Transcatheter aortic valve implantation (TAVI) is a viable treatment option severe aortic stenosis in patients deemed to be at high surgical risk. Despite the favorable results of this procedural technique, concomitant medical comorbidities contribute to peri- and post-procedural morbidity and mortality in patients undergoing TAVI.1 Additional procedural factors such as the need for valve predilation, prolonged or repeated rapid pacing also contribute to procedural risk, particularly in those with heart failure or pulmonary hypertension with resultant loss of ventricular interdependence.2 Some authors have shown that microvascular tissue perfusion is affected during rapid pacing while performing a transcatheter aortic valve replacement. Rapid ventricular pacing may lower coronary blood flow from 25% with an 8-second rapid-pacing run up to 50% at a 18-second rapid-pacing run.3 Although mean arterial pressure is recovered rapidly, mircovascular perfusion arrest (“no flow”) occurs in a significant proportion of patients and becomes more likely with prolonged rapid pacing.4 Also rapid pacing may promote ventricular arrhythmias and hypotension. When added to pulmonary hypertension, this effect could be catastrophic due to an imbalance on preload, afterload and contractility.5 Interestingly, prior reports have shown the feasibility of direct transcatheter valve implantation without valve predilation.6 To this effect, we describe the case of a patient deemed at extremely high surgical risk for conventional surgical aortic valve replacement, complicated by severe pulmonary hypertension, who underwent TAVI. It was decided by the Heart Team to not perform valve predilation before the procedure was begun in order to minimize the risks related to rapid ventricular pacing. During the procedure, it was necessary to improvise as the absence of valve predilation made it impossible to cross the annulus with the transcatheter aortic valve. We adopted the “buddy wire” technique as an effective tool to cross the aortic valve on this case with severe calcified aortic stenosis without valve predilation.
A 90-year-old frail man was referred to the Quebec Heart and Lung Institute for the management of rapidly progressing dyspnea (New York Heart Association class 4). Investigations revealed severe aortic stenosis with a mean transvalvular gradient of 70mmHg, aortic valve area: 0.4cm2, peak pulmonary artery systolic pressure of 75mmHg, left ventricular ejection fraction of 35%, and angiographically normal coronary arteries. Following Heart Team evaluation, his advanced age, markedly raised pulmonary artery pressures, frailty, and high STS-PROM and logistic EuroSCORE scores (8.1% and 20.6% respectively), TAVI was offered as an alternative treatment option. Furthermore, it was also felt that native valve predilation with rapid pacing would likely be poorly tolerated, largely due to his severe pulmonary hypertension and concomitant severe biventricular systolic dysfunction.
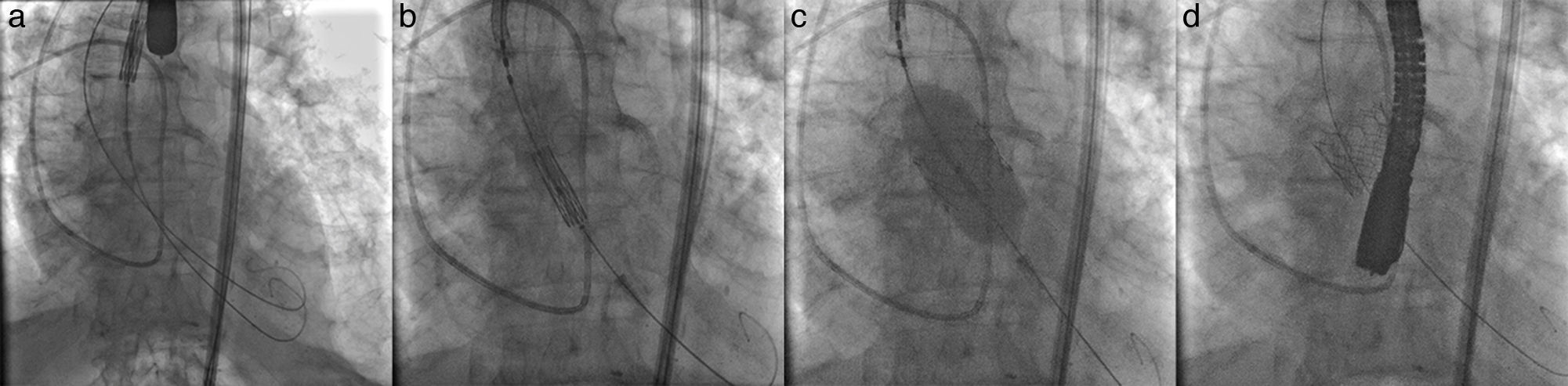
The procedure was performed via the right transfemoral approach, under general anesthesia, using fluoroscopic and echocardiographic guidance. The left femoral artery was used to advance a 5-Fr pigtail catheter as a reference catheter. Crossing of the aortic valve catheter was performed with an Argon 0.035″ straight guide wire (Argon Medical Devices Inc, Athens, TX, USA) followed by a 260cm Amplatz extra stiff guide wire (Cook Medical, Bloomington, IN, USA) positioned in the left ventricle. Subsequently, attempts were made to introduce a 26mm SAPIEN 3 balloon expandable valve (Edwards Lifesciences Inc, Irvine, CA, USA) within the aortic annulus. However this was unsuccessful due to the severity of aortic stenosis and valve calcification. Given the hesitancy to perform valve predilation due to the patient's fragile hemodynamic condition, via left transfemoral arterial access, a second Argon 0.035″ straight guide wire was advanced into the left ventricle and then exchanged for a 260cm Amplatz extra stiff guide wire (Fig. 1a). Using a “buddy wire” technique, the transcatheter heart valve was successfully positioned within the aortic annulus. The second guide wire was immediately withdrawn and the valve was adequately positioned within the aortic annulus (Fig. 1b). Prosthesis positioning was echocardiographically verified and a very short burst of rapid pacing with simultaneous balloon inflation was performed for valve deployment (Fig. 1c). Angiographic and echocardiographic control images showed satisfactory valve position (Fig. 1d). There was no residual aortic valve regurgitation. We observed left ventricular recovery without stunning and pulmonary arterial pressures were unchanged. Post-intervention, the patient's condition remained stable, without complication, and he was discharged from hospital 72h post-TAVI.
It has been shown that rapid pacing on TAVI has some consequences on coronary blood flow specially on those with 8-second runs or longer, causing left ventricle stunning and a period of hypotension right after the stimuli.7 Nonetheless, pulmonary arterial hypertension is a major risk factor for mortality during transcatheter valve implantation and cardiac surgery.1,8,9 The resultant increase in right ventricular afterload coupled with the inability of the right ventricle to adapt to this hemodynamic stress is closely tied with poor survival. Right heart failure, the end result of pulmonary arterial hypertension, is responsible for 70% of deaths in this patient group. Therefore, in patients whose ventricular interdependence is severely compromised, specific measures and extreme caution during TAVI are required for minimizing the risk of severe peri-procedural hemodynamic compromise. In such situations, avoiding or minimizing rapid pacing needs to be strongly considered. This however provides hindrance for optimal valve positioning. Nevertheless, this challenge may be overcome using the “buddy wire” technique for crossing the aortic annulus, thus avoiding aortic balloon valvuloplasty, but not the ability to successfully perform transcatheter aortic valve implantation.10
In conclusion, extreme heart failure or severe pulmonary hypertension can result in loss of ventricular interdependence, and subsequent increased morbidity and mortality may occur when repeated or prolonged rapid pacing during TAVI is undertaken leading to disastrous results. In the latter cases, it may be advisable to avoid predilation of the aortic valve. Yet, to overcome the difficulty of crossing the severely stenosed aortic valve, the “buddy wire” technique may be strongly considered to avoid valve predilation in cases where valve crossing with the endoprosthesis is difficult and valve predilation is not possible.