Alcoholic hepatitis is a clinical syndrome characterized by recent onset jaundice and/or the other signs of liver decompensation in people with heavy alcohol use [1]. Severe alcoholic hepatitis (SAH), which is defined as Maddrey's discriminant function (mDF)≥32, is a dismal condition with a 28-day mortality of 34 % in untreated patients [2]. In SAH patients, corticosteroids and pentoxifylline treatment were used. Recent meta-analyses showed that corticosteroid treatment in SAH improved 1-month mortality [2,3], However, in the Steroid or Pentoxifylline for Alcoholic Hepatitis (STOPAH) study, corticosteroid was associated with 28-day mortality reduction but not with 3-month and 6-month mortality in patients with SAH [4]. In addition, the survival benefit of pentoxifylline is uncertain [3,4], Therefore, new treatment strategies for SAH are needed.
In SAH patients, gut dysbiosis plays an important role. Alcohol consumption changes the gut microbiome and disrupts the intestinal epithelial barrier [5]. Gut dysbiosis in SAH leads to increased gut permeability and translocation of viable bacteria or their products, such as lipopolysaccharide (LPS) or peptidoglycan [6,7], These microbial products of portal or systemic circulation increase alcohol-induced liver injury and inflammation [7]. In practice, SAH patients with high serum LPS levels showed poor survival with frequent multiorgan failure and a lower corticosteroid response rate [8]. Therefore, gut microbiome modulation is considered a new target for SAH treatment.
Rifaximin is a poorly absorbable broad-spectrum antibiotic against gram-positive and gram-negative bacteria and has a low risk of bacterial resistance [9]. Rifaximin is used to treat hepatic encephalopathy or to treat recurrent or refractory Clostridium infections. In patients with hepatic encephalopathy, it is thought that rifaximin modulates the intestinal microbiome and reduces gut-derived neurotoxins, such as ammonia [10]. In patients with alcoholic liver cirrhosis, rifaximin improved systemic hemodynamics and renal function by reducing serum endotoxin levels [11]. In addition, rifaximin administration reduced the risk of developing complications related to portal hypertension and improved survival [12].
Therefore, we aimed to assess the effectiveness of adding rifaximin to corticosteroids or pentoxifylline in SAH patients in this prospective study.
2Materials and methods2.1Study patientsThis multicenter, randomized, open-label pilot trial was performed on patients hospitalized between September 2015 and January 2019 in 6 Korean hospitals. The eligibility criteria were an age of 18–75 years and chronic heavy drinking (>40 g/day for men and >30 g/day for women) with severe alcoholic hepatitis. Chronic heavy drinking was defined as active drinking until at least 3 months before presentation, and severe alcoholic hepatitis was defined as follows: 1) mDF ≥ 32, 2) recent onset jaundice within 3 months (bilirubin ≥ 3 mg/dL), 3) aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio ≥ 1.5, and 4) one or more of the following clinical findings: hepatic encephalopathy, ascites, hepatomegaly, and leukocytosis with predominantly neutrophilic differentiation. The exclusion criteria included 1) the existence of other etiologies of chronic liver disease, such as chronic hepatitis B, hepatitis C or autoimmune hepatitis, 2) acute viral hepatitis (hepatitis A or E), 3) drug-induced hepatotoxicity, 4) antibiotic or probiotic use within 8 weeks, 5) hepatocellular carcinoma (HCC) of stage 2 or more, 6) malignancy other than HCC, 7) pregnancy, 8) type 1 hepatorenal syndrome, 9) hepatic encephalopathy of West Haven grade 3 or 4, 10) severe infection, and 11) gastrointestinal bleeding with transfusion of more than 3 units of blood.
2.2Study designThe patients with SAH were scheduled to be treated with 40 mg of prednisolone per day or 400 mg of pentoxifylline taken three times daily in the steroid-ineligible patients for 4 weeks, irrespective of the treatment response assessed using the Lille model. The steroid-ineligibility criteria were uncontrolled bacterial infection, concomitant pancreatitis, acute kidney injury, and uncontrolled upper gastrointestinal bleeding. The patients meeting the criteria were randomized into two groups at a 1:1 ratio, with one group receiving a dosage of 400 mg of rifaximin three times daily and the control group receiving no additive treatment. Randomization was performed with a block size of four, with stratification according to treatment of SAH and hospital.
2.3Determination of serum LPS and LBP levelsSerum samples were obtained from the peripheral blood on the first day and 28th day of prednisolone or pentoxifylline treatment. Serum levels of LPS and LBP were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits following the manufacturer's instructions (Cusabio Biotech Co., Wuhan, China).
2.4OutcomeThe primary outcome was survival without liver transplantation at 6 months. Patients who received transplantation were regarded as dead. The secondary outcome was transplantation-free survival at 90 days.
2.5Statistical analysisContinuous variables were represented by means ± standard deviations (SDs) or medians (ranges), while categorical variables were presented as numbers and percentages. Comparisons between the two groups were made using Student's t-test or Mann-Whitney U test for continuous variables and chi-square test or Fisher's exact test for categorical variables. The period from the initial day of SAH treatment ± rifaximin until death or liver transplantation. For those patients who did not encounter the events of interest, their data were censored on the date of their last follow-up visit. The Kaplan–Meier method and Cox proportional hazards model were utilized to compare survival times between the two groups, with a two-sided test and a significance threshold of 0.05. Statistical significance was defined as a P value under 0.05. Data analysis was conducted using SPSS version 18.0 (SPSS, Inc. IBM Company, Chicago, IL, USA).
2.6Ethical statementAll participants provided written informed consent. This study received approval from the institutional review board at each participating hospital and adhered to the Good Clinical Practice guidelines and the Declaration of Helsinki. The study has been registered with ClinicalTrials.gov (NCT02485106). Each co-authors had access to the study data, and all reviewed and approved the final manuscript.
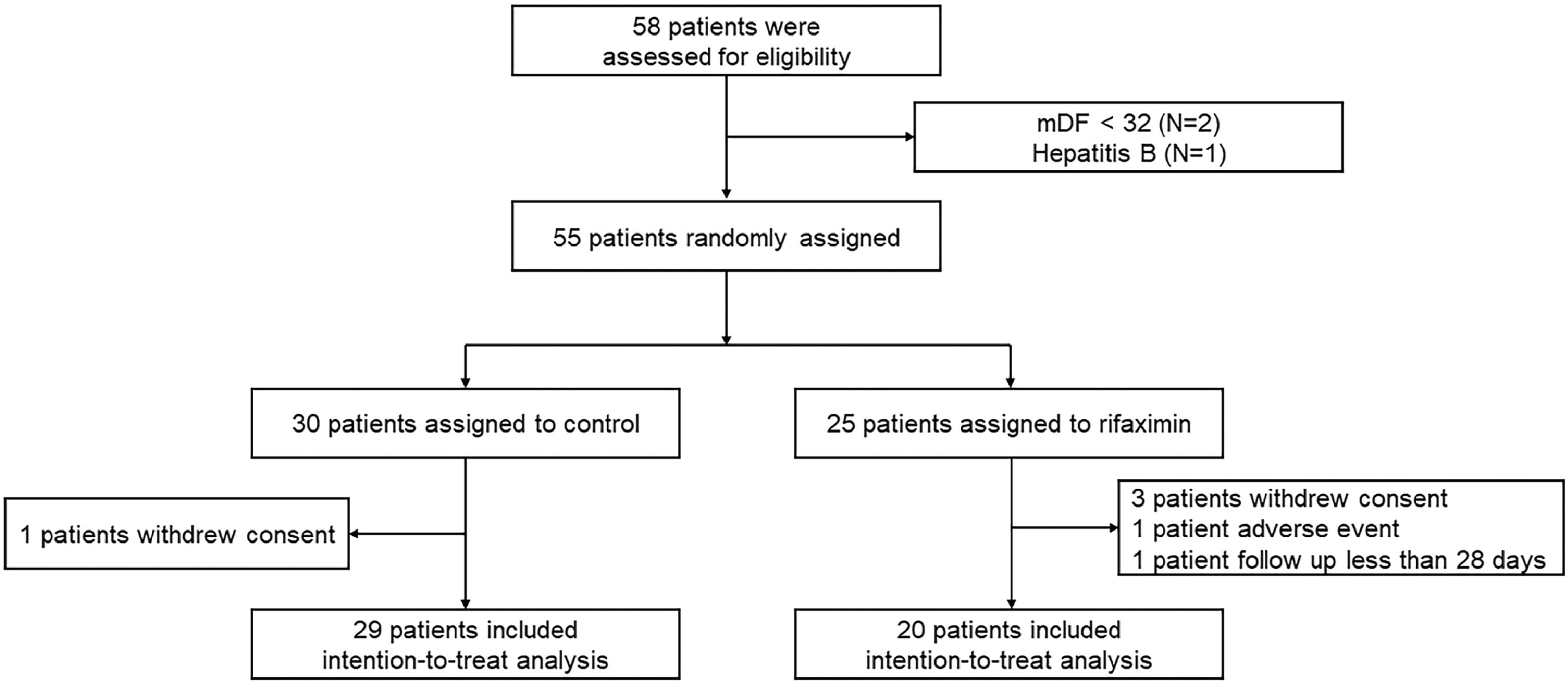
3Results3.1PatientsDuring the study period, 58 patients were screened, and after the application of the eligibility criteria, 55 patients were randomized to the two groups: 30 to the rifaximin treatment group and 25 to the control group. One patient in the rifaximin treatment group (withdrawal of consent) and 5 patients in the control group (3 patients withdrew consent, one patient had adverse events, and one patient was followed for less than 28 days) were excluded, and 29 patients in the rifaximin group and 20 patients in the control group were analyzed in this study (Fig. 1).
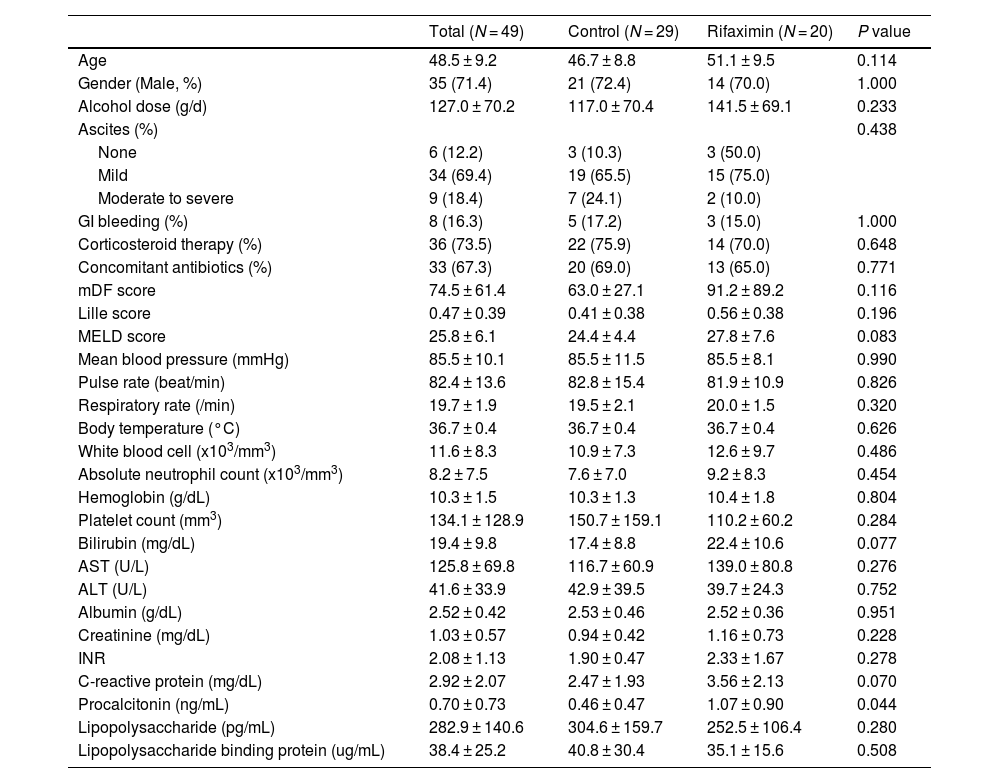
The baseline characteristics of the enrolled patients are reported in Table 1. The mean age was 48.5 ± 9.2 years, and 35 patients (71.4 %) were male. Thirty-six patients (73.5 %) were treated with corticosteroids, and concomitant antibiotics were used in 33 patients (67.3 %). There were no significant differences in the baseline characteristics between the control and rifaximin groups. However, the rifaximin treatment group had significantly higher serum procalcitonin levels than the control group (1.07 vs. 0.46 ng/mL, P = 0.044).
Baseline characteristics.
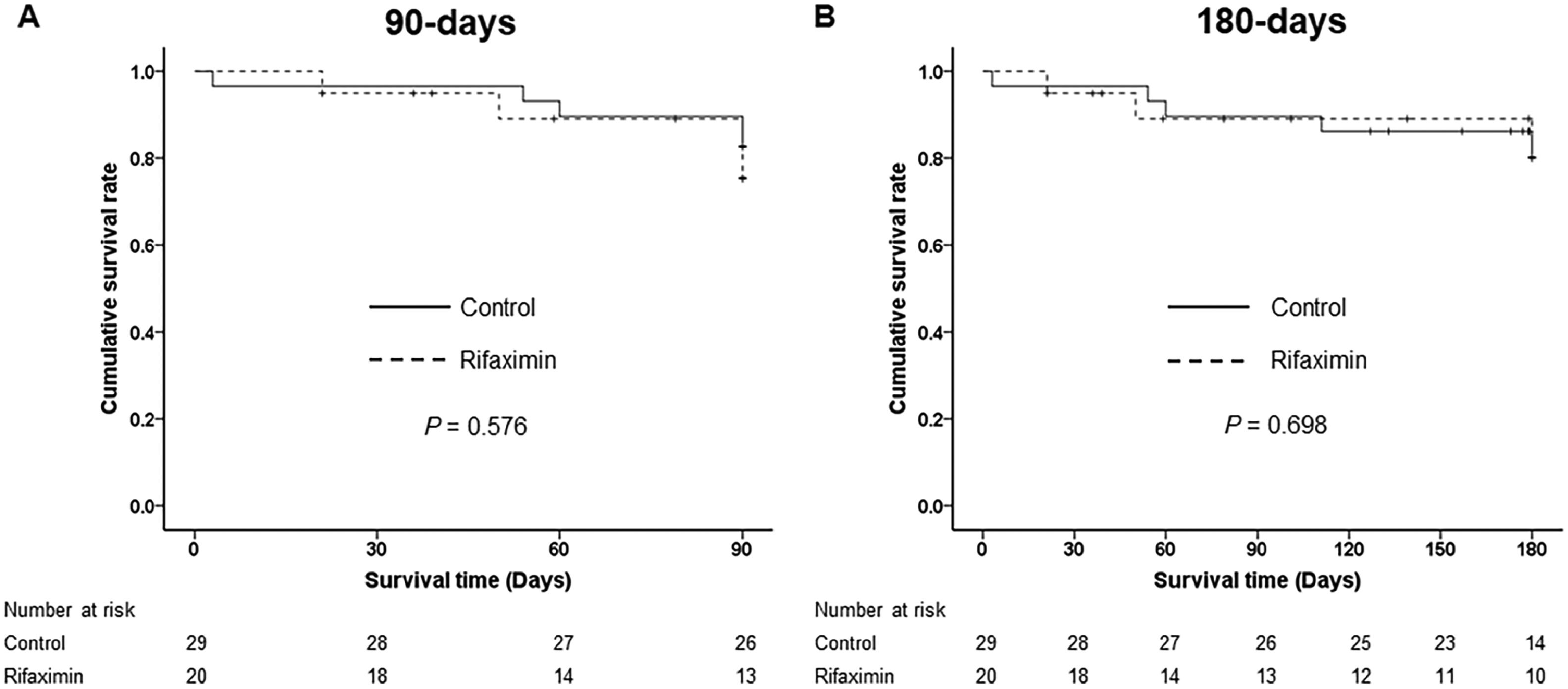
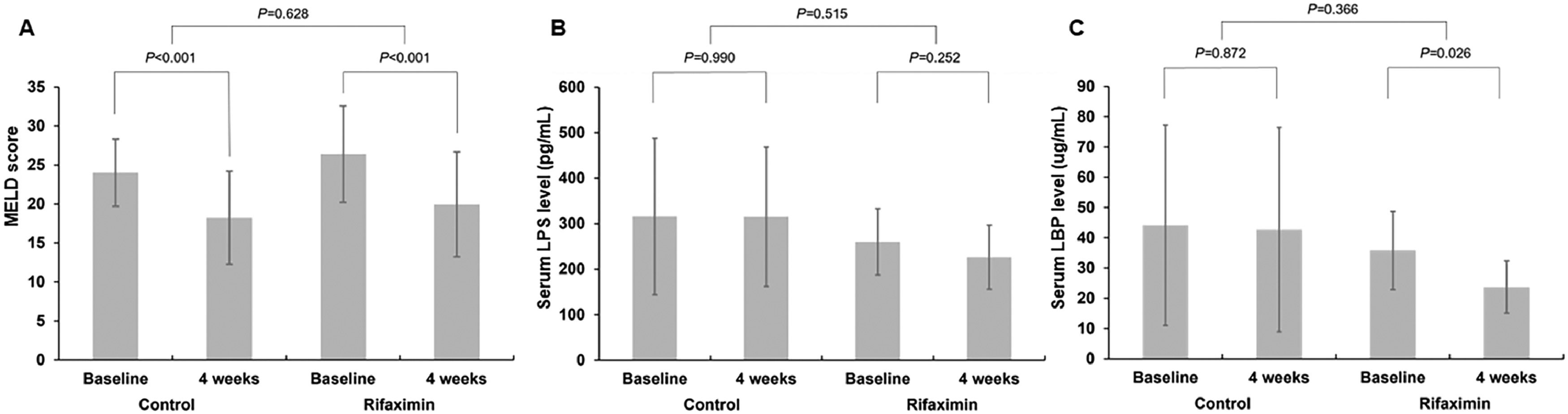
There were no significant differences in 90-day and 180-day LT-free survival between the control and rifaximin groups (82.8 % vs. 80.8 %, P = 0.576 at 90 days and 82.8 % vs. 80.8 %, P = 0.698 at 180 days, respectively) (Fig 2). At 4 weeks after enrollment, the MELD score was significantly decreased in both groups compared to baseline (−5.8 ± 4.1, P < 0.001 in the control group and −6.4 ± 5.0, P < 0.001 in the rifaximin group), but there was no significant difference in the improvement of MELD scores over 4 weeks between the two groups (Fig. 3A). The serum LPS levels were not decreased in either the control or rifaximin groups (−0.4 ± 136.9, P = 0.990 in the control group and −33.7 ± 81.8, P = 0.252 in the rifaximin group), and there was no significant difference between the two groups in the LPS decrease (P = 0.515) (Fig. 3B). The serum LBP levels were significantly decreased in the rifaximin group (−12.0 ± 14.3, P = 0.026). However, there was no significant change in the serum LBP levels in the control group (−1.4 ± 34.4, P = 0.872), and there was no significant difference in the decrease in serum LBP levels between the two groups (P = 0.366) (Fig. 3C).
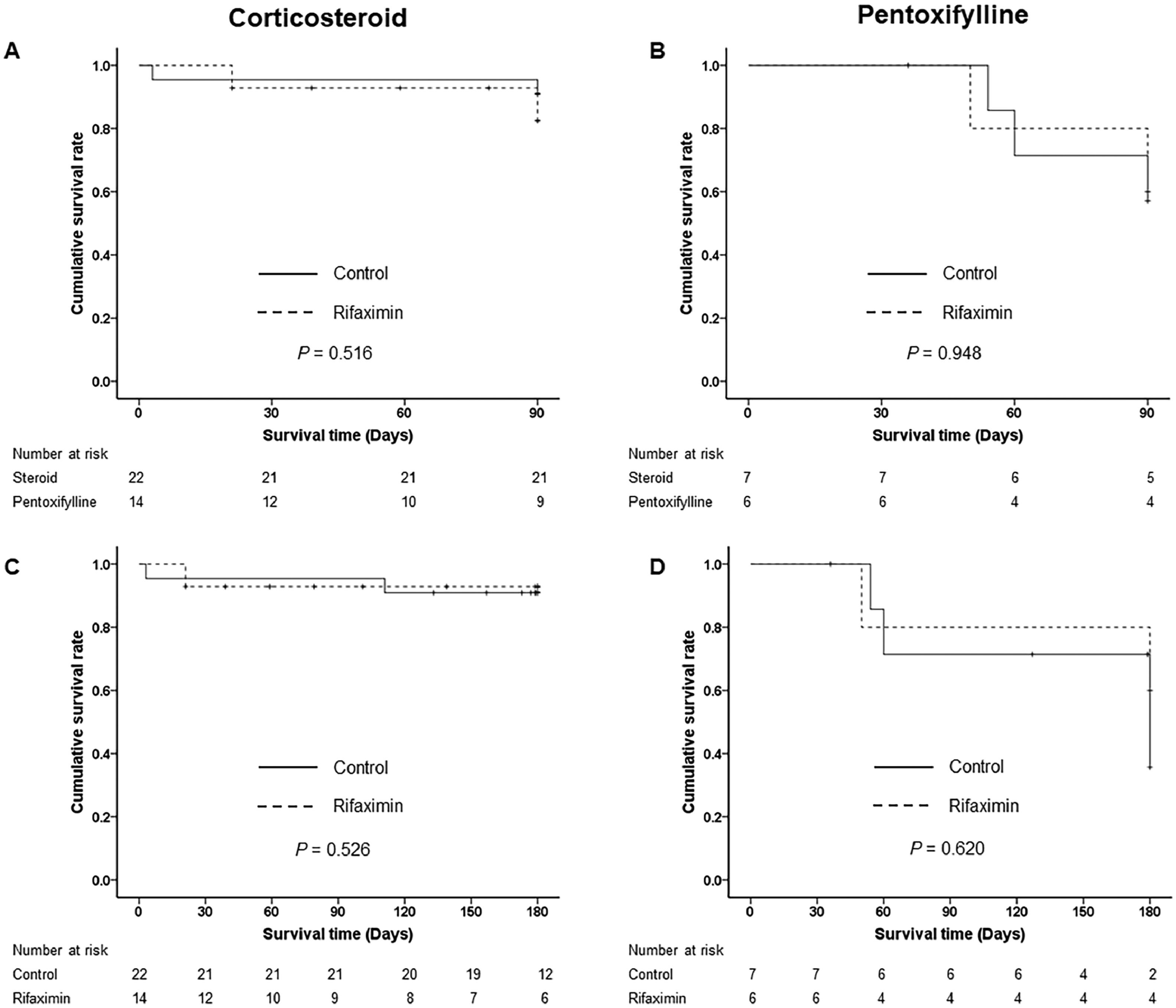
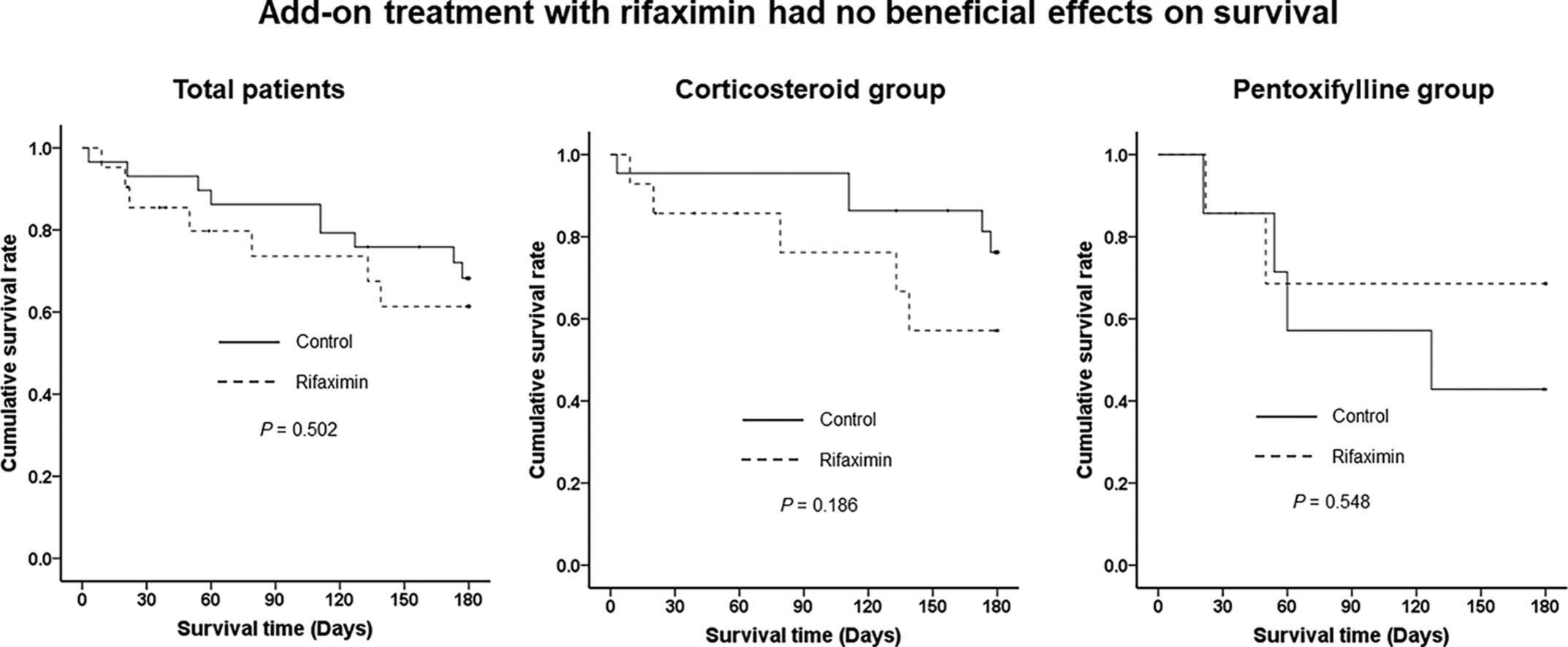
When we stratified by SAH treatment, there were no significant differences in 90-day and 180-day LT-free survival between the control and rifaximin treatment groups in either the corticosteroid (90.9 % vs. 85.7 %, P = 0.516 at 90 days and 90.9 % vs. 85.7 %, P = 0.526 at 180 days) or pentoxifylline treatment groups (57.1 % vs. 66.7 %, P = 0.948 at 90 days and 57.1 % vs. 66.7 % at 180 days) (Fig. 4). Those who were treated with corticosteroids had significantly better 90-day and 180-day LT-free survival than those who were treated with pentoxifylline (P = 0.030 and P = 0.036) (Supplementary Fig. 1).
Three-month and 6-month LT-free survival stratified by treatment of severe alcoholic hepatitis. (A) 3-month LT-free survival in the corticosteroid group. (B) 3-month LT-free survival in the pentoxifylline group. (C) 6-month LT-free survival in the corticosteroid group. (D) 6-month LT-free survival in the pentoxifylline group. Abbreviation: LT, liver transplantation.
There were no significant differences in 90-day and 180-day LT-free survival between the groups with and without concomitant antibiotics (P = 0.351 in 90-day and P = 0.286 in 180-day LT-free survival).
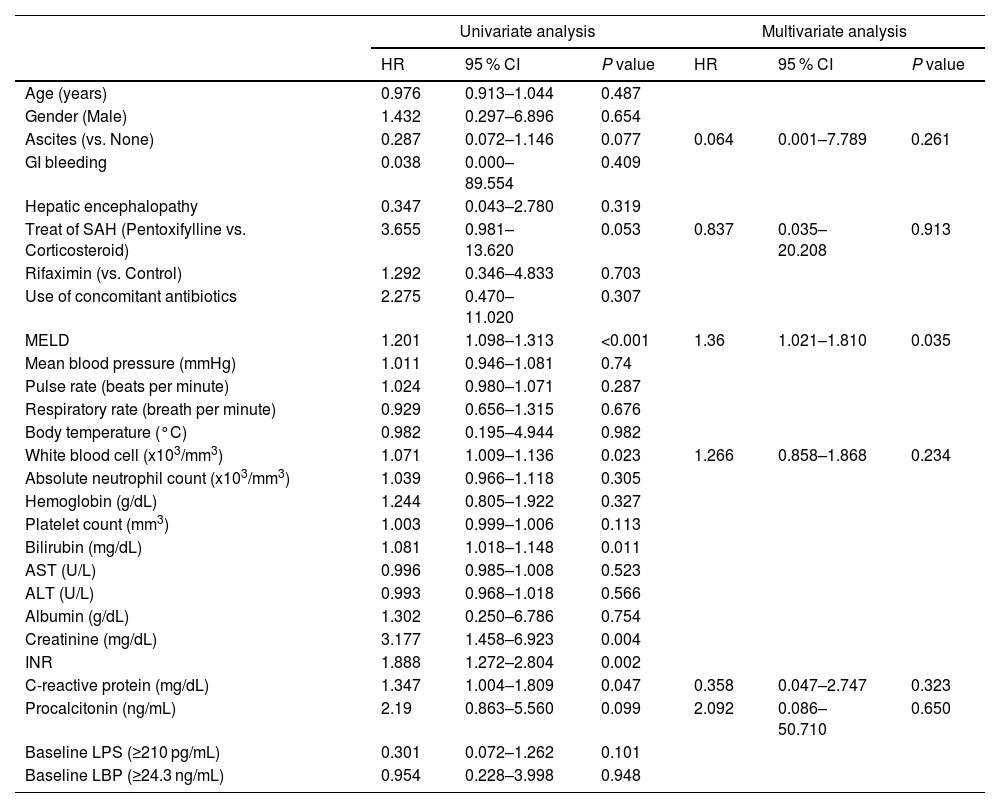
3.5Factors associated with 6-month LT-free survivalWe used the Cox proportional hazards model to identify risk factors associated with 6-month LT-free survival. In the univariate analysis, baseline MELD score, white blood cell count, serum bilirubin, creatinine, international normalized ratio (INR), and C-reactive protein level were significant factors, and no ascites, pentoxifylline treatment, and serum procalcitonin level were marginally significant factors (P values between 0.05 and 0.10). Rifaximin treatment was not a significant factor (hazard ratio (HR) 1.292, P = 0.703). We performed multivariate analysis with these factors except for the components of the MELD scores because the MELD score includes serum bilirubin and creatinine levels and INR. In the multivariate analysis, the MELD score was the only significant factor associated with 6-month LT-free survival in the patients with SAH (HR 1.36, P = 0.035) (Table 2).
Factors associated with 6-month liver transplantation-free survival.
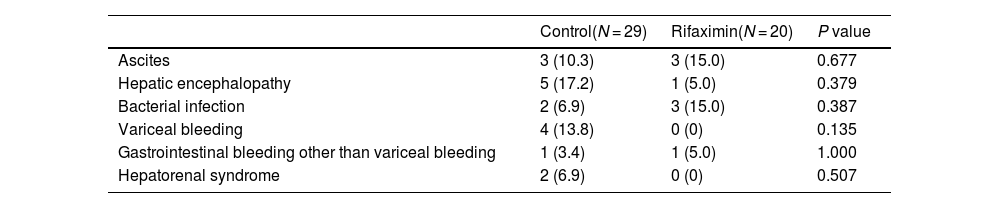
In the control group, hepatic encephalopathy was the most frequent complication (17.2 %), followed by variceal bleeding (13.8 %) and ascites (10.3 %) for 4 weeks. In the rifaximin group, bacterial infection and ascites were the most frequent complications (15.0 % and 15.0 %), followed by hepatic encephalopathy (5.0 %) and gastrointestinal bleeding other than variceal bleeding (5.0 %). There was no significant difference in the occurrence of liver-related complications between the two groups at 4 weeks (Table 3).
Liver-related complications.
The results of this pilot study indicate that rifaximin treatment does not improve the prognosis in patients with SAH. Rifaximin treatment was not effective in either the standard treatment group, corticosteroid group, or pentoxifylline group. In addition, rifaximin treatment did not affect the recovery of liver function or gut microbial translocation and did not reduce the occurrence of liver-related complications.
Rifaximin, a poorly absorbable antibiotic, is used for preventing recurrent hepatic encephalopathy, and its mechanism is presumed to reduce ammonia production by modulating the gut microbiome [13,14], In addition, some studies reported that rifaximin treatment improved portal hypertension and prognosis in patients with decompensated cirrhosis [12,15], However, previous studies on rifaximin treatment in SAH patients showed conflicting results. Jiménez et al. reported that the addition of rifaximin to standard treatment was associated with significantly lower bacterial infections and liver-related complications and a trend toward lower mortality without significant differences [16]. On the other hand, Kimer et al. showed that mortality and hepatic function were not affected by the addition of rifaximin and that there were no differences in inflammation and metabolism markers [17]. Our study also showed that add-on treatment with rifaximin in SAH patients had no beneficial effects on survival or the prevention of complications. However, studies on rifaximin in SAH, including our study, all had a small number of patients. Therefore, it is necessary to verify the effectiveness of rifaximin through a well-designed RCT that includes more patients in the future.
Enteric dysbiosis is thought to be a central component of alcoholic hepatitis [5,18], Transplantation of fecal microbiota of SAH patients increased the susceptibility to alcoholic liver disease[19], and healthy donor fecal microbiota transplantation improved prognosis in SAH patients [20]. Therefore, modulation of the intestinal microbiome is emerging as a promising therapeutic option. Rifaximin is also known to modify the gut microbiome by changes in the bile acid composition or microbiome function rather than the gut microbiome composition in patients with chronic liver disease [13,21,22], Bajaj et al. [22]. and Yu et al. [23]. showed a significant change in bacterial metabolic function without a significant change in the gut microbiome after rifaximin treatment in cirrhotic patients with hepatic encephalopathy. Pose et al. [24]. also showed that rifaximin and simvastatin treatment was associated with reduced change of plasma metabolite in decompensated cirrhosis patients. However, in the study that analyzed the fecal microbiome of SAH patients included in this study, there were no significant changes in not only in the composition of the gut microbiome but also in the metabolic pathway after rifaximin treatment [25]. Kimer et al. also reported that rifaximin treatment did not change inflammatory and metabolic markers in SAH patients [17]. Unlike cirrhosis, failure of rifaximin to change microbiome function would not have improved the prognosis in SAH patients. The difference in the outcome of rifaximin treatment in cirrhosis and SAH may have arisen from the gut microbial composition and function between the two diseases [26]. Therefore, research is needed to identify and modulate the SAH-specific gut microbiome and its functions that can improve the prognosis of alcoholic hepatitis.
Enhanced intestinal permeability also contributes to alcoholic hepatitis. Increased gut permeability leads to an increased load of gut-derived bacterial products such as LPS in the portal circulation and liver, and increased endotoxin leads to hepatic inflammation and fibrosis [5,27,28], Increased serum LPS also plays a role in the systemic inflammatory response and the development of complications of cirrhosis [29,30], Michelena et al. showed that serum LPS levels were correlated with the severity of AH and that increased serum LPS predicted the development of multiorgan failure and corticosteroid response [8]. Some studies reported that rifaximin treatment lowered serum endotoxin levels in alcohol-associated cirrhosis [11,22], However, rifaximin treatment did not significantly lower serum LPS and LBP levels in patients with SAH compared with the nontreated group in this study. This result suggests that rifaximin treatment is insufficient to improve gut permeability and reduce bacterial translocation in patients with SAH. The failure to reduce gut permeability after rifaximin treatment may also be associated with the failure to modulate the gut microbiome.
This study has several limitations. First, this study included too small a number of patients. Further studies including a sufficiently larger number of patients are warranted to prove the effectiveness of adding rifaximin in patients with SAH. Second, although this study is designed as an RCT, the possibility that the bias of the researcher or patients is involved should be considered because this is an open-label study without a placebo group. Third, pentoxifylline was included as the standard treatment for SAH. After the STOPAH study showed that pentoxifylline was ineffective in SAH [4], only corticosteroids were used as standard treatment for SAH. However, at the beginning of this study, pentoxifylline was able to be used as a standard treatment. Additionally, because this study used block randomization stratified by SAH treatment and adding rifaximin was ineffective in both pentoxifylline and corticosteroid groups, pentoxifylline treatment as standard treatment would not have affected the results of adding rifaximin in SAH. Fourth, a substantial number of patients used concomitant antibiotics. In this study, concomitant antibiotics did not affect the effectiveness of rifaximin. However, research is also needed on the differences in the type and severity of infections and the type of antibiotics used in the future.
5ConclusionsIn conclusion, adding rifaximin to standard treatment in patients with SAH did not improve short-term LT-free survival and did not prevent the development of liver-related complications. The only significant factor for short-term LT-free survival was the MELD score at baseline. However, given that this is a pilot study, further studies that are well-designed and include a larger number of patients are warranted.
Author contributionConceptualization: Do Seon Song, Young Kul Jung; Data curation: Do Seon Song, Young Kul Jung, Hyung Joon Yim, Hee Yeon Kim, Chang Wook Kim, Soon Sun Kim, Jae Youn Cheong, Hae Lim Lee, Sung Won Lee, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim; Formal analysis: Do Seon Song, Young Kul Jung, Hee Yeon Kim, Soon Sun Kim, Sung Won Lee, Jeong-Ju Yoo; Writing-original draft: Do Seon Song; Project administration: Hyung Joon Yim, Chang Wook Kim, Jae Youn Cheong, Sang Gyune Kim, Young Seok Kim; Supervision: Jin Mo Yang, Young Seok Kim. All authors read and approved the final manuscript.
Clinical trial registrationThe study was registered on ClinicalTrials.gov (number: NCT02485106).