics of adults (n = 4664) from the CHMS 2009–2019.
| Number of Participants | Weighted % or Mean (95 % CI) | |
|---|---|---|
| Age (years) | 4644 | 45 (34, 62) |
| Sex (%) | ||
| Male | 2359 | 51 (48, 53) |
| Geographical Residence (%) | ||
| Atlantic | 543 | 7 (6, 8) |
| Quebec | 1052 | 23 (21, 25) |
| Ontario | 1664 | 40 (37, 43) |
| Prairies | 295 | 6 (5, 8) |
| Alberta | 426 | 11 (9, 13) |
| British Columbia | 684 | 13 (12, 15) |
| Race (%) | ||
| White | 3634 | 75 (72, 78) |
| Black | 105 | 2 (2, 4) |
| Asian | 530 | 13 (11, 15) |
| Other | 251 | 7 (5, 8) |
| Missing | 144 | 3 (2, 4) |
| Income (%) | ||
| ≤$10,000 | 573 | 12 (10, 14) |
| $10,001–30,000 | 1138 | 25 (22, 27) |
| $30,001–65,000 | 1406 | 29 (27, 32) |
| $65,001–100,000 | 679 | 14 (12, 16) |
| > $100,000 | 348 | 7 (5, 8) |
| Missing | 520 | 13 (12, 16) |
| WC (cm) | 94.3 | 93.9, 94.8 |
| BMI (kg/m2) | 26.9 | 26.8, 27.1 |
| Alcohol Consumption*(%) | ||
| None | 1948 | 43 % (40, 46 %) |
| 1–3 | 989 | 22 % (19, 24 %) |
| 4–15 | 1376 | 28 % (26, 31 %) |
| 16+ | 351 | 7 % (6, 8 %) |
| Comorbidities (%) | ||
| T2DM | 270 | 5 % (4, 6 %) |
| Hypertension | 797 | 18 % (16, 20 %) |
| Panel Chemistry | ||
| AST (U/L) | 26.2 | 25.8, 26.6 |
| ALT (U/L) | 29.3 | 28.9, 29.8 |
| Albumin (g/L) | 43.8 | 43.7, 43.9 |
| GGT (U/L) | 29.9 | 28.9, 31.0 |
| HbA1c (%) | 5.57 | 5.55, 5.60 |
| HDL-C (mmol/l) | 1.4 | 1.3, 1.4 |
| Platelets (109/L) | 228 | 227, 230 |
| Triglycerides (mmol/L) | 1.4 | 1.3, 1.4 |
| WBC (109/L) | 6.3 | 6.3, 6.4 |
Note:.
Alcohol consumption is defined as the number of drinks per week.
Abbreviations: CI, Confidence Interval; WC, Waist Circumference; BMI, Body Mass Index [kg/height(m)2]; T2DM, Type 2 Diabetes Mellitus; AST, Aspartate transaminase; ALT, Alanine transaminase; GGT; Gamma-glutamyltransferase; HbA1c; Hemoglobin A1c; HDL-C, High-density lipoprotein cholesterol; WBC, White blood cells.
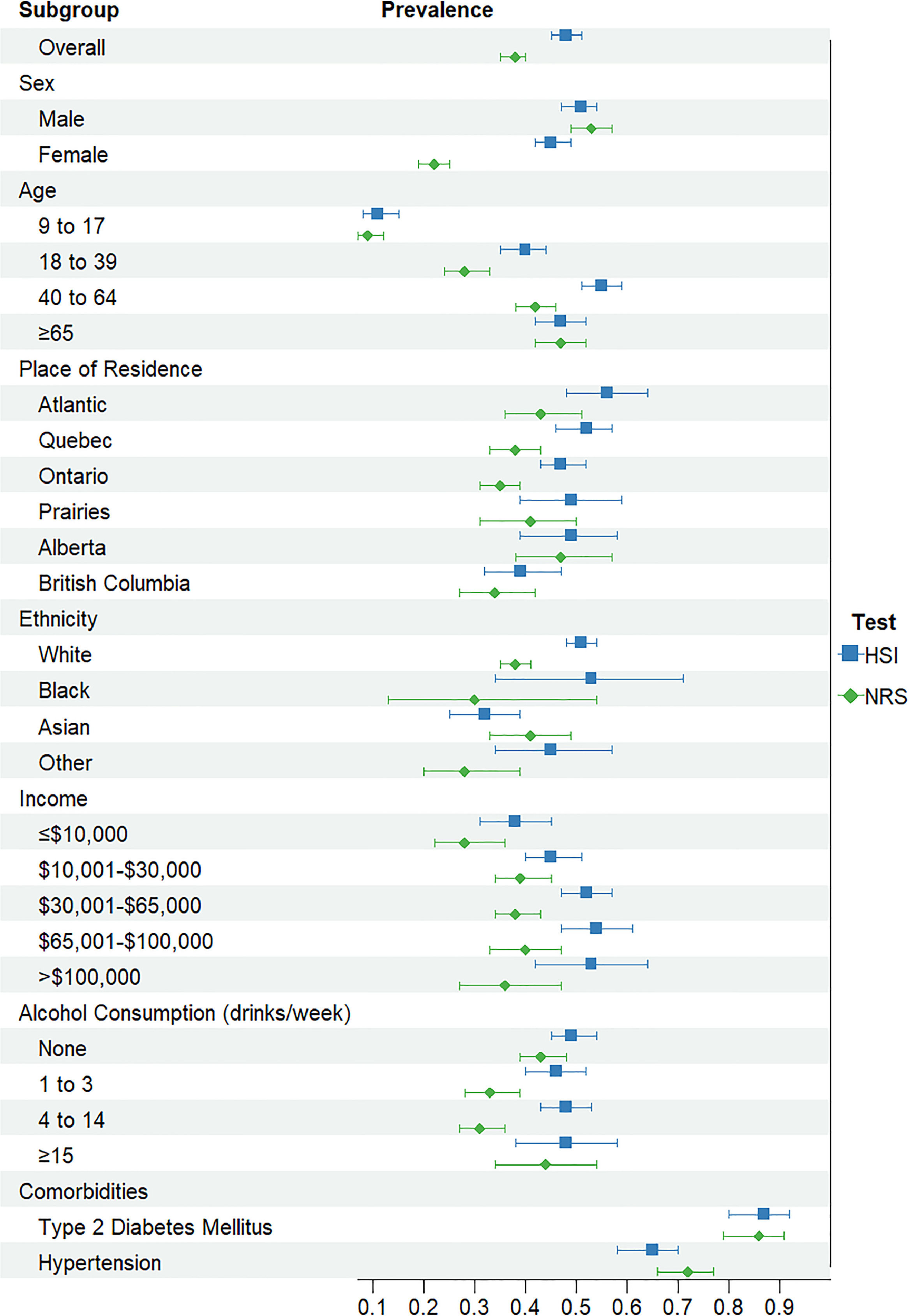
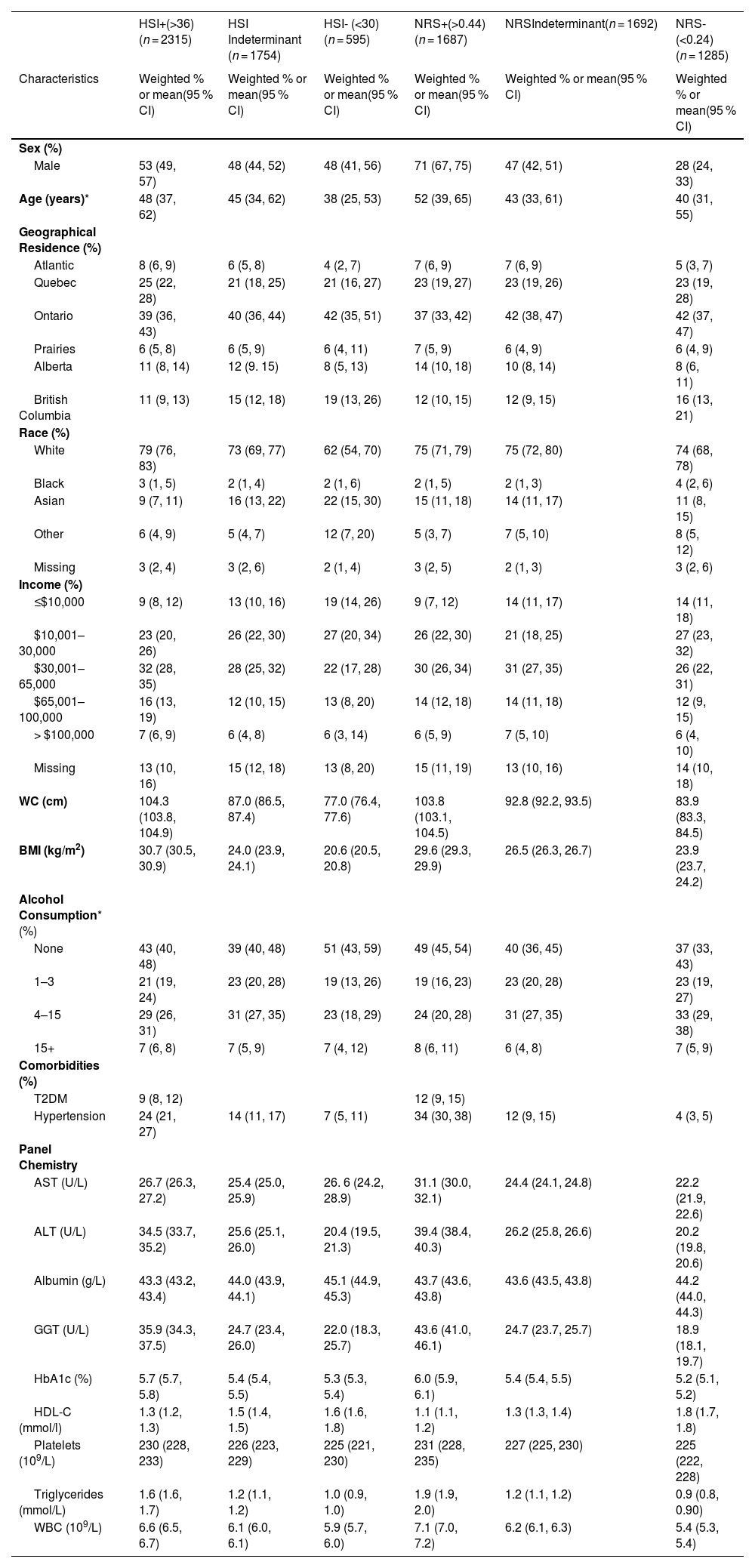
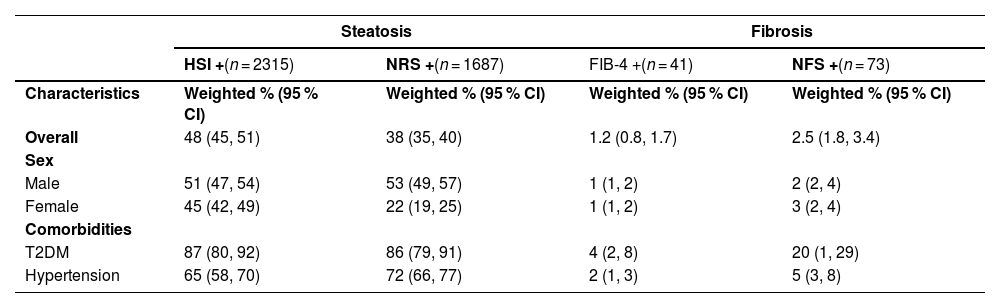
The adult population identified as having steatosis based on HSI (n = 2315) was 53 % male, with a median age of 48 years old, 79 % were white, 32 % had an income of $30,000–65,000, 57 % reported drinking alcohol, 9 % had T2DM, and 24 % had hypertension (Table 2). The overall prevalence of steatosis varied based on the NITs but was high across sociodemographic and clinical factors (Fig. 1& Supplemental Table 1). There was agreement between HSI and NRS, with a kappa statistic of 0.62. Based on the HSI, 2315 adults met the criteria for steatosis, representing 16 million Canadian adults or an overall prevalence of 48 % (95 % CI: 45–51 %). A total of 1687 adults met the criteria for steatosis, according to the NRS, with an overall prevalence of 38.0 % (95 % CI: 35.0–40.3 %). Overall, the HSI estimated higher prevalence than NRS, particularly for females, 45 % (95 % CI: 42–49 %) compared to 22 % (95 % CI: 19–25 %), respectively. Between 9 % (95 % CI: 7–12 %) and 11 % (95 % CI: 8–15 %) of children had evidence of steatosis, based on the NRS and HSI, respectively and prevalence increased by age groups.
Characteristics of the adult population (n = 4664) by HSI and the NRS cut-offs
| HSI+(>36) (n = 2315) | HSI Indeterminant (n = 1754) | HSI- (<30) (n = 595) | NRS+(>0.44)(n = 1687) | NRSIndeterminant(n = 1692) | NRS-(<0.24)(n = 1285) | |
|---|---|---|---|---|---|---|
| Characteristics | Weighted % or mean(95 % CI) | Weighted % or mean(95 % CI) | Weighted % or mean(95 % CI) | Weighted % or mean(95 % CI) | Weighted % or mean(95 % CI) | Weighted % or mean(95 % CI) |
| Sex (%) | ||||||
| Male | 53 (49, 57) | 48 (44, 52) | 48 (41, 56) | 71 (67, 75) | 47 (42, 51) | 28 (24, 33) |
| Age (years)* | 48 (37, 62) | 45 (34, 62) | 38 (25, 53) | 52 (39, 65) | 43 (33, 61) | 40 (31, 55) |
| Geographical Residence (%) | ||||||
| Atlantic | 8 (6, 9) | 6 (5, 8) | 4 (2, 7) | 7 (6, 9) | 7 (6, 9) | 5 (3, 7) |
| Quebec | 25 (22, 28) | 21 (18, 25) | 21 (16, 27) | 23 (19, 27) | 23 (19, 26) | 23 (19, 28) |
| Ontario | 39 (36, 43) | 40 (36, 44) | 42 (35, 51) | 37 (33, 42) | 42 (38, 47) | 42 (37, 47) |
| Prairies | 6 (5, 8) | 6 (5, 9) | 6 (4, 11) | 7 (5, 9) | 6 (4, 9) | 6 (4, 9) |
| Alberta | 11 (8, 14) | 12 (9. 15) | 8 (5, 13) | 14 (10, 18) | 10 (8, 14) | 8 (6, 11) |
| British Columbia | 11 (9, 13) | 15 (12, 18) | 19 (13, 26) | 12 (10, 15) | 12 (9, 15) | 16 (13, 21) |
| Race (%) | ||||||
| White | 79 (76, 83) | 73 (69, 77) | 62 (54, 70) | 75 (71, 79) | 75 (72, 80) | 74 (68, 78) |
| Black | 3 (1, 5) | 2 (1, 4) | 2 (1, 6) | 2 (1, 5) | 2 (1, 3) | 4 (2, 6) |
| Asian | 9 (7, 11) | 16 (13, 22) | 22 (15, 30) | 15 (11, 18) | 14 (11, 17) | 11 (8, 15) |
| Other | 6 (4, 9) | 5 (4, 7) | 12 (7, 20) | 5 (3, 7) | 7 (5, 10) | 8 (5, 12) |
| Missing | 3 (2, 4) | 3 (2, 6) | 2 (1, 4) | 3 (2, 5) | 2 (1, 3) | 3 (2, 6) |
| Income (%) | ||||||
| ≤$10,000 | 9 (8, 12) | 13 (10, 16) | 19 (14, 26) | 9 (7, 12) | 14 (11, 17) | 14 (11, 18) |
| $10,001–30,000 | 23 (20, 26) | 26 (22, 30) | 27 (20, 34) | 26 (22, 30) | 21 (18, 25) | 27 (23, 32) |
| $30,001–65,000 | 32 (28, 35) | 28 (25, 32) | 22 (17, 28) | 30 (26, 34) | 31 (27, 35) | 26 (22, 31) |
| $65,001–100,000 | 16 (13, 19) | 12 (10, 15) | 13 (8, 20) | 14 (12, 18) | 14 (11, 18) | 12 (9, 15) |
| > $100,000 | 7 (6, 9) | 6 (4, 8) | 6 (3, 14) | 6 (5, 9) | 7 (5, 10) | 6 (4, 10) |
| Missing | 13 (10, 16) | 15 (12, 18) | 13 (8, 20) | 15 (11, 19) | 13 (10, 16) | 14 (10, 18) |
| WC (cm) | 104.3 (103.8, 104.9) | 87.0 (86.5, 87.4) | 77.0 (76.4, 77.6) | 103.8 (103.1, 104.5) | 92.8 (92.2, 93.5) | 83.9 (83.3, 84.5) |
| BMI (kg/m2) | 30.7 (30.5, 30.9) | 24.0 (23.9, 24.1) | 20.6 (20.5, 20.8) | 29.6 (29.3, 29.9) | 26.5 (26.3, 26.7) | 23.9 (23.7, 24.2) |
| Alcohol Consumption* (%) | ||||||
| None | 43 (40, 48) | 39 (40, 48) | 51 (43, 59) | 49 (45, 54) | 40 (36, 45) | 37 (33, 43) |
| 1–3 | 21 (19, 24) | 23 (20, 28) | 19 (13, 26) | 19 (16, 23) | 23 (20, 28) | 23 (19, 27) |
| 4–15 | 29 (26, 31) | 31 (27, 35) | 23 (18, 29) | 24 (20, 28) | 31 (27, 35) | 33 (29, 38) |
| 15+ | 7 (6, 8) | 7 (5, 9) | 7 (4, 12) | 8 (6, 11) | 6 (4, 8) | 7 (5, 9) |
| Comorbidities (%) | ||||||
| T2DM | 9 (8, 12) | 12 (9, 15) | ||||
| Hypertension | 24 (21, 27) | 14 (11, 17) | 7 (5, 11) | 34 (30, 38) | 12 (9, 15) | 4 (3, 5) |
| Panel Chemistry | ||||||
| AST (U/L) | 26.7 (26.3, 27.2) | 25.4 (25.0, 25.9) | 26. 6 (24.2, 28.9) | 31.1 (30.0, 32.1) | 24.4 (24.1, 24.8) | 22.2 (21.9, 22.6) |
| ALT (U/L) | 34.5 (33.7, 35.2) | 25.6 (25.1, 26.0) | 20.4 (19.5, 21.3) | 39.4 (38.4, 40.3) | 26.2 (25.8, 26.6) | 20.2 (19.8, 20.6) |
| Albumin (g/L) | 43.3 (43.2, 43.4) | 44.0 (43.9, 44.1) | 45.1 (44.9, 45.3) | 43.7 (43.6, 43.8) | 43.6 (43.5, 43.8) | 44.2 (44.0, 44.3) |
| GGT (U/L) | 35.9 (34.3, 37.5) | 24.7 (23.4, 26.0) | 22.0 (18.3, 25.7) | 43.6 (41.0, 46.1) | 24.7 (23.7, 25.7) | 18.9 (18.1, 19.7) |
| HbA1c (%) | 5.7 (5.7, 5.8) | 5.4 (5.4, 5.5) | 5.3 (5.3, 5.4) | 6.0 (5.9, 6.1) | 5.4 (5.4, 5.5) | 5.2 (5.1, 5.2) |
| HDL-C (mmol/l) | 1.3 (1.2, 1.3) | 1.5 (1.4, 1.5) | 1.6 (1.6, 1.8) | 1.1 (1.1, 1.2) | 1.3 (1.3, 1.4) | 1.8 (1.7, 1.8) |
| Platelets (109/L) | 230 (228, 233) | 226 (223, 229) | 225 (221, 230) | 231 (228, 235) | 227 (225, 230) | 225 (222, 228) |
| Triglycerides (mmol/L) | 1.6 (1.6, 1.7) | 1.2 (1.1, 1.2) | 1.0 (0.9, 1.0) | 1.9 (1.9, 2.0) | 1.2 (1.1, 1.2) | 0.9 (0.8, 0.90) |
| WBC (109/L) | 6.6 (6.5, 6.7) | 6.1 (6.0, 6.1) | 5.9 (5.7, 6.0) | 7.1 (7.0, 7.2) | 6.2 (6.1, 6.3) | 5.4 (5.3, 5.4) |
Note:.
Alcohol consumption is defined as the number of drinks per week.
Abbreviations: HSI; Hepatic Steatosis Index; NRS, NAFLD Ridge Score; CI, Confidence Interval; WC, Waist Circumference; BMI, Body Mass Index [kg/height(m)2]; T2DM, Type 2 Diabetes Mellitus; AST, Aspartate transaminase; ALT, Alanine transaminase; GGT; Gamma-glutamyltransferase; HbA1c; Hemoglobin A1c; HDL-C, High-density lipoprotein cholesterol; WBC, White blood cells.
Steatosis prevalence.
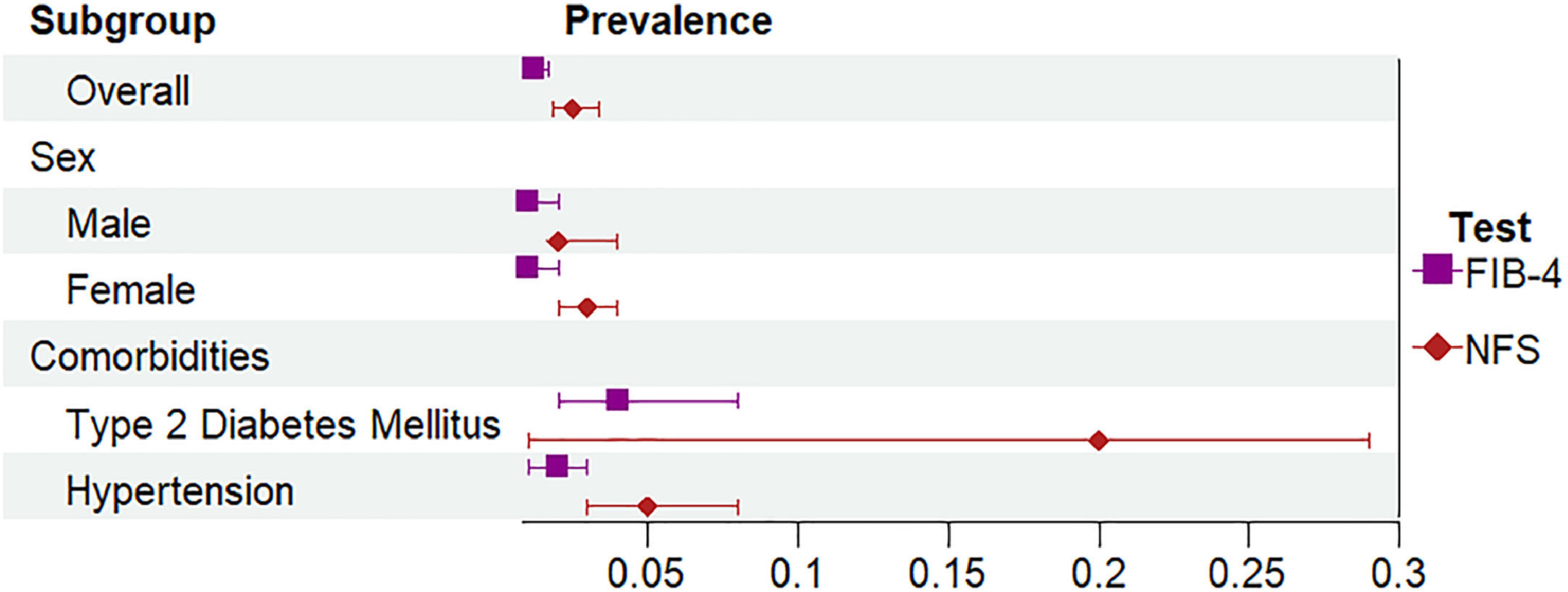
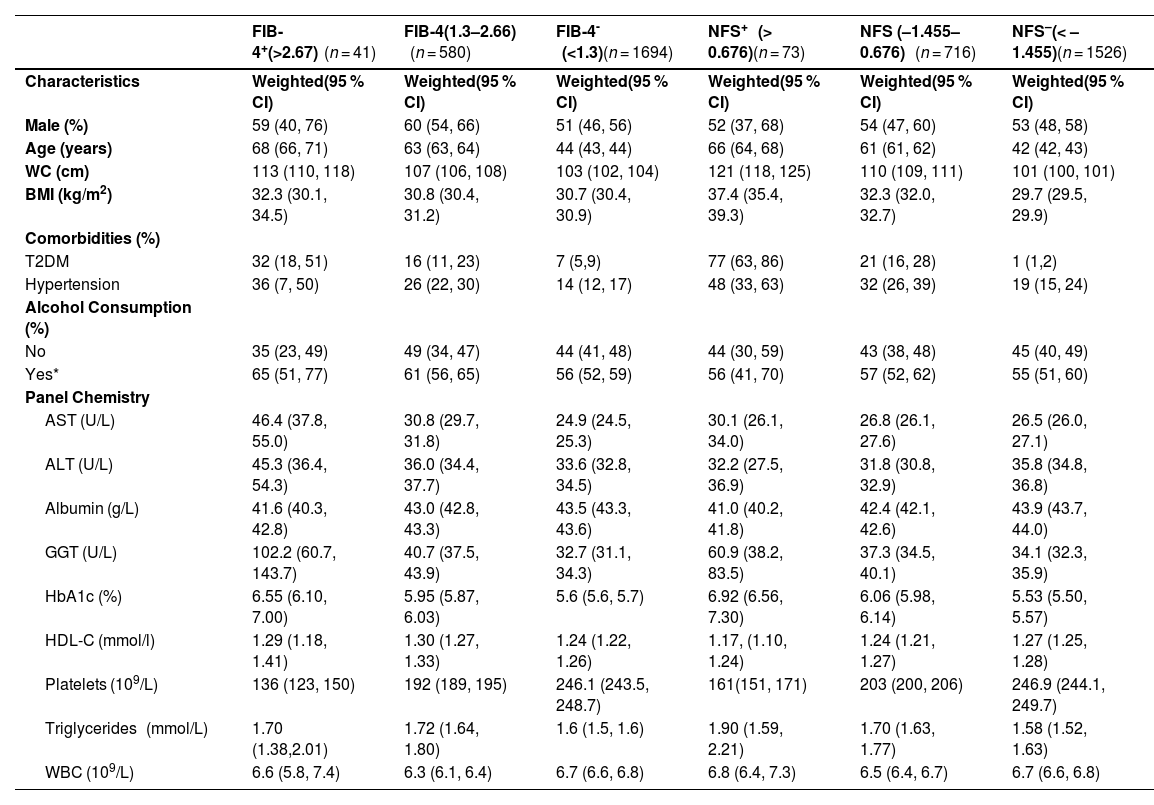
The adult population that was identified as high risk of advanced fibrosis (n = 41) based on FIB-4 was 59 % male, with a median age of 68 years old; 65 % reported drinking alcohol, 32 % had T2DM, and 36 % had hypertension (Table 3). Although prevalence of advanced liver fibrosis varied based on the NITs, kappa statistics indicated substantial agreement (kappa: 0.79 between NFS and FIB-4). The overall prevalence of advanced fibrosis was 1.2 % (95 % CI 0.8–1.7 %) based on FIB-4. Using the NFS, 73 adults were identified as having a high risk of liver fibrosis, representing a prevalence of 2.5 % (95 % CI 1.8–3.4 %) (Fig. 2 and Table 4). Overall, these estimates represent 195,000–406,200 people across Canada with steatosis who are at high risk of advanced liver disease. The percentage of participants estimated to have an indeterminate probability of having advanced fibrosis varied between 25.0 % using the FIB-4 and 31.5 % using the NFS (Table 3). People with T2DM were more likely to have liver fibrosis compared to those without T2DM (FIB-4: RR 5.8 (95 %CI: 3.1, 10.6) & NFS: RR 27.4, 95 %CI: 16.4, 45.9)). Those with hypertension were also more likely to have liver fibrosis (FIB-4: RR 2.9 (95 %CI: 1.6, 5.3) & NFS: RR 2.9 (95 %CI: 1.8, 4.5)).
Characteristics of the adult population with evidence of steatosis (based on HSI; n = 2315) by FIB-4 and NFS cut-offs.
| FIB-4+(>2.67) (n = 41) | FIB-4(1.3–2.66) (n = 580) | FIB-4- (<1.3)(n = 1694) | NFS+ (> 0.676)(n = 73) | NFS (–1.455–0.676) (n = 716) | NFS–(< – 1.455)(n = 1526) | |
|---|---|---|---|---|---|---|
| Characteristics | Weighted(95 % CI) | Weighted(95 % CI) | Weighted(95 % CI) | Weighted(95 % CI) | Weighted(95 % CI) | Weighted(95 % CI) |
| Male (%) | 59 (40, 76) | 60 (54, 66) | 51 (46, 56) | 52 (37, 68) | 54 (47, 60) | 53 (48, 58) |
| Age (years) | 68 (66, 71) | 63 (63, 64) | 44 (43, 44) | 66 (64, 68) | 61 (61, 62) | 42 (42, 43) |
| WC (cm) | 113 (110, 118) | 107 (106, 108) | 103 (102, 104) | 121 (118, 125) | 110 (109, 111) | 101 (100, 101) |
| BMI (kg/m2) | 32.3 (30.1, 34.5) | 30.8 (30.4, 31.2) | 30.7 (30.4, 30.9) | 37.4 (35.4, 39.3) | 32.3 (32.0, 32.7) | 29.7 (29.5, 29.9) |
| Comorbidities (%) | ||||||
| T2DM | 32 (18, 51) | 16 (11, 23) | 7 (5,9) | 77 (63, 86) | 21 (16, 28) | 1 (1,2) |
| Hypertension | 36 (7, 50) | 26 (22, 30) | 14 (12, 17) | 48 (33, 63) | 32 (26, 39) | 19 (15, 24) |
| Alcohol Consumption (%) | ||||||
| No | 35 (23, 49) | 49 (34, 47) | 44 (41, 48) | 44 (30, 59) | 43 (38, 48) | 45 (40, 49) |
| Yes* | 65 (51, 77) | 61 (56, 65) | 56 (52, 59) | 56 (41, 70) | 57 (52, 62) | 55 (51, 60) |
| Panel Chemistry | ||||||
| AST (U/L) | 46.4 (37.8, 55.0) | 30.8 (29.7, 31.8) | 24.9 (24.5, 25.3) | 30.1 (26.1, 34.0) | 26.8 (26.1, 27.6) | 26.5 (26.0, 27.1) |
| ALT (U/L) | 45.3 (36.4, 54.3) | 36.0 (34.4, 37.7) | 33.6 (32.8, 34.5) | 32.2 (27.5, 36.9) | 31.8 (30.8, 32.9) | 35.8 (34.8, 36.8) |
| Albumin (g/L) | 41.6 (40.3, 42.8) | 43.0 (42.8, 43.3) | 43.5 (43.3, 43.6) | 41.0 (40.2, 41.8) | 42.4 (42.1, 42.6) | 43.9 (43.7, 44.0) |
| GGT (U/L) | 102.2 (60.7, 143.7) | 40.7 (37.5, 43.9) | 32.7 (31.1, 34.3) | 60.9 (38.2, 83.5) | 37.3 (34.5, 40.1) | 34.1 (32.3, 35.9) |
| HbA1c (%) | 6.55 (6.10, 7.00) | 5.95 (5.87, 6.03) | 5.6 (5.6, 5.7) | 6.92 (6.56, 7.30) | 6.06 (5.98, 6.14) | 5.53 (5.50, 5.57) |
| HDL-C (mmol/l) | 1.29 (1.18, 1.41) | 1.30 (1.27, 1.33) | 1.24 (1.22, 1.26) | 1.17, (1.10, 1.24) | 1.24 (1.21, 1.27) | 1.27 (1.25, 1.28) |
| Platelets (109/L) | 136 (123, 150) | 192 (189, 195) | 246.1 (243.5, 248.7) | 161(151, 171) | 203 (200, 206) | 246.9 (244.1, 249.7) |
| Triglycerides (mmol/L) | 1.70 (1.38,2.01) | 1.72 (1.64, 1.80) | 1.6 (1.5, 1.6) | 1.90 (1.59, 2.21) | 1.70 (1.63, 1.77) | 1.58 (1.52, 1.63) |
| WBC (109/L) | 6.6 (5.8, 7.4) | 6.3 (6.1, 6.4) | 6.7 (6.6, 6.8) | 6.8 (6.4, 7.3) | 6.5 (6.4, 6.7) | 6.7 (6.6, 6.8) |
Note: All Data is reported as Mean and 95 % CI, with select subgroups presenting weighted percentage.
Yes to alcohol consumption refers to reporting at least one drink per week.
Abbreviations: FIB-4, FIB-4 Index; NFS; NAFLD Fibrosis Score; CI, Confidence Interval; WC, Waist Circumference; BMI, Body Mass Index [kg/height(m)2]; T2DM, Type 2 Diabetes Mellitus; AST, Aspartate transaminase; ALT, Alanine transaminase; GGT; Gamma-glutamyltransferase; HbA1c; Hemoglobin A1c; HDL-C, High-density lipoprotein cholesterol; WBC, White blood cells.
Prevalence of steatosis and fibrosis among the adult population in Canada
Note: HSI+ indicates HSI >36. NRS+ indicates NRS > 0.44. FIB-4+ indicates FIB-4 > 2.67. NFS+ indicates NFS > 0.676.
Abbreviations: HSI; Hepatic Steatosis Index; NRS, NAFLD Ridge Score; FIB-4, FIB-4 Index; NFS; NAFLD Fibrosis Score; CI, Confidence Interval; T2DM, Type 2 Diabetes Mellitus.
This is the first study to estimate the national prevalence of hepatic steatosis and advanced fibrosis in Canada. We estimate between 1 in 3 and 1 in 2 adults have steatosis based on two validated NITs, of whom 195,000–406,200 Canadians could have advanced liver fibrosis. This study offers a comprehensive analysis of the prevalence of steatosis in subgroups reporting high prevalence across age groups, sex, province, race, income, alcohol consumption, and comorbid conditions. Results suggest that the prevalence of steatosis increases with age and is heightened in males. Furthermore, our data also indicates that when using NITs to evaluate prevalence by sub-groups, the choice of NITs should be evaluated.
Since no representative surveys include transient elastography or liver ultrasounds in Canada, NITs are the only means of estimating generalizable prevalence estimates for hepatic steatosis and fibrosis in the general population. Our study design was similar to other nationally representative studies conducted using blood-based NITs in the US [13,18,28], France [29], Australia [30], Mexico [31], and Korea [32]. The samples studied ranged from 695 [31] to 102,344 participants[29], and similarly all were cross-sectional by design. The choice of NITs varied based on the available components to calculate various NITs. Six out of seven of these studies excluded people with a history of chronic liver diseases. In the United States, Jones et al., used the National Health and Nutrition Examination Survey (NHANES) and found the overall prevalence of steatosis was 53.5 % in the United States using the HSI, reporting higher rates among males compared to females [18]. Studies among people at higher risk include people with T2DM, where prevalence estimates for steatosis range from 45 % to 85 %, and the prevalence of significant liver fibrosis (based on FIB-4) was 3 %, which aligns with our results [33,34]. A separate recent study using the United States Fatty Liver Index (US-FLI) found the prevalence of steatosis increased with age until 75 years old; 20.4 % (18<45 years), 29.2 % (45-<65 years) 40.6 % (65-<75 years) and 34.8 % (≥75 years) [13]. While we reported the same trend, the prevalence of steatosis using the NRS and HSI was higher than that of US-FLI. This has been reported elsewhere when all three NITs were compared to each other [21]. Also consistent with other studies we found that prevalence differed by race where Asian people had the lower prevalence compared to people self-identifying as Black or White [13].
The prevalence of steatosis between HSI and NRS differs overall, but the differences by subgroup are not statistically significant except when examining sex. NITs include various demographics, such as age, ethnicity, sex, and clinical indicators that differ by sample populations [35]. NITs such as the USFLI and the FLI do not incorporate comorbid conditions such as T2DM and hypertension into the respective formulas, whereas the HSI and NRS do. Sex is included as an indicator for HSI, which may have prognostic advantages by accounting for the differences in visceral and liver fat accumulation between males and females [36–38]. In addition, the HSI and NFS have overlapping variables (T2DM and BMI). Thus, steatosis defined by HSI may overestimate NFS advanced fibrosis.
We focused our study on the sequela of SLD instead of the prevalence of the different sub-types of SLD (i.e. MASLD, MetALD and ALD) based on the most recent nomenclature. Interestingly, we found between 24 and 29 % of people with evidence of steatosis could meet the definition of MetALD by drinking 4–14 drinks per week, with an additional 7–8 % by drinking >15 drinks per day. Among people at risk of liver fibrosis, between 56 and 65 % were drinking at least one drink per week when the recommendation for this group of individuals is abstinence.
The data utilized for this study leveraged a national survey representative of the Canadian population, increasing the generalizability of our prevalence estimates, which is the strength of our study. By applying the sampling weights, we could appropriately adjust for complex survey designs, such as oversampling, survey non-response, and post-stratification. Survey weights helped improve our survey estimates' accuracy by ensuring that our conclusions were more representative of the Canadian population. Steatotic liver disease has become a significant liver condition with an impact at the societal level, leading to higher healthcare costs and impairing a patient's health-related quality of life [10,39,40]. Reporting prevalence estimates of steatosis and fibrosis is therefore crucial in guiding policies and implementing appropriate resource utilization [41–43]. Furthermore prevalence estimates can provide insight into and improve the levels of preparedness to prevent and manage the burden of the spectrum of steatotic liver disease [12]. With the implementation of comprehensive national and subnational guidelines, policymakers and healthcare professionals can effectively allocate resources, including the distribution of health services such as elastography across the country [44]. Reporting age and sex-specific prevalence of steatosis and fibrosis is also important to better inform modelling studies and global meta-analysis. While non-invasive tests are not currently considered to be the gold standard for hepatic steatosis and MASH diagnosis, they are readily available, inexpensive, and can be used for epidemiological studies of steatosis and advanced fibrosis prevalence [45,46].
There are several limitations to our study. NITs were used and are known to be imperfect [47]. NITs can yield “indeterminate” scores that range between 30 and 50 % of the study population, posing a significant limitation and requiring secondary diagnostic testing for this population [45,48]. In epidemiological studies, follow-up diagnostic testing is not possible. The incorporation of BMI in HSI may pose challenges in identifying people with lean MASLD or can result in increased prevalence when examining people with elevated BMI, over 40 [46]. Though the FIB-4 index is one of the most utilized NITs in identifying fibrosis, it has lower sensitivity in older individuals, which has led to proposed adjustments to cut-off values [45]. Moreover, poor diagnostic performance among individuals under the age of 35 has been noted for both the FIB-4 index and the NFS [49]. Neither HSI, NRS, NFS, nor FIB-4 index have been validated in children, warranting further validation studies to assess their accuracy [50,51]. The application of the NRS is novel, with limited subsequent validation studies, leaving a gap in the validation o primarily Caucasian populations [52]. However, studies conducted in Sweden [21]. and Italy [19] utilized the NRS in a population that was not predominately Asian, and found high steatosis burden similar to our results. Given that provinces in Canada, such as British Columbia[53] and Ontario[54], have restricted access to AST to liver specialists (hepatologists and gastroenterologists), the NRS allows for an alternative method of estimating the prevalence of hepatic steatosis without using AST in the formula.
The CHMS is a voluntary survey and, therefore, is not immune to the “healthy participants bias,” where healthier people are more likely to agree to participate. This is evident in our study since only 5 % of our sample population self-identified as having T2DM despite the Canadian prevalence estimated at 10 %. This difference has been reported elsewhere[55] and may be attributed to the reliance on self-reported data in the CHMS and the younger demographic of our study population. Additionally, we excluded people with HIV/AIDS and hepatitis C and B. Although this is not a requirement for the new definitions of SLD, this allowed us to directly compare our results with previous studies conducted when definitions of NAFLD required the exclusion of other aetiologies of liver disease. Given very few people met this exclusionary criterion, we don't believe this altered the main results of our study. Finally, certain sub-group analyses (i.e., based on BMI and other co-morbidities) were not possible due to privacy protocols (suppression of small cells) set by Statistics Canada.
5ConclusionsCurrently, no routine screening guidelines for steatotic liver disease exist in Canada, leaving most patients with advanced fibrosis unaware of their condition. This study supports a high prevalence of steatosis in the general population across Canada and confirms people with T2DM are at increased risk of advanced fibrosis. Prevalence studies are essential for raising awareness and advocating for the inclusion of chronic liver disease on national public health agendas.
FundingGiada Sebastiani is supported by a Senior Salary Award from Fonds de Recherche Québec – Santé (FRQ-S) (#296306). Mark Swain holds the Cal Wenzel Family Foundation Endowed Chair in Hepatology.
The Canadian Health Measures Survey was developed in partnership with Health Canada and the Public Health Agency of Canada and with the assistance of several advisory committees, stakeholders, experts, reviewers and supporters. The authors would like to acknowledge all of the CHMS staff, whose collective efforts led to the successful survey launch in March 2007. We would also like to thank the Queen's Research Data Center staff, including Christopher Russel, Yasmine Amirkhakhali, and Jing Liang. Duy Dihn, for his careful review of the manuscript.