Relationships and interactions among waist circumference (WC), body mass index (BMI) and high-sensitivity C-reactive protein (hs-CRP) with steatotic liver disease (SLD) in children have rarely been studied as a whole. We aimed to investigate the association among WC, BMI and hs-CRP with SLD and its related metabolic indictors.
Materials and MethodsA total of 10,776 children aged 10–15 years were screened in our study. Anthropometric data, biochemical parameters and ultrasound assessments were collected. Metabolic indictors between children with and without SLD were compared. The correlation of waist circumference Z score (ZWC), body mass index Z score (ZBMI) and hs-CRP with SLD and its related metabolic indictors, and the interactive effect between ZWC with hs-CRP and ZBMI with hs-CRP upon SLD, respectively, was tested.
ResultsA total of 543 children with normal BMI (n = 287) and high BMI(n = 256) were examined. Hs-CRP, ZWC and ZBMI were all found to significantly correlate with SLD and its related metabolic indexes. The interaction effect analysis showed that ZWC and male was independent risk factor of SLD with OR (95 %CI) of 23.431 (7.253, 75.697) and 7.927(2.766,22.713), respectively, whereas the same effect wasn't found in ZBMI. The cut-off value of ZWC for the prediction of SLD was 1.494 and 1.541 in boys and girls, respectively.
ConclusionsIncreased WC, BMI and hs-CRP exerts adverse effect in pediatric SLD and its related metabolic indictors. WC and male gender could be independent risk factors for SLD, and WC was a powerful index for the prediction of SLD in children aged 10–15 years.
Non-alcoholic fatty liver disease (NAFLD), updated to metabolic dysfunction-associated steatotic liver disease (MASLD) recently, was considered to be the most common liver disease in children and adolescents, which affects about 10 % of the general pediatric population and could be up to 30–40 % in children with overweight or obesity [1,2]. NAFLD/MASLD not only is closely associated with childhood intra-and extrahepatic comorbidities, such as insulin resistance and dyslipidemia, but also it progresses and affects adulthood. Diagnosis of MASLD requires the presence of both “Steatotic Liver Disease” (SLD) and at least one cardiometabolic risk factor in the presence of hepatic steatosis, the finding of any of a cardiometabolic risk factor if there are no other causes of hepatic steatosis [3]. Nevertheless, the data on MASLD was limited, especially in children, and it has been shown that there is little difference between MASLD and NAFLD in the general population [4,5]. Since the present study was carried out before the concept of MASLD was proposed, our analysis focused on SLD as well as the metabolic abnormalities related to SLD. Data both on MASLD and NAFLD were referenced.
Approximately 9.03 % of Chinese children aged 7–18 years were suspected to have NAFLD [6]. Severely obese children were found more likely to develop more severe liver disease [7]. Growing evidence showed that the occurrence of hepatic steatosis is usually prompted by obesity-related metabolic alterations, which lead to expansion of lipid stores in the liver and then cause oxidative stress and inflammation. Pediatric cardiovascular diseases (CVD), metabolic syndrome (MetS), type 2 diabetes (T2DB) and renal comorbidities could be induced by NAFLD [8–11], which was renamed as MASLD now. All obese children aged 9–11 years are recommended to be screened for MASLD and those with high risk factors, such as a family history of MASLD, should be screened more earlier [12].
Excessive adipose tissue is a significant risk factor of MASLD, which is usually indicated by body mass index (BMI). It has been demonstrated that adiposity gain in childhood could be used for the prediction of adolescent adverse liver outcomes [13]. The association between the risk of MASLD with obesity was thought to mainly attributed to the dysfunction of adipose tissue and the development of insulin resistance in obese children [14]. Abnormal adipose tissue function could cause abnormal secretion of adipokines, especially leptin and adiponectin, which were believed to have pro-inflammatory and anti-inflammatory effects [15].
Numerous studies have shown that abdominal fat played a crucial role in obesity-related inflammation, insulin resistance and liver fat accumulation [16–18]. Increased waist circumference (WC) was an independent risk factor for increased liver fat in overweight/obese adolescents [19]. WC, insulin resistance and triglycerides were found to be the most relevant factors in explaining the SLD related metabolic dysfunction [20]. Moreover, WC and insulin resistance increased as liver abnormalities progressed in children [21].
In addition, high-sensitivity C-reactive protein (hs-CRP) was found to be another independent risk factor associated with MASLD [22]. Nevertheless, the interaction between WC with hs-CRP and BMI with hs-CRP on the occurrence of SLD in children have rarely been reported. Moreover, the high cost or concerns of radiation hazards limited the application of computed tomography (CT), ultrasound and dual-energy X-ray absorptiometry (DEXA) in the primary health screening of children. Therefore, an effective screening tool, which is simple, accurate, inexpensive, reproducible and non-invasive, is urgently needed for early detection of SLD for children and adolescents. In this study, we examined the association among WC Z score (ZWC), hs-CRP and BMI Z score (ZBMI) with SLD and its related metabolic indictors, as well as the impacts of interaction between ZWC with hs-CRP and ZBMI with hs-CRP on the risk of SLD. We also investigated the predictive effect of ZWC on SLD in this age group of Chinese pediatric population.
2Materials and methods2.1Study populationBetween the years 2010 and 2012, a total of 10,776 children in grades 4–9 from 26 primary or middle schools in two districts of Shanghai were screened by a simple cluster sampling method. Trained teachers examined the data of children's height and weight, and BMI of each child was calculated. Children were excluded if they were: 1) diagnosed with major congenital disease or chromosome abnormality and skeletal dysplasia or asymmetrical short stature. These children may have age-inappropriate WC and BMI; 2) diagnosed with genetic metabolic disease, which may cause bias in metabolic indicators or ultrasound results.
2.2Anthropometric measurementsPhysical examination was conducted by trained personnel using standard methods and instruments. Participants were barefoot and in light clothing. Measurements of height and weight were accurate to 0.1 cm and 0.1 kg, respectively. WC was defined as the narrowest level between the lower border of the rib cage and the iliac crest, and its measurement was accurate to 0.1 cm. Blood pressure (BP) was measured by the standard method using a mercury sphygmomanometer. BMI was calculated as body weight (kg) divided by height squared (m2).
Ultrasonography was applied to detect hepatic steatosis, which was divided into 4 grades of severity according to the examination results [23]: absent, mild, moderate and severe.
2.3Biochemical measurementsAfter fasting overnight for at least 8-h, fasting blood samples were collected for laboratory examination. A Hitachi 7180 analyzer (Hitachi High Technologies Corp, Japan) was used to detect total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), fasting blood-glucose (FBG), hs-CRP, the glycated hemoglobin percentage (HbA1c%), alanine aminotransferase (ALT) and aspartate aminotransferase (AST). A Roche E601 analyzer (Roche, Switzerland) was applied to examine the electrochemical luminescence (ECL) of insulin and C-peptide(C-P).
2.4Definitions and calculations2.4.1DefinitionsAccording to the reference value of Chinese children and adolescents, BMI and WC for age and sex were calculated by Lambda-Mu-Sigma (LMS) method [24–26] and expressed as ZBMI and ZWC respectively.
Overall obesity or overweight was defined as ≥ 95th percentile of BMI or ≥ 85th and <95th percentile of BMI for age and sex, respectively [27]. Abdominal obesity was defined as >90th percentile of WC for age and sex according to the International Diabetes Federation (IDF) [28]. Hypertension was defined as systolic pressure ≥ 130 mmHg or diastolic pressure ≥ 85 mm Hg. Elevated plasma glucose (hyperglycemia) was defined as fasting blood glucose (FBG) ≥ 5.6 mmol/L. Elevated TG and low HDL-C was defined as ≥ 1.70 mmol/L and < 1.03 mmol/L, respectively. TC ≥ 5.18 mmol/L or LDL-C > 3.37 mmol/L was considered as abnormal [29]. Dyslipidemia was defined with any one of the aforementioned abnormal lipids profiles.
2.4.2CalculationsThe homeostatic model assessment of insulin resistance (HOMA-IR) = HOMA-IR= fasting plasma glucose (mmol/L) ×fasting serum insulin(mU/L) / 22.5 [30].
2.5Statistical analysisStatistical analysis was carried out using SPSS22.0. Data of normal distributions were displayed as mean ±SD and tested by independent sample t tests, while skewed distribution data was displayed as median with interquartile range and tested by the Mann-Whitney U test. The categorical variables were expressed as number (percentage) and tested by Chi-square test. Correlations of ZWC, hs-CRP, ZBMI with SLD severity and its related variables were analyzed by Spearman rank correlation. Receiver operating characteristic (ROC) analyses and Youden index (sensitivity +specificity − 1) were applied to determine the areas under the curve (AUC) and the optimal ZWC cut-off values for SLD. Binary logistic regression analysis was used to correct the confounding factors and analyze the interaction effects. A value of p < 0.05 was considered statistically significant.
2.6Ethical statementsBefore informed consent was signed, all the children and their heir parents or the other guardians were told that physical measurements, liver ultrasound and blood tests would be taken. Written informed consent was obtained from each subject and their parents or other guardians. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Ethics Committee. All procedures involving the research study participants were approved by the ethics committee of Children's Hospital of Fudan University.
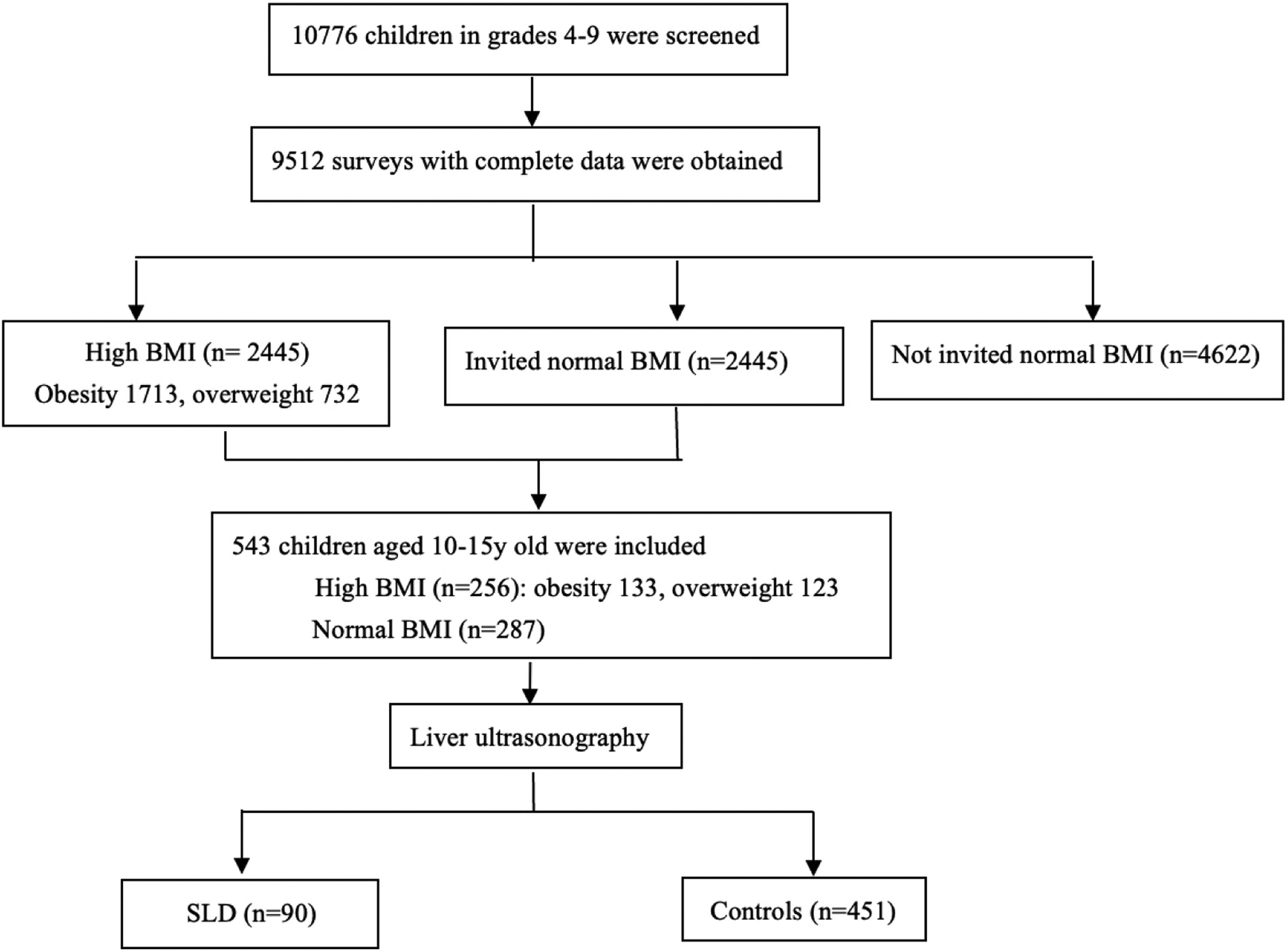
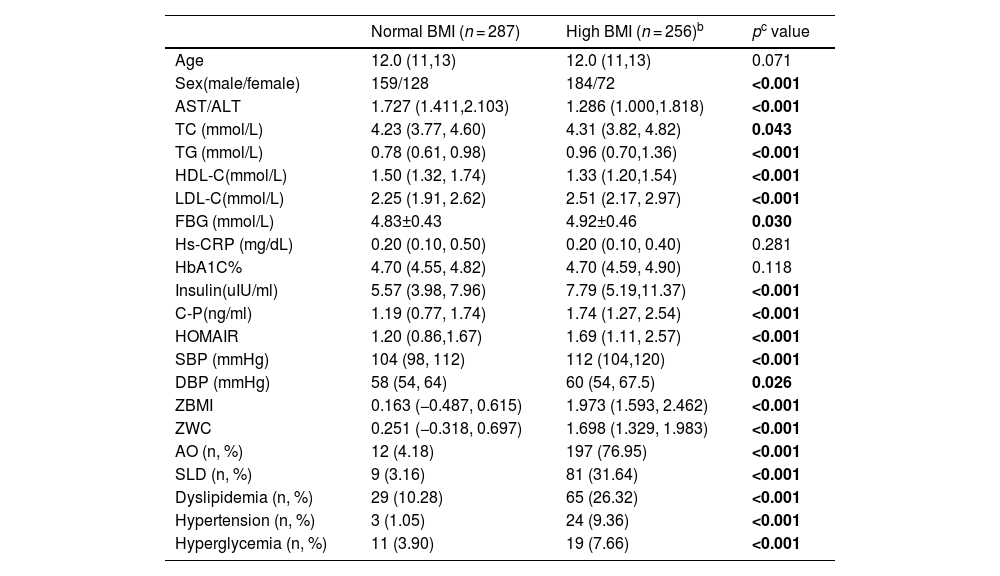
3Results3.1Baseline characteristics of enrolled subjectsAs shown in enrollment flowchart (Fig. 1), a total of 9512 surveys with complete data were obtained and 2445 children were identified as either overweight (n = 1713, 18.01 %) or obese (n = 732, 7.70 %) according to Chinese criterion of overweight and obesity in children [27]. All the 2445 children with high BMI, including overweight and obese children, and randomly selected 2445 children with normal BMI were invited for further medical examinations (including physical measurements, liver ultrasound and blood tests). The present study eventually included 287 children with normal BMI and 256 children with high-BMI children (133 and 123 were obese or overweight, respectively), whose guardians provided the written informed consent. The baseline characteristics of the participants and the prevalence of some cardiometabolic components among boys and girls are shown in Table 1. The numbers of some variables in the table may not add up due to data lose, which were excluded from the corresponding analyses. The percentage of males in the high BMI group was significantly higher that of normal BMI group (χ2 = 15.784, p < 0.001). In comparison to the children with normal BMI group, children with high BMI had significantly higher levels of TC, TG, LDL-C, FBG, insulin, C-P, HOMAIR, SBP, DBP and ZWC (all p < 0.05). Moreover, high BMI group presented remarkable increased prevalence of AO, SLD, dyslipidemia and hypertension than normal BMI group (all p < 0.001). However, HDL-C and the ratio of AST/ALT were significantly lower in high BMI group in comparison to normal BMI group (all p < 0.001). There was no significant difference in age composition, the levels of hs-CRP and HbA1C% between the two groups (all p > 0.05).
The baseline characteristics of the participants divided by BMIa.
| Normal BMI (n = 287) | High BMI (n = 256)b | pc value | |
|---|---|---|---|
| Age | 12.0 (11,13) | 12.0 (11,13) | 0.071 |
| Sex(male/female) | 159/128 | 184/72 | <0.001 |
| AST/ALT | 1.727 (1.411,2.103) | 1.286 (1.000,1.818) | <0.001 |
| TC (mmol/L) | 4.23 (3.77, 4.60) | 4.31 (3.82, 4.82) | 0.043 |
| TG (mmol/L) | 0.78 (0.61, 0.98) | 0.96 (0.70,1.36) | <0.001 |
| HDL-C(mmol/L) | 1.50 (1.32, 1.74) | 1.33 (1.20,1.54) | <0.001 |
| LDL-C(mmol/L) | 2.25 (1.91, 2.62) | 2.51 (2.17, 2.97) | <0.001 |
| FBG (mmol/L) | 4.83±0.43 | 4.92±0.46 | 0.030 |
| Hs-CRP (mg/dL) | 0.20 (0.10, 0.50) | 0.20 (0.10, 0.40) | 0.281 |
| HbA1C% | 4.70 (4.55, 4.82) | 4.70 (4.59, 4.90) | 0.118 |
| Insulin(uIU/ml) | 5.57 (3.98, 7.96) | 7.79 (5.19,11.37) | <0.001 |
| C-P(ng/ml) | 1.19 (0.77, 1.74) | 1.74 (1.27, 2.54) | <0.001 |
| HOMAIR | 1.20 (0.86,1.67) | 1.69 (1.11, 2.57) | <0.001 |
| SBP (mmHg) | 104 (98, 112) | 112 (104,120) | <0.001 |
| DBP (mmHg) | 58 (54, 64) | 60 (54, 67.5) | 0.026 |
| ZBMI | 0.163 (−0.487, 0.615) | 1.973 (1.593, 2.462) | <0.001 |
| ZWC | 0.251 (−0.318, 0.697) | 1.698 (1.329, 1.983) | <0.001 |
| AO (n, %) | 12 (4.18) | 197 (76.95) | <0.001 |
| SLD (n, %) | 9 (3.16) | 81 (31.64) | <0.001 |
| Dyslipidemia (n, %) | 29 (10.28) | 65 (26.32) | <0.001 |
| Hypertension (n, %) | 3 (1.05) | 24 (9.36) | <0.001 |
| Hyperglycemia (n, %) | 11 (3.90) | 19 (7.66) | <0.001 |
Abbreviations: BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood-glucose; hs-CRP, high sensitive C-reactive protein; HbA1c%, the glycated hemoglobin percentage; C peptide, C-P; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance; ZBMI, body mass index Z score; ZWC, waist circumference Z score; AO, abdominal obesity; SLD, steatotic fatty liver disease.
Numbers of some variables in the table may not add up due to data lose: the AO data was not lost in either group of children; the SLD data of 1 child (0.35 %) in the normal BMI group was lost and none was lost in the high BMI group; the blood lipid data of 5 children (1.74 %) in the normal BMI group and 9 (3.52 %)in the high BMI group were lost; the blood pressure data of 1child (0.35 %) in the normal BMI group was lost and none was lost in the high BMI group; the FBG data of 5 children (1.74 %) in the normal BMI group and 8 children (3.13 %) in the high BMI group were lost.
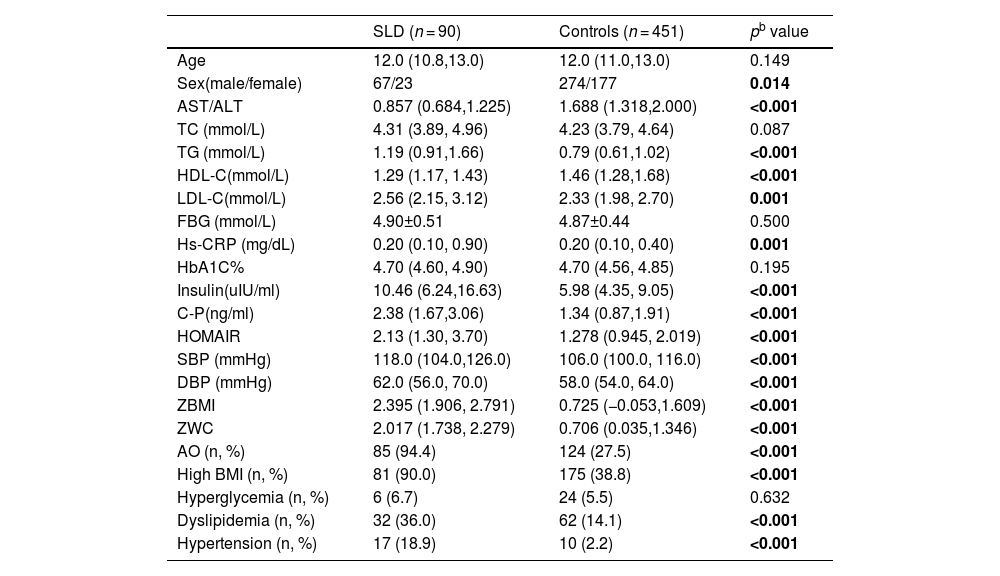
As shown in Table 2, the male children were more susceptible to SLD compared with the female children. (χ2=6.035, p = 0.014). The children with SLD presented significantly higher levels of TG, LDL-C, hs-CRP, insulin, C-P, HOMAIR, SBP, DBP, ZBMI and ZWC (all p < 0.01), while significantly lower levels of HDL-C and the ratio of AST/ALT (all p < 0.001) in comparison to children without SLD. The prevalence of AO, high BMI, dyslipidemia and hypertension were found significantly higher in SLD children in comparison to non-SLD participants (all p < 0.001). No significant difference of the levels of TC, FBG and HbA1C, as well as the prevalence of hyperglycemia, was found between the two groups (all p > 0.05).
Comparison of the anthropometric and cardiometabolic profiles of children with and without SLD (controls) a.
| SLD (n = 90) | Controls (n = 451) | pb value | |
|---|---|---|---|
| Age | 12.0 (10.8,13.0) | 12.0 (11.0,13.0) | 0.149 |
| Sex(male/female) | 67/23 | 274/177 | 0.014 |
| AST/ALT | 0.857 (0.684,1.225) | 1.688 (1.318,2.000) | <0.001 |
| TC (mmol/L) | 4.31 (3.89, 4.96) | 4.23 (3.79, 4.64) | 0.087 |
| TG (mmol/L) | 1.19 (0.91,1.66) | 0.79 (0.61,1.02) | <0.001 |
| HDL-C(mmol/L) | 1.29 (1.17, 1.43) | 1.46 (1.28,1.68) | <0.001 |
| LDL-C(mmol/L) | 2.56 (2.15, 3.12) | 2.33 (1.98, 2.70) | 0.001 |
| FBG (mmol/L) | 4.90±0.51 | 4.87±0.44 | 0.500 |
| Hs-CRP (mg/dL) | 0.20 (0.10, 0.90) | 0.20 (0.10, 0.40) | 0.001 |
| HbA1C% | 4.70 (4.60, 4.90) | 4.70 (4.56, 4.85) | 0.195 |
| Insulin(uIU/ml) | 10.46 (6.24,16.63) | 5.98 (4.35, 9.05) | <0.001 |
| C-P(ng/ml) | 2.38 (1.67,3.06) | 1.34 (0.87,1.91) | <0.001 |
| HOMAIR | 2.13 (1.30, 3.70) | 1.278 (0.945, 2.019) | <0.001 |
| SBP (mmHg) | 118.0 (104.0,126.0) | 106.0 (100.0, 116.0) | <0.001 |
| DBP (mmHg) | 62.0 (56.0, 70.0) | 58.0 (54.0, 64.0) | <0.001 |
| ZBMI | 2.395 (1.906, 2.791) | 0.725 (−0.053,1.609) | <0.001 |
| ZWC | 2.017 (1.738, 2.279) | 0.706 (0.035,1.346) | <0.001 |
| AO (n, %) | 85 (94.4) | 124 (27.5) | <0.001 |
| High BMI (n, %) | 81 (90.0) | 175 (38.8) | <0.001 |
| Hyperglycemia (n, %) | 6 (6.7) | 24 (5.5) | 0.632 |
| Dyslipidemia (n, %) | 32 (36.0) | 62 (14.1) | <0.001 |
| Hypertension (n, %) | 17 (18.9) | 10 (2.2) | <0.001 |
Abbreviations: SLD, steatotic fatty liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood-glucose; hs-CRP, high sensitive C-reactive protein; HbA1c%, the glycated hemoglobin percentage; C peptide, C-P; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance; ZBMI, body mass index Z score; ZWC, waist circumference Z score; AO, abdominal obesity.
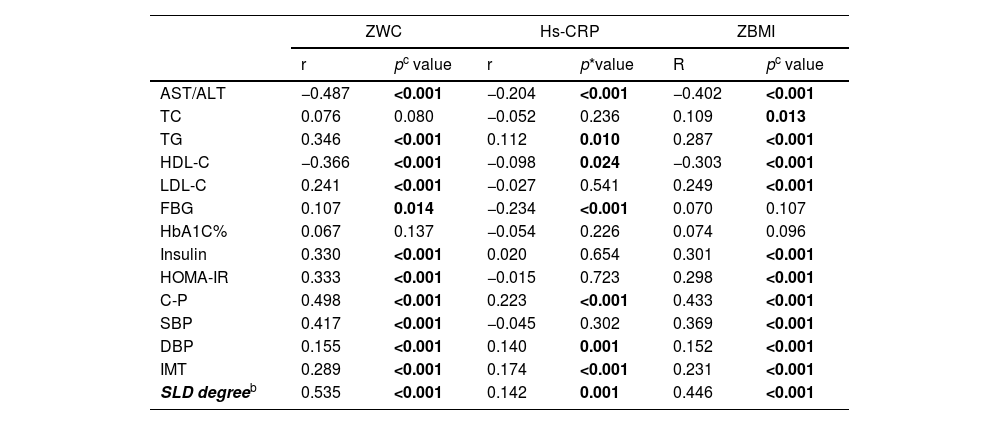
As shown in Table 3, all the three parameters showed significantly negative correlation with AST/ALT and HDL-C (all p < 0.05). while significantly positive correlations between TG, C-P, DBP and the SLD degree, respectively (all p < 0.05). Both ZWC and ZBMI showed significantly positive association with, LDL-C, insulin, HOMA-IR, C-P and SBP (all p < 0.001), respectively. Besides, significantly positive correlation was determined between ZWC with FBG and ZBMI with TC, while significantly negative correlation was examined between hs-CRP with FBG (all p < 0.05). No significant correlations were found between ZWC, hs-CRP and ZBMI with HbA1C% (all p > 0.05).
Correlation coefficients of ZWC, ZBMI, hs-CRP with SLD and its related indicatorsa.
| ZWC | Hs-CRP | ZBMI | ||||
|---|---|---|---|---|---|---|
| r | pc value | r | p*value | R | pc value | |
| AST/ALT | −0.487 | <0.001 | −0.204 | <0.001 | −0.402 | <0.001 |
| TC | 0.076 | 0.080 | −0.052 | 0.236 | 0.109 | 0.013 |
| TG | 0.346 | <0.001 | 0.112 | 0.010 | 0.287 | <0.001 |
| HDL-C | −0.366 | <0.001 | −0.098 | 0.024 | −0.303 | <0.001 |
| LDL-C | 0.241 | <0.001 | −0.027 | 0.541 | 0.249 | <0.001 |
| FBG | 0.107 | 0.014 | −0.234 | <0.001 | 0.070 | 0.107 |
| HbA1C% | 0.067 | 0.137 | −0.054 | 0.226 | 0.074 | 0.096 |
| Insulin | 0.330 | <0.001 | 0.020 | 0.654 | 0.301 | <0.001 |
| HOMA-IR | 0.333 | <0.001 | −0.015 | 0.723 | 0.298 | <0.001 |
| C-P | 0.498 | <0.001 | 0.223 | <0.001 | 0.433 | <0.001 |
| SBP | 0.417 | <0.001 | −0.045 | 0.302 | 0.369 | <0.001 |
| DBP | 0.155 | <0.001 | 0.140 | 0.001 | 0.152 | <0.001 |
| IMT | 0.289 | <0.001 | 0.174 | <0.001 | 0.231 | <0.001 |
| SLD degreeb | 0.535 | <0.001 | 0.142 | 0.001 | 0.446 | <0.001 |
Abbreviations: ZWC, waist circumference Z score; ZBMI, body mass index Z score; hs-CRP, high sensitive C-reactive protein; SLD, Steatotic fatty liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; FBG, fasting blood-glucose; HbA1c%, the glycated hemoglobin percentage; C peptide, C-P; SBP, systolic blood pressure; DBP, diastolic blood pressure; HOMA-IR, homeostatic model assessment of insulin resistance.
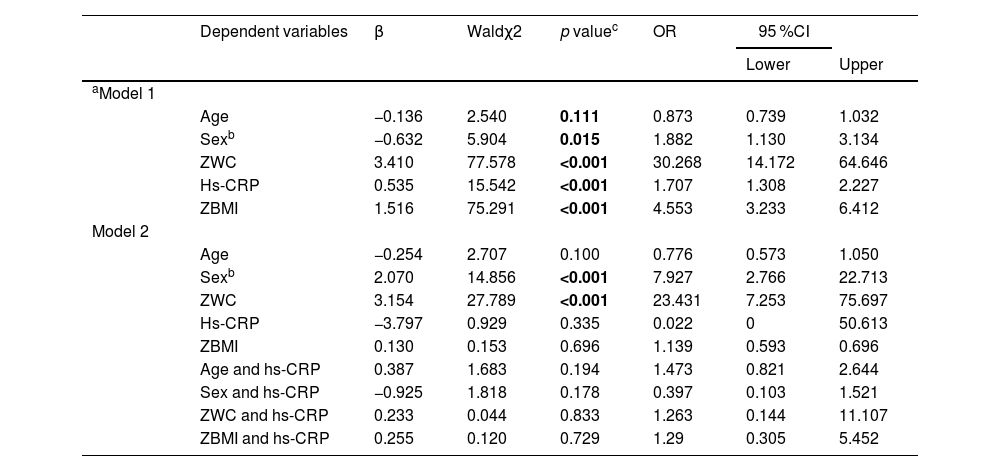
By using the binary regression analysis, the influence of ZWC, hs-CRP and ZBMI on SLD, as well as the interactions of ZWC with hs-CRP and ZBMI with hs-CRP on SLD was investigated. Table 4 presented the associations of SLD with the explanatory variables in 2 models. Model 1 represents the correlations between SLD with ZWC, hs-CRP and ZBMI with adjustment of age and sex, respectively. In model 2, the analysis of correlation between SLD with above mentioned variables and the interaction of age with hs-CRP, sex with hs-CRP, ZWC with hs-CRP and ZBMI with hs-CRP were conducted after age and sex adjustment. The results of model 1 showed that ZWC, hs-CRP and ZBMI significantly positively correlated to SLD with OR (95 %CI) of 30.268 (14.172,64.646), 1.707 (1.308–2.227) and 4.553 (3.233,6.412), respectively, and boys had a higher risk of SLD (OR1.882, 95 % CI:1.130–3.134). The results of model 2 showed that both ZWC and male gender were independent risk factors of SLD with OR (95 %CI) of 23.431 (7.253, 75.697) and 7.927(2.766,22.713), respectively. No significance was found for ZBMI, the interactive effect between ZWC with hs-CRP, as well as ZBMI with hs-CRP on the development of SLD (p > 0.05).
Multivariable association of ZWC, ZBMI and hs-CRP with SLD by binary logistic regression analysis.
| Dependent variables | β | Waldχ2 | p valuec | OR | 95 %CI | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| aModel 1 | ||||||||
| Age | −0.136 | 2.540 | 0.111 | 0.873 | 0.739 | 1.032 | ||
| Sexb | −0.632 | 5.904 | 0.015 | 1.882 | 1.130 | 3.134 | ||
| ZWC | 3.410 | 77.578 | <0.001 | 30.268 | 14.172 | 64.646 | ||
| Hs-CRP | 0.535 | 15.542 | <0.001 | 1.707 | 1.308 | 2.227 | ||
| ZBMI | 1.516 | 75.291 | <0.001 | 4.553 | 3.233 | 6.412 | ||
| Model 2 | ||||||||
| Age | −0.254 | 2.707 | 0.100 | 0.776 | 0.573 | 1.050 | ||
| Sexb | 2.070 | 14.856 | <0.001 | 7.927 | 2.766 | 22.713 | ||
| ZWC | 3.154 | 27.789 | <0.001 | 23.431 | 7.253 | 75.697 | ||
| Hs-CRP | −3.797 | 0.929 | 0.335 | 0.022 | 0 | 50.613 | ||
| ZBMI | 0.130 | 0.153 | 0.696 | 1.139 | 0.593 | 0.696 | ||
| Age and hs-CRP | 0.387 | 1.683 | 0.194 | 1.473 | 0.821 | 2.644 | ||
| Sex and hs-CRP | −0.925 | 1.818 | 0.178 | 0.397 | 0.103 | 1.521 | ||
| ZWC and hs-CRP | 0.233 | 0.044 | 0.833 | 1.263 | 0.144 | 11.107 | ||
| ZBMI and hs-CRP | 0.255 | 0.120 | 0.729 | 1.29 | 0.305 | 5.452 | ||
Abbreviations: ZWC, waist circumference Z score; ZBMI, body mass index Z score; hs-CRP, high sensitive C-reactive protein; SLD, Steatotic fatty liver disease.
Model-1 evaluated the association between ZWC, hs-CRP and ZBMI with SLD after adjusting age and sex, respectively. Model-2 evaluated the association between SLD with ZWC, ZBMI, hs-CRP, the interaction effects of age with hs-CRP, sex with hs-CRP, ZWC with hs-CRP and ZBMI with hs-CRP on the development of SLD after age and sex were adjusted.
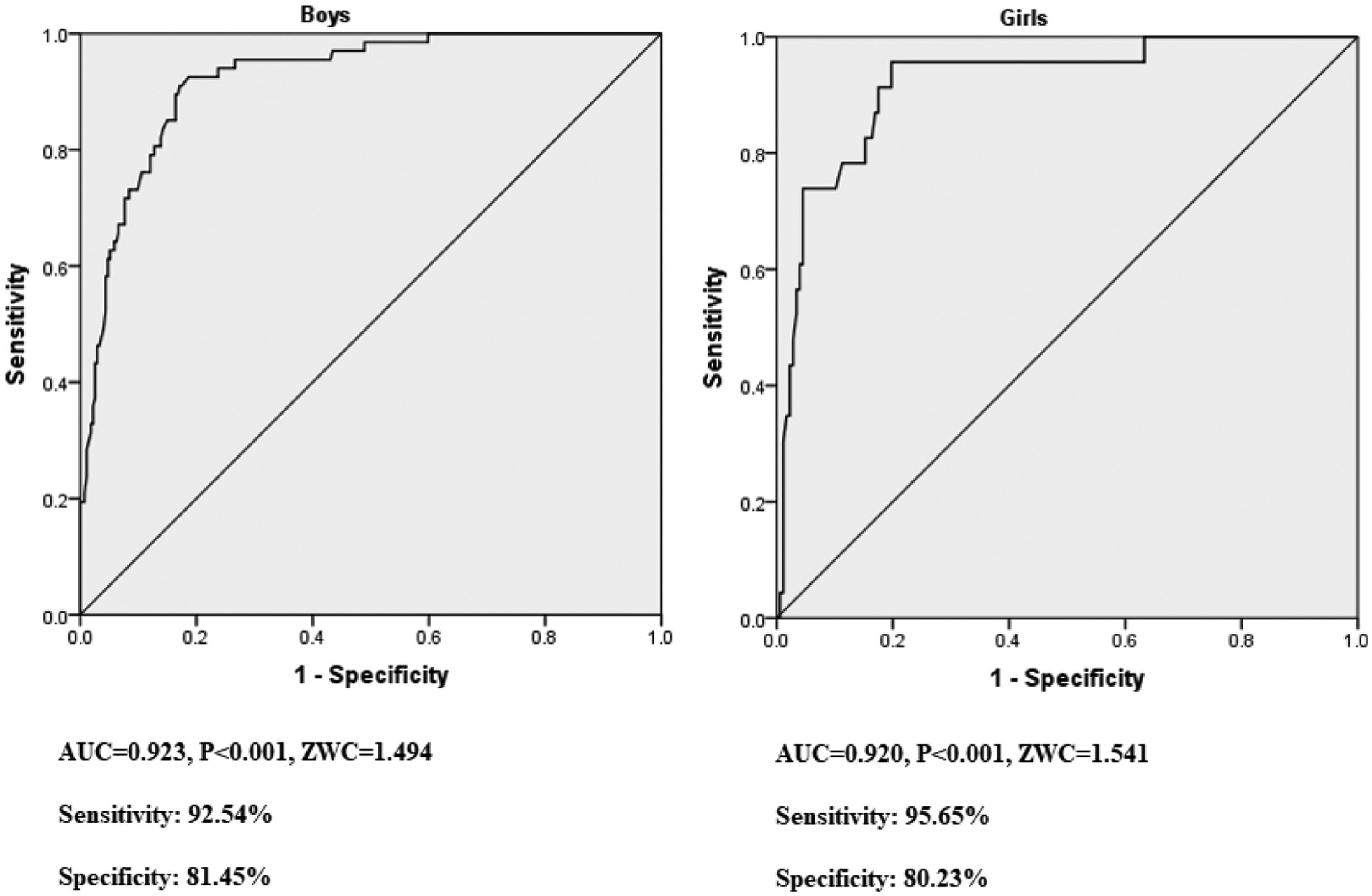
The AUCs were calculated and the optimal cutoff points for ZWC were analyzed to identify SLD in both boys and girls. As shown in Fig. 2, the AUC (OR, 95 %CI) of the boys and girls was 0.923 (0.889–0.949, p < 0.001) and 0.920 (0.874–0.954, p < 0.001), respectively. The cut-off value of ZWC with the maximum sensitivity plus specificity was 1.494 and 1.541 in boys and girls, respectively. With these cut-off values, the sensitivity and specificity for boys was 92.54 % and 81.45 %, while those for girls was 95.65 % and 80.23 %, respectively.
4DiscussionThe present study assessed the association among WC, hs-CRP, BMI and SLD, as well as SLD related metabolic indictors in children aged 10–15 years. The predictive effect of WC for SLD was also evaluated. The results suggested that WC, hs-CRP and BMI were all significantly correlated to SLD and its related metabolic indictors in the pediatric population. Children with SLD showed obviously more metabolic abnormalities and greater risk for insulin resistance, dyslipidemia, hypertension and increased inflammation. ZWC presented to be an independent risk factor of SLD, while hs-CRP and ZBMI played interactive effect on SLD. Our study offered a new insight into the elaborate association among WC, inflammation, BMI and SLD in children as a whole.
It has been proven that hepatic steatosis closely associates with insulin resistance and majority of MASLD patients had dyslipidemia which was thought to result from hepatic and peripheral insulin resistance [31,32]. Excess liver fat accumulation during the occurrence of SLD can lead to macrophage/ Kupffer cells activation, which increases insulin resistance and liver inflammation. Moreover, numerous studies have demonstrated that SLD patients had significantly higher prevalence of hypertension than general population, and about 49.5 % of adult patients aged 50–75 years with hypertension developed SLD [33]. Meanwhile, the occurrence and long-term existence of hypertension was found to promote the development of SLD conversely [34]. It has been reported that MASLD linked to various aspects of CVD in children aged 10–17years old, including cardiac dysfunctions, atherosclerosis, and hypertension [35–37]. SLD may induce hypertension by activating systemic inflammation and insulin resistance, as well as the renin-angiotensin system (RAS)-sympathetic nervous system (SNS) [38,39]. In the present study, we showed that the children with SLD had obviously higher risk of dyslipidemia, insulin resistance, hypertension and increased inflammation in comparison to those without SLD, providing direct evidence that the development of SLD associates with a series of metabolic abnormalities. That is, children with SLD had a higher risk of progressing to MASLD.
ALT and AST are biomarkers for the detection of hepatocellular injury and liver function. There were plenty of studies showed that AST/ALT ratio could be related to multiple liver diseases [40,41]. Furthermore, AST/ALT ratio has been reported to independently associate with increased risk of both all-cause mortality and cardiovascular mortality in patients with a mean age 70 years with T2DB [42]. However, rare studies have been reported regarding the relationship among AST/ALT ratio, WC, hs-CRP, BMI and SLD in children. One study from Chinese population displayed that the adolescents aged 11–16 years with the lowest AST/ALT ratio had 6.02 times higher risk of metabolic syndrome in comparison to their highest counterpart, and AST/ALT ratio was negatively associated with FBG [43]. Another study observed that the ALT level of overweight children aged 1–12 years was significantly lower than that of obese children, whose liver enzymes at the upper limit of normal were likely to show signs of fatty liver disease [44]. Experts even suggested that screening for elevated ALT in severely obese children could begin at age 2 [45]. AST/ALT ratio was found significantly lower in children with higher BMI and SLD in our study, as well as significantly negatively associated with ZWC, hs-CRP and ZBMI. Taken together, the change of AST/ALT ratio is highly probably related to SLD and its related cardiometabolic disorders in children.
It is well documented that WC, hs-CRP and obesity play fundamental roles during the development of SLD and are closely related to CVD and T2DB in adulthood. Extensive studies have investigated the correlations of WC and BMI with SLD, or systemic inflammation with SLD [14,46]. However, the association and interaction among WC, hs-CRP and BMI with SLD and its related metabolic indictors have rarely been thoroughly analyzed. In the current study, we observed that WC, hs-CRP and BMI were all significantly associated with glucolipid metabolism disorder, elevated blood pressure and SLD severity. Furthermore, logistic regression analysis showed that WC could act as an independent risk factor for SLD, while no significance was found for ZBMI, the interactive effect between ZWC with hs-CRP, as well as ZBMI with hs-CRP on the development of SLD. Although WC, BMI, and hs-CRP were all strongly associated with SLD and its related metabolic indictors in the preliminary analysis, only WC and male gender were independent risk factors for SLD after adjustment of age, sex and interactions. The different influential effects between WC and BMI on SLD shows that WC has more obvious effect on SLD in children aged 10–15 years old. It suggested that WC would be a better clinical indicator than BMI for screening and monitoring SLD and its related metabolic abnormalities in this age group of children. The reason may be that although BMI can reflect body fat content, it cannot reflect body fat distribution, which has been proved to act a greater effect than body fat content on cardiometabolic diseases [47].Abdominal fat can release free fatty acids into the portal vein, form TG in the liver and then promote hepatocyte inflammation, which is believed to be the most important factor leading to liver injury in SLD [48].
It is worth mentioning that our study found that male children aged 10–15 years not only have a significantly higher risk of overweight or obesity, but also a significantly higher risk of SLD. Even after the adjustment of age ZWC, ZBMI and hs-CRP, male gender remained an independent risk factor for SLD in our study, suggesting that more attention should be paid to male children in the prevention and management of SLD and MASLD.
Although it remained controversial, CRP has long been believed to have a crucial role in glucose metabolism. The risk of T2DB was observed to increase in the population with mean age of 52.7 years with elevated CRP levels even after the potential confounding factors were adjusted [49]. It has been reported that T2DB patients aged 7–17 years had higher hs-CRP, which positively correlated with many cardiometabolic indictors [50]. Kato K et al. reported that the highest serum CRP quartile was independently relevant to higher HbA1c and marginally correlated with increased risk of impaired glucose tolerance plus diabetes in a population with mean age 52 years. However, CRP levels were unrelated to a higher risk of developing impaired fasting glucose according to their analyses. In our study, we also found a weak inverse correlation between hs-CRP and FBG. High levels of C-P usually coexist with insulin, which was thought to promote atherogenesis and then lead to increased risk for CVD [51]. In the current study, a significantly positive correlation between hs-CRP and C-P was observed. The possible reason for the negative correlation between hs-CRP with FBG and positive correlation between C-P and hs-CRP could be the compensatory capacity of islet cells, which can regulate temporarily raised FBG during childhood and adolescence.
The association between WC and SLD has been extensively studied in adults. WC was even reported as a simpler and more manageable index than fatty liver index for detection of SLD with comparable accuracy [52]. Although previous studies showed that waist-to-height ratio was a useful index for SLD in children and adolescents with mean age of 15.5 years (with 70.1 % of sensitivity and 76.9 % of specificity) [53] and obese adolescents aged 12–19 years with higher quartiles of WC were more likely to develop SLD than those with lower quartile [54] of WC, the current study to our knowledge determined the predictive effect of WC for SLD in children aged 10–15 years for the first time. Similar to the results of a previous study on adults with mean age of 39.4 years, in which increased quartiles of WC was observed to cause increased SLD in a dose-response manner [55], we also found a dose-response relationship between ZWC and SLD severity. Taken together, all the studies offered evidence that increased WC plays a key role in SLD development in both children and adults, and the management of WC is crucial for SLD prevention and early detection.
In summary, although the associations of WC, BMI, and hs-CRP with SLD have been already known and the analyses of MASLD risk factors have been extensively studied, we conducted overall analyses of the correlations not only between ZWC, hs-CRP, ZBMI with SLD but also with various aspects involved in the development of MASLD, including glucose metabolism, lipid metabolism, liver and kidney function and blood pressure. Our study is helpful to comprehensively understand the important role of these indicators in the process of SLD progressing to MASLD. Furthermore, the interaction effects between age and hs-CRP, sex and hs-CRP, ZWC and hs-CRP, as well as ZBMI and hs-CRP on SLD were also explored, and the predictive effect of ZWC on SLD was analyzed. We not only found the association of ZWC, hs-CRP and ZBMI with SLD and its related metabolic abnormalities, but also provided strong evidence that WC and male gender were independent risk factors for SLD, while the same effect wasn't observed for BMI.
The new nomenclature of MASLD represents a range of metabolic abnormalities from simple intrahepatic fat accumulation to steatohepatitis, which may eventually progress to cirrhosis and hepatocellular carcinoma [56]. However, the metabolic dysfunction indictors are complex and correlated, which are less convenient for routine health screening in children. Therefore, WC measurement as a simple, non-invasive, and comparatively accurate assessment method is not only essential to ensure early detection of SLD and MASLD in children, but also critical for the intervention effect monitoring. The present study showed that WC could be an independent predictor for pediatric SLD. Based on a relatively large sample of Chinese children population, our data may be used as a reference for studies on SLD and MASLD in children and adolescents, especially in Asian pediatric population. However, there are limitations in our study. First, the sample size should be even larger, which would have made the conclusions more convincing. Secondly, liver biopsy has been thought to be the gold standard for SLD diagnosis. In our study, SLD was diagnosed by ultrasonography to avoid invasive and expensive procedure, which may reduce the diagnostic accuracy.
5ConclusionsThe present study showed that increased WC, BMI and hs-CRP exerted adverse effects on pediatric SLD and its related cardiometabolic conditions, which could cause a high MASLD risk. WC and male gender were independent risk factors for SLD, while the same effect wasn't observed for BMI. Moreover, WC is a powerful index for the prediction of SLD in children aged 10–15 years.
FundingThe study was supported by the Key Projects in the National Science & Technology Pillar Program during the Eleventh Five-year Plan Period (No.2009BAI80B03), and the Clinical Research Fund of the Internal Peace Maternity and Child Health Hospital (No.YN201908), Shanghai Jiao Tong University. The founders had no role in the design, analysis or writing of this article.
Authors contributionsQiaoling Wu was responsible for the participant recruitment, data collection, statistical analysis, drafting and editing of the manuscript. Chundan Gong were involved in the participant recruitment and data collection. Yongmei Peng designed and supervised the study. All authors read and approved the final manuscript.