Exercise-induced premature ventricular complexes (EiPVCs) are often considered as benign arrhythmias, although they are associated with a high risk of all-cause death in the general healthy population. However, an intermediate pathophysiological process remains unclear, particularly in patients with known cardiovascular disease. The aim of this study was to find an association between EiPVCs, the occurrence of life-threatening ventricular arrhythmias (LACO), and all-cause mortality in patients with cardiovascular disease.
MethodsThis was an observational study of a cohort of patients with coronary artery disease (CAD) or idiopathic cardiomyopathy (ICM). Stress testing was performed as a part of the routine cardiovascular evaluation. The occurrence of EiPVCs was evaluated during exercise testing (ET). At follow-up, long-term occurrence of LACO was evaluated. A bivariate and multivariate analysis was performed.
ResultsOut of the total of 1442 patients analysed, 700 (49%) had EiPVCs. During 14 years of following-up after ET, 106 LACO outcomes were observed. Long-term all-cause mortality was 4% (n=61). A bivariate analysis showed that patients with EiPVCs had an increased risk for LACO (RR=2.81, 95% CI; 1.9–4.3, P<.001), and for mortality (RR=2.1, CI95% 1.2–3.4, P<.01). Occurrence of LACO was also associated with a higher mortality risk (RR=5.7, 95% CI; 3.4–9.4, P<.001). After a post hoc analysis, LACO remained as a highly predictive variable for mortality.
ConclusionPatients with EiPVCs have a high risk of LACO and all-cause mortality. The presence of LACO could be an intermediate stage between EiPVCs and mortality in subjects with cardiovascular disease.
La extrasistolia ventricular inducida por ejercicio (EVIE) suele considerarse una arritmia benigna, sin embargo, ha sido asociada a mayor mortalidad en población general. Hasta hoy no se ha encontrado el proceso fisiopatológico involucrado, particularmente en pacientes con enfermedad cardiovascular. El objetivo del estudio fue establecer una asociación entre EVIE con la ocurrencia de arritmias ventriculares potencialmente malignas (APM) y letalidad a largo plazo, en sujetos con enfermedad cardiovascular.
MétodoEstudiamos una cohorte de pacientes con enfermedad coronaria o miocardiopatía dilatada, que realizaron una prueba de ejercicio al inicio del estudio. Inicialmente evaluamos la ocurrencia de EVIE, APM y letalidad a largo plazo y posteriormente se realizó un estudio bivariable y multivariable.
ResultadosSe incluyeron 1,442 pacientes de los cuales 700 presentaron EVIE (49%). Durante 14 años de seguimiento, 106 sujetos presentaron APM y la mortalidad total fue de 61 casos (4%). El estudio bivariable mostró que aquellos pacientes con EVIE tuvieron un riesgo de presentar APM de 2.81 (IC95% de 1.9 a 4.3, p<0.001) y de mortalidad de 2.1 (IC95% de 1.2 a 3.4, p<0.01). Los individuos con APM tuvieron mayor riesgo de mortalidad (RR= 5.7, IC95% de 3.4 a 9.4, p<0.001). Después de un análisis multivariable, la APM continuó siendo una variable altamente predictiva de mortalidad.
ConclusiónLos pacientes con EVIE tienen un riesgo elevado de presentar APM y de mortalidad a largo plazo. Los resultados sugieren que las APM podrían representar un estado intermedio entre la presencia de EVIE y la mortalidad en pacientes con enfermedad cardiovascular.
Premature ventricular complexes are traditionally considered as benign arrhythmias, but this may not be the case. Some studies reported that these arrhythmias observed in apparently healthy patients, are associated with a long-term high risk of all-cause mortality, mainly due to unrecognized coronary heart disease.1–4 Exercise testing (ET) is particularly useful to identify heart rhythm disorders, especially those associated with cardiac sudden death.5,6 A recent meta-analysis showed that exercise-induced premature ventricular complexes (EiPVCs) are associated with an increased risk of all-cause death in the general population.7 However, the long-term worsening of EiPVCs into a high-risk arrhythmia events in patients with heart disease remains unclear, specifically the development of life-threatening arrhythmia. Therefore, the aim of this study was to find an association between EiPVCs, the occurrence of life-threatening ventricular arrhythmias and all-cause mortality in patients with cardiovascular disease.
MethodsThis was an observational study, and we studied a cohort of patients with recognized coronary artery disease (CAD) or idiopathic cardiomyopathy (ICM), older than 18 years old, who initially performed stress testing as a part of their routine cardiovascular evaluation.
The occurrence of EiPVCs was evaluated during exercise testing (ET). All patients performed a symptoms limited ET, using a Schiller CS-200© device with a treadmill. An electrocardiographic signal (ECG) was recorded throughout the test and blood pressure (BP) was measured every minute during exercise and at 1st, 3rd, 5th and 8th min of recovery, using a calibrated aneroid sphygmomanometer. ECG leads were placed according to the Mason-Likar method. Initially, the skin was cleaned with a cotton swab saturated with alcohol and a gentle abrasion of the skin layer was made with a fine sandpaper. All ETs started with a rest period of 3min, and a Balke ramp protocol was selected for the exercise phase. Once maximal exercise was reached, the subjects walked 3 more minutes at 2km/h at a 0% elevation. They then rested in the supine position for 5min. A heart defibrillator with a fully stocked resuscitation cart were present at all times. Written consents were obtained from all patients.
The heart rate (HR) was obtained from the continuous ECG signal. Maximum exercise tolerance was measured in metabolic equivalents (MET). EiPVCs were defined as isolated ectopic beats, trigeminism, bigeminism or couplets. Patients with ventricular tachycardia were excluded from the analysis. Statistical analysis was performed using SPSS 21 software. Nominal and categorical variables were presented as frequencies and percentages, and they were compared using the Chi square test. Numerical continuous variables are presented as mean and standard deviation and data distribution was analyzed with the Kolmogorov–Smirnov test. A t-test for independent variables was used to compare those variables with Gaussian distribution, and for those variables without normal distribution, the Mann–Whitney U test was used.
At a follow-up, long-term Life-threatening Arrhythmia Combined Outcome (LACO) was evaluated, and it included those patients who survived the occurrence of an episode of ventricular fibrillation/flutter or sustained monomorphic ventricular tachycardia, that were attended at the emergency room, and/or that received a cardio-defibrillator (ICD). Survival status was obtained directly from medical records. A bivariate analysis was conducted between EiPVCs, clinical characteristics and those variables that were considered statistically significant. Then, those variables were included in a multivariable Cox-regression proportional hazard model. Kaplan and Meier survival curves were plotted according to the presence of EiPVCs and LACO. All p values less than 0.05 were considered stochastically significant.
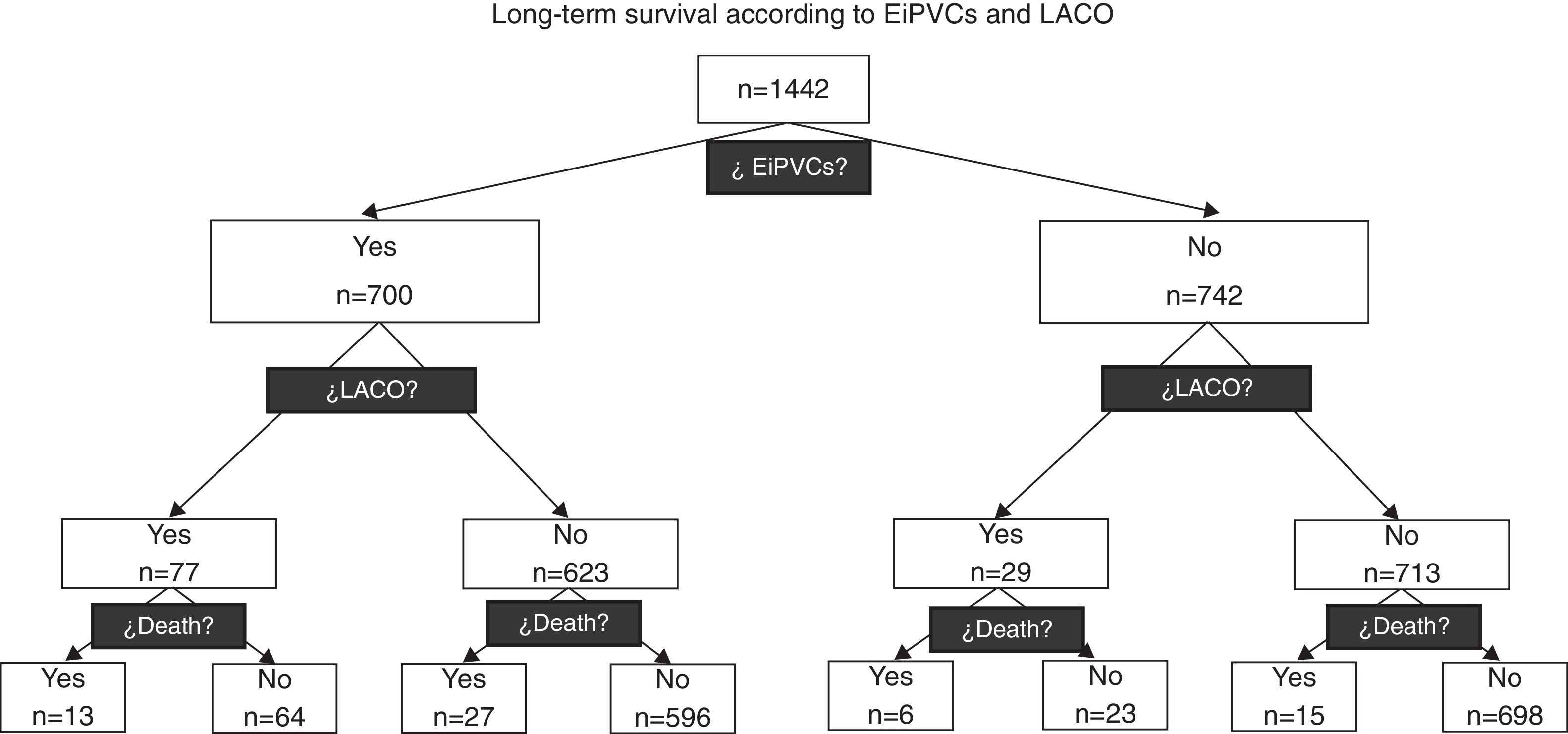
ResultsA total of 1442 patients were included and 700 (49%) of them presented EiPVCs. No ventricular fibrillation episode was observed. During 14 years of following-up, 106 (7%) LACO and 61 (4%) deaths were observed, both outcomes were clustered according to EiPVCs, and 250 patients were lost (17.3%), see Fig. 1. At first glance, we observed that patients with EiPVCs were older, heavier, with lower LVEF (left ventricle ejection fraction) and they used to take digoxin, diuretics and antiarrhythmic drugs. Demographic characteristics (Table 1).
In this diagram we can observe how the occurrence of Life-threatening Arrhythmia Combined Outcome (LACO) and mortality are distributed according to the presence of exercise-induced premature ventricular complexes (EiPVCs). Patients with EiPVCs have a higher relative risk for LACO (RR=2.81; IC95% from 1.9 to 4.3).
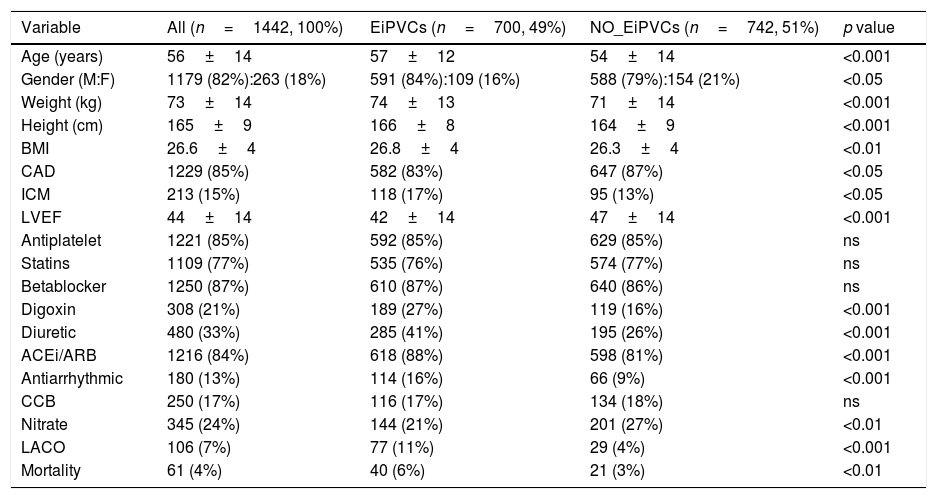
Demographic characteristics.
| Variable | All (n=1442, 100%) | EiPVCs (n=700, 49%) | NO_EiPVCs (n=742, 51%) | p value |
|---|---|---|---|---|
| Age (years) | 56±14 | 57±12 | 54±14 | <0.001 |
| Gender (M:F) | 1179 (82%):263 (18%) | 591 (84%):109 (16%) | 588 (79%):154 (21%) | <0.05 |
| Weight (kg) | 73±14 | 74±13 | 71±14 | <0.001 |
| Height (cm) | 165±9 | 166±8 | 164±9 | <0.001 |
| BMI | 26.6±4 | 26.8±4 | 26.3±4 | <0.01 |
| CAD | 1229 (85%) | 582 (83%) | 647 (87%) | <0.05 |
| ICM | 213 (15%) | 118 (17%) | 95 (13%) | <0.05 |
| LVEF | 44±14 | 42±14 | 47±14 | <0.001 |
| Antiplatelet | 1221 (85%) | 592 (85%) | 629 (85%) | ns |
| Statins | 1109 (77%) | 535 (76%) | 574 (77%) | ns |
| Betablocker | 1250 (87%) | 610 (87%) | 640 (86%) | ns |
| Digoxin | 308 (21%) | 189 (27%) | 119 (16%) | <0.001 |
| Diuretic | 480 (33%) | 285 (41%) | 195 (26%) | <0.001 |
| ACEi/ARB | 1216 (84%) | 618 (88%) | 598 (81%) | <0.001 |
| Antiarrhythmic | 180 (13%) | 114 (16%) | 66 (9%) | <0.001 |
| CCB | 250 (17%) | 116 (17%) | 134 (18%) | ns |
| Nitrate | 345 (24%) | 144 (21%) | 201 (27%) | <0.01 |
| LACO | 106 (7%) | 77 (11%) | 29 (4%) | <0.001 |
| Mortality | 61 (4%) | 40 (6%) | 21 (3%) | <0.01 |
Demographic variables are described as mean±standard deviation or n (%) according variable type. EiPVCs (exercise-induced premature ventricular complexes), BMI (body mass index), CAD (coronary artery disease), ICM (idiopathic cardiomyopathy), LVEF (left ventricle ejection fraction as percentage), ACEi (angiotensin converting enzyme inhibitor), ARB (Angiotensin Receptor Blocker), CCB (Calcium Channel Blocker), LACO (Life-threatening Arrhythmia Combined Outcome).
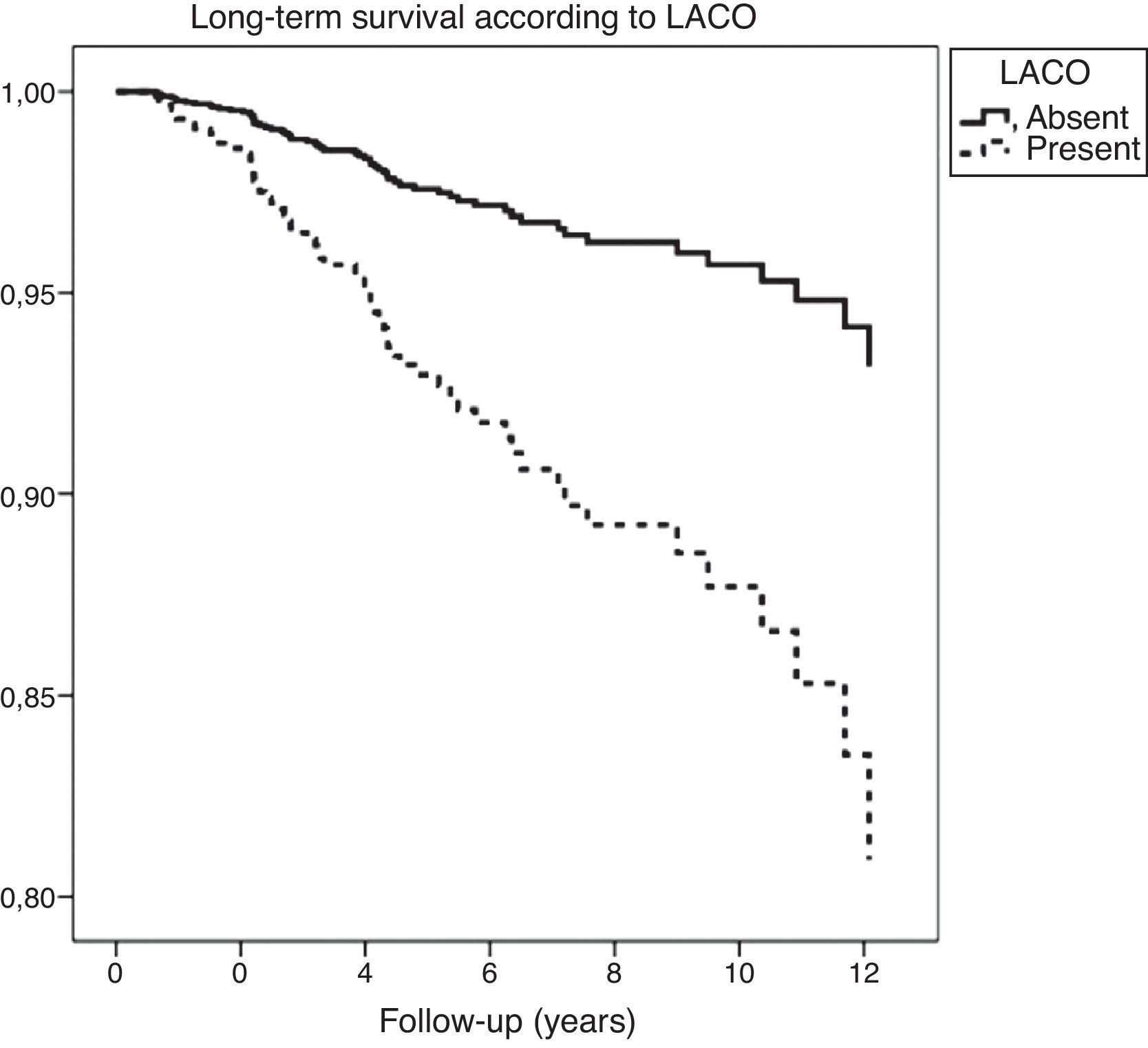
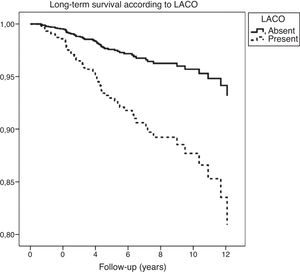
On one hand, patients with EiPVCs showed an increased long-term risk of LACO (RR=2.81, CI95% 1.9–4.3, p<0.001) and of mortality (RR=2.1, CI95% 1.2–3.4, p<0.01). On the other hand, bi-variate analysis showed several clinical characteristics that were associated with a higher risk of mortality, including both EiPVCs and LACO (Table 2). LACO was also related with higher mortality (RR=5.7, CI95% 3.4–9.4, p<0.001). Survival curves are displayed elsewere (Fig. 2). After post hoc analysis, variables that remained statistically associated with mortality were digoxin use, LACO and maximal exercise tolerance (METs).
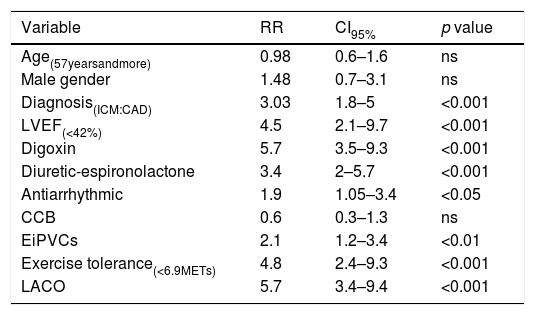
The relative risk of mortality according to clinical and exercise testing variables.
| Variable | RR | CI95% | p value |
|---|---|---|---|
| Age(57yearsandmore) | 0.98 | 0.6–1.6 | ns |
| Male gender | 1.48 | 0.7–3.1 | ns |
| Diagnosis(ICM:CAD) | 3.03 | 1.8–5 | <0.001 |
| LVEF(<42%) | 4.5 | 2.1–9.7 | <0.001 |
| Digoxin | 5.7 | 3.5–9.3 | <0.001 |
| Diuretic-espironolactone | 3.4 | 2–5.7 | <0.001 |
| Antiarrhythmic | 1.9 | 1.05–3.4 | <0.05 |
| CCB | 0.6 | 0.3–1.3 | ns |
| EiPVCs | 2.1 | 1.2–3.4 | <0.01 |
| Exercise tolerance(<6.9METs) | 4.8 | 2.4–9.3 | <0.001 |
| LACO | 5.7 | 3.4–9.4 | <0.001 |
Abbreviations: LACO (Life-threatening Arrhythmia Combined Outcome), RR (relative risk), CI (confidence interval), ns (non significant difference), ICM (idiopathic cardiomyopathy), CAD (coronary artery disease), LVEF (left ventricle ejection fraction), CCB (Calcium Channel Blocker), EiPVCs (exercise-induced premature ventricular complexes), METs (metabolic equivalents).
Ventricular tachyarrhythmia is a heterogeneous entity with wide variation in clinical course and prognosis. Several researching groups established that healthy individuals with ventricular ectopy during exercise showed a higher risk of all cause or cardiovascular mortality than their counterparts without arrhythmia, but they have not yet found a clear pathophysiological explanation. Morshedi et al. have induced ventricular ectopy in apparently healthy subjects using intense and acute exercise. They found that EiPVCs had a higher long-term mortality than their counterparts without arrhythmia. This risk persisted independently of EiPVC's kind or severity.1 Physical exercise is a useful tool for triggering cardiac arrhythmia due to some physio-pathological changes, including ischemia and an elevated sympathetic nervous system flow with higher concentrations of serum catecholamines.5
Exercise induced ventricular arrhythmias in athletes are relatively rare. Verdile et al., have found that 7% of healthy athletes presented premature ventricular beats during exercise, and only 0.7% showed more than 10 PVC or another more complex ventricular arrhythmias. No events or cardiovascular disease occurred in these athletes over a follow-up period of 7.4±5 years.8
In our study, we hypothesized that a life-threatening arrhythmia (LACO) can be an intermediate stage between EiPVCs and mortality, in subjects with cardiovascular disease. Therefore, first we established that patients with EiPVCs had a higher risk for all-cause mortality. After that, we found a statistically strong association between EiPVCs and LACO. Finally, this last group of individuals also had a higher risk of mortality.
It has been reported that the presence of complex ventricular arrhythmia is associated with a higher mortality in patients with coronary artery disease, especially in those individuals with risk factors like genetics, abnormalities of QT interval, left ventricular dysfunction, coronary artery disease and early repolarization.9–11 Our research had initially revealed, in bivariate analysis, some statistical differences between patients with and without EiPVC like age, gender, BMI, baseline diagnostic (CAD vs ICM), LVEF, and pharmacological therapy. Surprisingly, we found that betablocker therapy has no statistically association for EiPVC at baseline, neither for main outcome. However, after post hoc analysis (Cox), mostly all these associations have disappeared. There were no statistical differences according to revascularization procedure either. We think that EiPVCs could progress into more dangerous ventricular rhythm disorders, associated to more severe myocardial damage, digoxin use and marked reduced maximal exercise tolerance. This last variable is well known to be strongly predictive for all-cause mortality.
Although the presence of EiPVC seems to be innocuous, it appears that specific treatment for this kind of arrhythmias should be more aggressive. Tran et al. have recently published a review describing how EiPVCs are related to a decrease of left ventricular function, and how pharmacological therapy or even catheter ablation, may suppress arrhythmia with a reversion of this kind of cardiomyopathy.12 Our results suggest that individuals with EiPVCs could evolve to a more severe arrhythmic condition before dying, specifically patients with heart disease. It is therefore recommended that patients with EiPVCs should be evaluated more closely, looking for prescribe an adequate therapy just in time, in order to prevent sudden cardiac death.
These findings accomplished some, but not all criteria for ascertaining causality (i.e. statistical association, temporal sequence of facts, reasoning by analogy, consistency, biological plausibility and logical coherence).13,14 The main limitation for this research is that we only established a statistical association between variables without a biochemical or physiological evidence. Therefore, further research is required to establish causality in areas such as dose-response and the specificity of risk factors and outcome association. It is also necessary to establish specific pathophysiological mechanisms that could explain how this phenomenon of worsening arrhythmia could develop. Serum markers, neuro- humoral signaling or electrophysiological processes may provide clues in understanding these mechanisms.
ConclusionPatients with EiPVCs have a high risk for all-cause mortality. The presence of a life-threatening arrhythmia (LACO) could be an intermediate stage between EiPVCs and mortality in subjects with cardiovascular disease.
Ethical disclosuresProtection of people and animalsThe authors state that no human or animal experiments have been performed for this research.
Confidentiality of dataThe authors state that the data presented in this work have been obtained from a database, which is treated in accordance with the current legislation regarding the confidentiality of the data.
Right to privacy and informed consentThe authors state that the data presented in this work have been obtained from a database, which is treated in accordance with the current legislation regarding the confidentiality of the data.
FundingNone.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank all the people that work at the Cardiac Rehabilitation and Electro-cardiology departments in the National Institute of Cardiology in Mexico for their daily work.