To describe current management and clinical outcomes in patients hospitalized with an acute coronary syndrome (ACS) in Mexico.
MethodsRENASICA III was a prospective multicenter registry of consecutive patients hospitalized with an ACS. Patients had objective evidence of ischemic heart disease; those with type II infarction or secondary ischemic were excluded. Study design conformed to current quality recommendations.
ResultsA total of 123 investigators at 29 tertiary and 44 community hospitals enrolled 8296 patients with an ACS (4038 with non-ST-elevation myocardial infarction/unstable angina [NSTEMI/UA], 4258 with ST-elevation myocardial infarction [STEMI]). The majority were younger (62±12years) and 76.0% were male. On admission 80.5% had ischemic chest pain lasting >20min and clinical stability. Left ventricular dysfunction was more frequent in NSTEMI/UA than in those with STEMI (30.0% vs. 10.7%, p<0.0001). In STEMI 37.6% received thrombolysis and 15.0% primary PCI. PCI was performed in 39.6% of NSTEMI/UA (early strategy in 10.8%, urgent strategy in 3.0%). Overall hospital death rate was 6.4% (8.7% in STEMI vs. 3.9% in NSTEMI/UA, p<0.001). The strongest independent predictors of hospital mortality were cardiogenic shock (odds ratio 22.4, 95% confidence interval 18.3–27.3) and ventricular fibrillation (odds ratio 12.5, 95% confidence interval 9.3–16.7).
ConclusionThe results from RENASICA III establish the urgent need to develop large-scale regional programs to improve adherence to guideline recommendations in ACS, including rates of pharmacological thrombolysis and increasing the ratio of PCI to thrombolysis.
describir abordaje terapéutico actual y evolución en pacientes hospitalizados con un síndrome coronario agudo (SCA) en México.
MétodosRENASICA III registro multicéntrico prospectivo de pacientes consecutivos con un SCA. Todos tuvieron demostración objetiva de enfermedad coronaria; se excluyeron infarto tipo II o isquemia secundaria. El diseño incluyó recomendaciones actuales de calidad.
Resultados123 investigadores en 29 hospitales de tercer nivel y en 44 de segundo ingresaron 8296 pacientes, 4038 con infarto del miocardio sin elevación del ST/angina inestable (IMSEST/AI) y 4258 con infarto del miocardio y elevación del ST (IMEST). La mayoría fueron jóvenes (62 ± 12 años) y el 76% del sexo masculino. Al ingreso 80.5% tuvo dolor torácico con perfil isquémico >20 minutos y estabilidad clínica. Se observó mayor disfunción ventricular en grupo con IMSEST/AI que en aquellos con IMEST (30.0% vs 10.7%, p <0.0001). En IMEST el 37.6% recibió trombolisis y el 15% angioplastía primaria. Este procedimiento se realizó en el 39.6% de los pacientes con IMSEST/AI (estrategia temprana 10.8%, estrategia urgente 3.0%). La mortalidad hospitalaria fue del 6.4% (8.7% IMEST vs. 3.9% IMSEST/AI, p <0.001). Los predictores independientes con mayor poder para mortalidad fueron choque cardiogénico (RM 22.4, 95% IC 18.3–27.3) y fibrilación ventricular (RM 12.5, 95% IC 9.3–16.7).
Conclusiónlos resultados del RENASICA III establecen la urgente necesidad de desarrollar en SCA programas regionales a gran escala para mejorar el apego a la guías y recomendaciones, incluyendo mayor porcentaje de trombolisis e incrementar la proporción de angioplastia primaria.
Knowledge of the epidemiological characteristics, therapeutic trends, and risk stratification of patients presenting with acute coronary syndromes (ACS) in Mexico is derived from the national registries: RENASICA I (Registro Nacional de Sindromes Coronarios Agudos),1 RENASICA II,2 and RENASCA (National Registry of patients with ACS in the IMSS).3 The results from the ACCESS (ACute Coronary Events – a multinational Survey of current management Strategies) registry,4 conducted in 11,731 ACS patients in Africa, Latin America, and the Middle East, confirmed the results from the national Mexican registries,1–3 highlighting persistent underuse of reperfusion therapies.
In the current era of therapeutic transition in ACS, it is important to identify changes in practice in terms of reperfusion approaches and antithrombotic strategies. RENASICA III was a prospective observational Mexican registry, the aims of which are to describe current management and clinical outcomes in patients with the broad spectrum of ACS types treated in everyday clinical practice. The results from RENASICA III will expand on the information provided by RENASICA I and RENASICA II.
MethodsRegistry designRENASICA III was established by the Mexican Cardiology Society, with unrestricted support provided by Sanofi. The full protocol, including definitions, has been published.5 In brief, RENASICA III was a prospective, multicenter registry involving adult men and women hospitalized with ACS in Mexico. In-hospital data were collected from patients in tertiary and community hospitals across both the public and private healthcare systems. The principal investigators were selected from community and tertiary hospitals located throughout Mexico.
The study population comprised a consecutive, prospective cohort of patients with a high clinical suspicion of ACS who were admitted to hospital. The initial diagnosis was established by the physician-in-charge on the basis of the patient's clinical and electrocardiographic characteristics. Four groups of patients were identified according to the electrocardiographic findings: normal or non-specific electrocardiogram (ECG), ST depression, transient ST elevation,6 and persistent ST elevation. Patients with a final diagnosis of ACS and objective evidence of ischemic heart disease (identified using invasive or non-invasive tests) were included in the registry and those with type II infarction were excluded. ACS nomenclature (ST-elevation myocardial infarction [STEMI] and non–ST-elevation myocardial infarction [NSTEMI] or unstable angina [UA]) was used at admission and, for epidemiologic reasons, UA or myocardial infarction at discharge.
The registry structure, data collection, and data analysis were based on current quality recommendations, including 11 criteria proposed by Gitt et al.7 The registry was conducted in accordance with national and international regulations. The protocol was approved by the institutional ethics committees in all participating centers and all patients provided informed consent.
The data were sent to the registry-coordinating center via a web site (http://www.renasica.mx/). Electronic case report forms were reviewed by the coordinating center to determine data quality. All participating sites had regular access to a data-entry clerk. Data-quality monitoring was conducted at 20% of the centers, chosen at random. Technical support was provided through periodic software updates. Investigators at all sites underwent training in the use of the electronic database and in the study definitions, and had real-time access to technical advice from the coordinating center through a 01-800 number. Follow-up by telephone and during site visits was performed through the coordinating center. In specific cases follow-up was done by the physician-in-charge. An executive committee set out the publication policies to allow an expedited process for publication or presentation at national and international meetings.
Sample size determinationA sample size with statistical significance was obtained using data from RENASCA3 and RENASICA II.2 Fifty percent of the patients in RENASCA3 did not receive adequate treatment and only 8% underwent percutaneous coronary intervention (PCI); consequently, 30% of patients developed left ventricular dysfunction. In RENASICA II, 15% of the patients had PCI and 17% developed left ventricular failure as an early complication. This difference between the two studies allowed us to calculate the smaller sample size necessary to evaluate this outcome (i.e. left ventricular dysfunction). A sample of 182 patients in each group (STEMI and NSTEMI/UA) would give a statistical power of 80%, with an alpha value of 0.05, delta value of 13%, 1:1 ratio of exposed: unexposed, and a confidence interval (CI) of 95%. Allowing for an estimated 20% loss to follow-up, the final number of patients chosen per group was 220. A lower delta value for mortality of 2% required 3975 patients to be treated (or not) with PCI; thus 8000 subjects had to be enrolled in the registry.
Statistical analysisData are expressed as percentages, mean±standard deviation (SD), and odds ratios (ORs) with 95% CIs. Differences between continuous variables with a normal distribution were examined using Student's t test. The Wilcoxon rank-sum test was used for non-normal continuous variables. The chi-square test, Fisher's exact test, or Yates’ correction was used to analyze categorical variables, as appropriate. A two-tailed test with a p-value ≤0.05 was considered statistically significant. Logistic regression analysis was used to identify independent predictors of hospital mortality on the basis of those with a p-value of ≤0.05 on univariate regression analysis, with adjustment for potential confounders to avoid confusion, the relationship between historical variables for atherosclerosis and cardiovascular events was examined through logistic regression. With the Cox proportional risk multivariable model, the relationship between each of these variables was analyzed. Kaplan–Meier survival curves were used to determine the risk of mortality, and differences between the curves were determined using the log-rank statistic. The Cox proportional risk model was used for adjusted survival analysis.
ResultsStudy populationBetween November 2012 and November 2013, 8296 consecutive ACS patients with proven ischemic heart disease were enrolled by 123 investigators at 29 tertiary and 44 community hospitals (63% government health system centers, 29% private practices, and 8% other health systems; hospitals were located across 91% of the Mexican territory). Of these patients, 4038 were diagnosed with NSTEMI/UA and 4258 with STEMI. All tertiary hospitals had the capability to perform percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG) surgery.
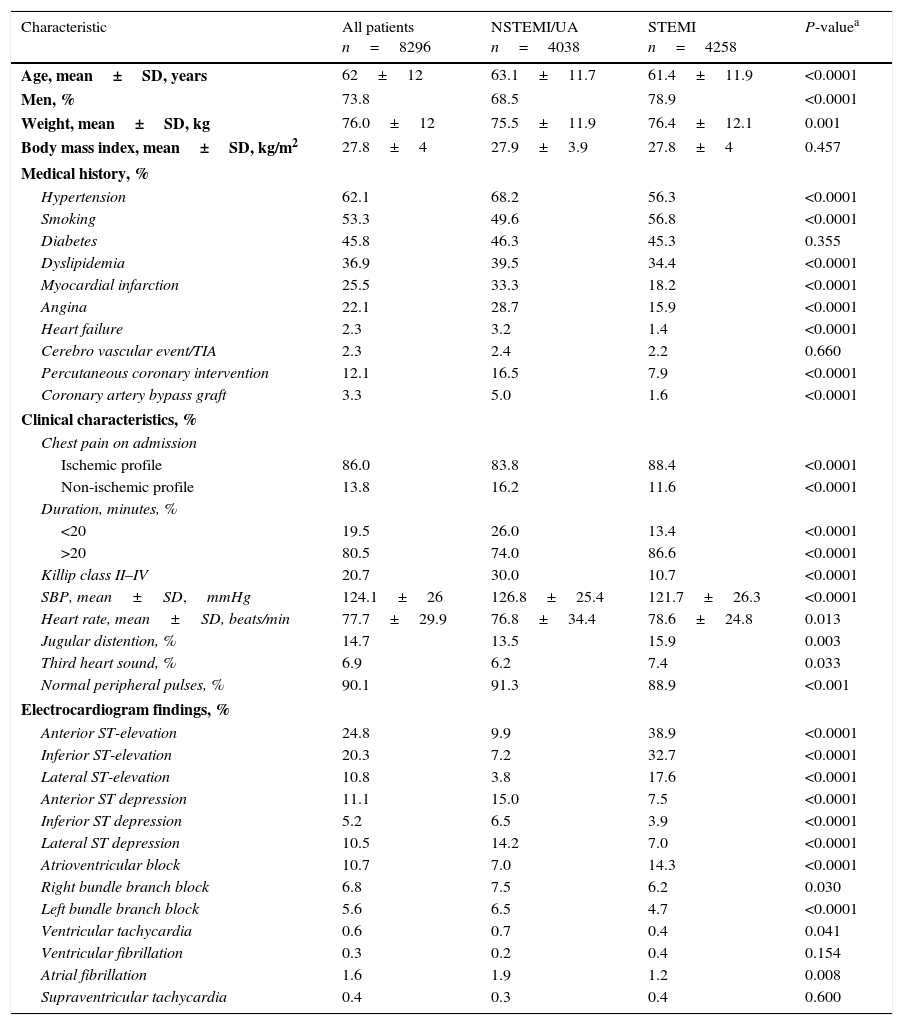
The mean±SD age of the population was 62.0±12 years, 73.8% were male, and the mean body mass index was 27.8±4.0kg/m2 (Table 1). The prevalence of major risk factors for ischemic heart disease was high in both ACS groups. Patients with STEMI were younger than those with NSTEMI/UA and a higher percentage smoked tobacco. Patients with NSTEMI/UA were more likely to have angina, dyslipidemia, heart failure, hypertension, previous myocardial infarction, or to have undergone a previous PCI or CABG (all p<0.0001).
Characteristics of study population, overall and according to type of acute coronary syndrome.
| Characteristic | All patients n=8296 | NSTEMI/UA n=4038 | STEMI n=4258 | P-valuea |
|---|---|---|---|---|
| Age, mean±SD, years | 62±12 | 63.1±11.7 | 61.4±11.9 | <0.0001 |
| Men, % | 73.8 | 68.5 | 78.9 | <0.0001 |
| Weight, mean±SD, kg | 76.0±12 | 75.5±11.9 | 76.4±12.1 | 0.001 |
| Body mass index, mean±SD, kg/m2 | 27.8±4 | 27.9±3.9 | 27.8±4 | 0.457 |
| Medical history, % | ||||
| Hypertension | 62.1 | 68.2 | 56.3 | <0.0001 |
| Smoking | 53.3 | 49.6 | 56.8 | <0.0001 |
| Diabetes | 45.8 | 46.3 | 45.3 | 0.355 |
| Dyslipidemia | 36.9 | 39.5 | 34.4 | <0.0001 |
| Myocardial infarction | 25.5 | 33.3 | 18.2 | <0.0001 |
| Angina | 22.1 | 28.7 | 15.9 | <0.0001 |
| Heart failure | 2.3 | 3.2 | 1.4 | <0.0001 |
| Cerebro vascular event/TIA | 2.3 | 2.4 | 2.2 | 0.660 |
| Percutaneous coronary intervention | 12.1 | 16.5 | 7.9 | <0.0001 |
| Coronary artery bypass graft | 3.3 | 5.0 | 1.6 | <0.0001 |
| Clinical characteristics, % | ||||
| Chest pain on admission | ||||
| Ischemic profile | 86.0 | 83.8 | 88.4 | <0.0001 |
| Non-ischemic profile | 13.8 | 16.2 | 11.6 | <0.0001 |
| Duration, minutes, % | ||||
| <20 | 19.5 | 26.0 | 13.4 | <0.0001 |
| >20 | 80.5 | 74.0 | 86.6 | <0.0001 |
| Killip class II–IV | 20.7 | 30.0 | 10.7 | <0.0001 |
| SBP, mean±SD,mmHg | 124.1±26 | 126.8±25.4 | 121.7±26.3 | <0.0001 |
| Heart rate, mean±SD, beats/min | 77.7±29.9 | 76.8±34.4 | 78.6±24.8 | 0.013 |
| Jugular distention, % | 14.7 | 13.5 | 15.9 | 0.003 |
| Third heart sound, % | 6.9 | 6.2 | 7.4 | 0.033 |
| Normal peripheral pulses, % | 90.1 | 91.3 | 88.9 | <0.001 |
| Electrocardiogram findings, % | ||||
| Anterior ST-elevation | 24.8 | 9.9 | 38.9 | <0.0001 |
| Inferior ST-elevation | 20.3 | 7.2 | 32.7 | <0.0001 |
| Lateral ST-elevation | 10.8 | 3.8 | 17.6 | <0.0001 |
| Anterior ST depression | 11.1 | 15.0 | 7.5 | <0.0001 |
| Inferior ST depression | 5.2 | 6.5 | 3.9 | <0.0001 |
| Lateral ST depression | 10.5 | 14.2 | 7.0 | <0.0001 |
| Atrioventricular block | 10.7 | 7.0 | 14.3 | <0.0001 |
| Right bundle branch block | 6.8 | 7.5 | 6.2 | 0.030 |
| Left bundle branch block | 5.6 | 6.5 | 4.7 | <0.0001 |
| Ventricular tachycardia | 0.6 | 0.7 | 0.4 | 0.041 |
| Ventricular fibrillation | 0.3 | 0.2 | 0.4 | 0.154 |
| Atrial fibrillation | 1.6 | 1.9 | 1.2 | 0.008 |
| Supraventricular tachycardia | 0.4 | 0.3 | 0.4 | 0.600 |
On admission 80.5% of patients had ischemic chest pain lasting >20min and clinical stability (Table 1). Killip class II–IV5 (Appendix B) was more frequent in patients with NSTEMI/UA than in those with STEMI (p<0.0001). Mitral and septal rupture murmur and cardiac rub were infrequent in NSTEMI/UA (1.5%, 0.1%, 0.1%, respectively) and STEMI (1.1%, 0.2%, 0.1%).
Patients with STEMI more likely have had anterior, inferior, and lateral ST-elevation, whereas anterior, inferior, and lateral ST depressions were more frequent in patients with NSTEMI/UA (Table 1). The prevalence of atrial fibrillation was low in both groups, although it was numerically more frequent in patients with NSTEMI/UA.
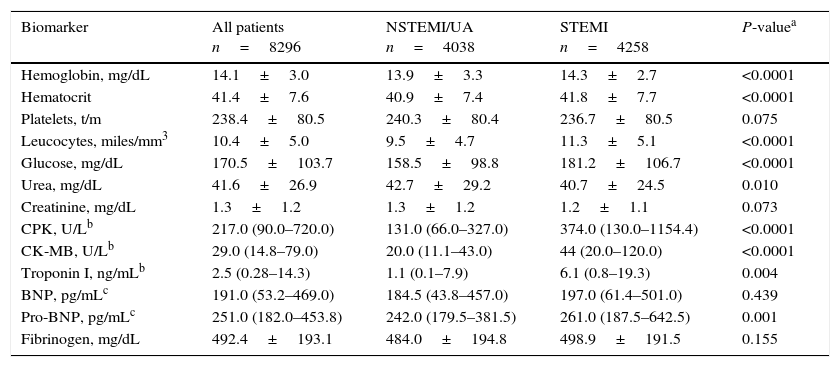
The plasma concentrations of various biomarkers, including hemoglobin, hematocrit, leucocytes, glucose, creatine phosphokinase, CK-MB, troponin I and NT-proBNP, were higher in patients with STEMI compared to those with NSTEMI/UA, whereas concentrations of fibrinogen and creatinine were similar (Table 2). BNP was obtained in 28% and NT-proBNP in 22.9% of the overall population.
Biomarker measurements.
| Biomarker | All patients n=8296 | NSTEMI/UA n=4038 | STEMI n=4258 | P-valuea |
|---|---|---|---|---|
| Hemoglobin, mg/dL | 14.1±3.0 | 13.9±3.3 | 14.3±2.7 | <0.0001 |
| Hematocrit | 41.4±7.6 | 40.9±7.4 | 41.8±7.7 | <0.0001 |
| Platelets, t/m | 238.4±80.5 | 240.3±80.4 | 236.7±80.5 | 0.075 |
| Leucocytes, miles/mm3 | 10.4±5.0 | 9.5±4.7 | 11.3±5.1 | <0.0001 |
| Glucose, mg/dL | 170.5±103.7 | 158.5±98.8 | 181.2±106.7 | <0.0001 |
| Urea, mg/dL | 41.6±26.9 | 42.7±29.2 | 40.7±24.5 | 0.010 |
| Creatinine, mg/dL | 1.3±1.2 | 1.3±1.2 | 1.2±1.1 | 0.073 |
| CPK, U/Lb | 217.0 (90.0–720.0) | 131.0 (66.0–327.0) | 374.0 (130.0–1154.4) | <0.0001 |
| CK-MB, U/Lb | 29.0 (14.8–79.0) | 20.0 (11.1–43.0) | 44 (20.0–120.0) | <0.0001 |
| Troponin I, ng/mLb | 2.5 (0.28–14.3) | 1.1 (0.1–7.9) | 6.1 (0.8–19.3) | 0.004 |
| BNP, pg/mLc | 191.0 (53.2–469.0) | 184.5 (43.8–457.0) | 197.0 (61.4–501.0) | 0.439 |
| Pro-BNP, pg/mLc | 251.0 (182.0–453.8) | 242.0 (179.5–381.5) | 261.0 (187.5–642.5) | 0.001 |
| Fibrinogen, mg/dL | 492.4±193.1 | 484.0±194.8 | 498.9±191.5 | 0.155 |
Data given as median (percentiles 25–75) by Mann–Whitney U test.
c pg/mL, picograms per milliliter.
CK-MB, creatinine kinase MB fraction; CPK, total creatinine phosphokinase; BNP, brain natriuretic peptide; Pro-BNP, pro-brain natriuretic peptide – N terminal. NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina; UL, units per liter.
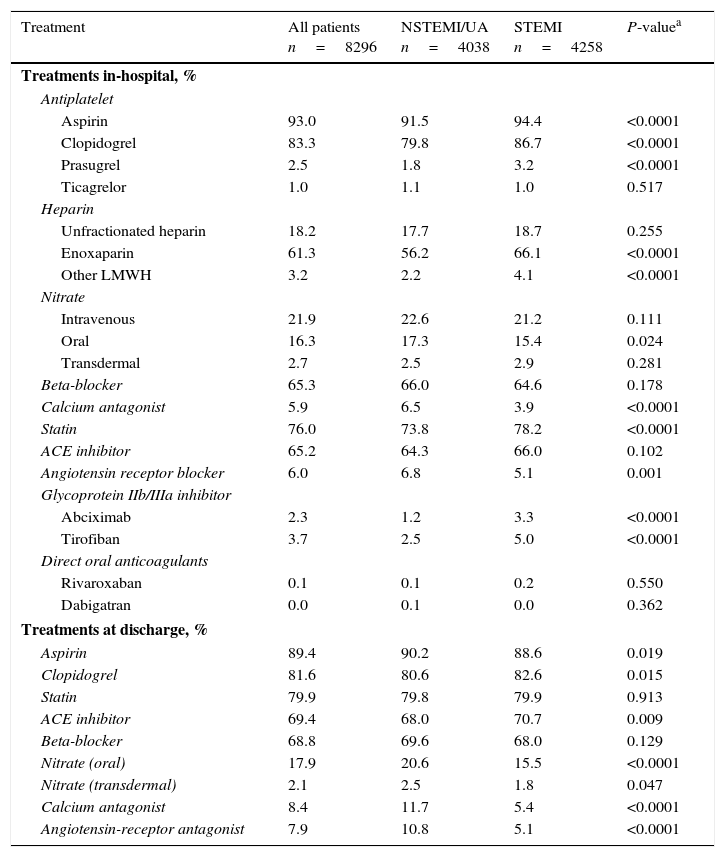
During hospitalization patients with STEMI were more likely than those with NSTEMI/UA to receive aspirin, clopidogrel or prasugrel, a glycoprotein IIb/IIIa inhibitor, low-molecular weight heparin, and statins. Patients with NSTEMI/UA were more likely to receive angiotensin receptor blockers, calcium antagonists, and oral nitrates (Table 3). Non-vitamin K oral anticoagulants were used in <1% of patients in both groups.
Treatments in-hospital and at discharge.
| Treatment | All patients n=8296 | NSTEMI/UA n=4038 | STEMI n=4258 | P-valuea |
|---|---|---|---|---|
| Treatments in-hospital, % | ||||
| Antiplatelet | ||||
| Aspirin | 93.0 | 91.5 | 94.4 | <0.0001 |
| Clopidogrel | 83.3 | 79.8 | 86.7 | <0.0001 |
| Prasugrel | 2.5 | 1.8 | 3.2 | <0.0001 |
| Ticagrelor | 1.0 | 1.1 | 1.0 | 0.517 |
| Heparin | ||||
| Unfractionated heparin | 18.2 | 17.7 | 18.7 | 0.255 |
| Enoxaparin | 61.3 | 56.2 | 66.1 | <0.0001 |
| Other LMWH | 3.2 | 2.2 | 4.1 | <0.0001 |
| Nitrate | ||||
| Intravenous | 21.9 | 22.6 | 21.2 | 0.111 |
| Oral | 16.3 | 17.3 | 15.4 | 0.024 |
| Transdermal | 2.7 | 2.5 | 2.9 | 0.281 |
| Beta-blocker | 65.3 | 66.0 | 64.6 | 0.178 |
| Calcium antagonist | 5.9 | 6.5 | 3.9 | <0.0001 |
| Statin | 76.0 | 73.8 | 78.2 | <0.0001 |
| ACE inhibitor | 65.2 | 64.3 | 66.0 | 0.102 |
| Angiotensin receptor blocker | 6.0 | 6.8 | 5.1 | 0.001 |
| Glycoprotein IIb/IIIa inhibitor | ||||
| Abciximab | 2.3 | 1.2 | 3.3 | <0.0001 |
| Tirofiban | 3.7 | 2.5 | 5.0 | <0.0001 |
| Direct oral anticoagulants | ||||
| Rivaroxaban | 0.1 | 0.1 | 0.2 | 0.550 |
| Dabigatran | 0.0 | 0.1 | 0.0 | 0.362 |
| Treatments at discharge, % | ||||
| Aspirin | 89.4 | 90.2 | 88.6 | 0.019 |
| Clopidogrel | 81.6 | 80.6 | 82.6 | 0.015 |
| Statin | 79.9 | 79.8 | 79.9 | 0.913 |
| ACE inhibitor | 69.4 | 68.0 | 70.7 | 0.009 |
| Beta-blocker | 68.8 | 69.6 | 68.0 | 0.129 |
| Nitrate (oral) | 17.9 | 20.6 | 15.5 | <0.0001 |
| Nitrate (transdermal) | 2.1 | 2.5 | 1.8 | 0.047 |
| Calcium antagonist | 8.4 | 11.7 | 5.4 | <0.0001 |
| Angiotensin-receptor antagonist | 7.9 | 10.8 | 5.1 | <0.0001 |
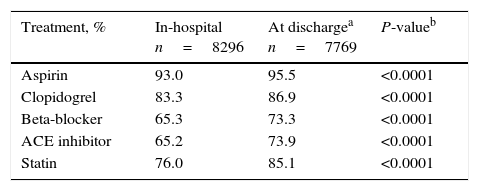
At discharge from hospital, aspirin, nitrates, calcium antagonists, and angiotensin receptor blockers were more frequently used in patients with NSTEMI/UA, whereas patients with STEMI were more likely to receive clopidogrel and angiotensin converting enzyme inhibitors (Table 3). The use of beta-blockers and statins was similar in both groups. In patients discharged alive, the use of ACE inhibitors, aspirin, beta-blockers, clopidogrel, and statins was higher at discharge than during hospitalization (all p<0.0001) (Table 4).
The overall mean±SD length of hospitalization was 7.1±7.8 days (6.8±7.2 days in NSTEMI/UA vs. 7.4±8.5 days in STEMI).
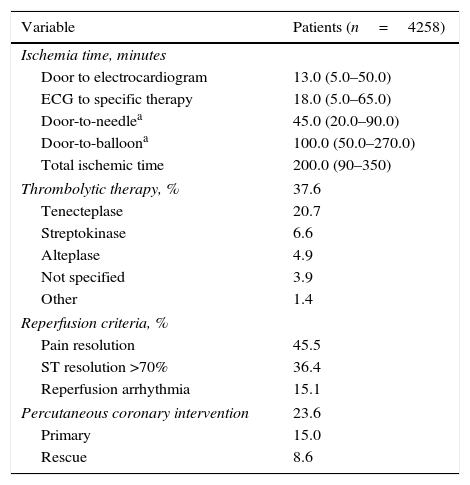
Reperfusion approachesThe median (25th–75th percentiles) ischemia times in patients with STEMI were 45.0 (20.0–90.0) min for door-to-needle and 100.0 (50.0–270.0) min for door to balloon (Table 5). In the STEMI group, 37.6% of patients received thrombolytic therapy and 15.0% underwent primary PCI (Table 5). Rescue PCI was performed in 8.6% of patients with STEMI. Pharmacological reperfusion was conducted primarily in the emergency department (70.3%) and less frequently in the prehospital setting (17.9%) or coronary care unit (10.0%). Tenecteplase was the most frequently used fibrinolytic agent, followed by streptokinase and alteplase.
Pharmacological and mechanical reperfusion in patients with STEMI.
| Variable | Patients (n=4258) |
|---|---|
| Ischemia time, minutes | |
| Door to electrocardiogram | 13.0 (5.0–50.0) |
| ECG to specific therapy | 18.0 (5.0–65.0) |
| Door-to-needlea | 45.0 (20.0–90.0) |
| Door-to-balloona | 100.0 (50.0–270.0) |
| Total ischemic time | 200.0 (90–350) |
| Thrombolytic therapy, % | 37.6 |
| Tenecteplase | 20.7 |
| Streptokinase | 6.6 |
| Alteplase | 4.9 |
| Not specified | 3.9 |
| Other | 1.4 |
| Reperfusion criteria, % | |
| Pain resolution | 45.5 |
| ST resolution >70% | 36.4 |
| Reperfusion arrhythmia | 15.1 |
| Percutaneous coronary intervention | 23.6 |
| Primary | 15.0 |
| Rescue | 8.6 |
Data given as median (percentiles 25–75).
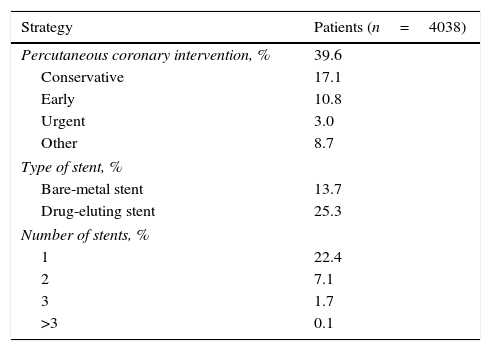
PCI was performed in 39.6% of patients with NSTEMI/UA, with an early strategy used in 10.8% and an urgent strategy in 3.0% (Table 6). Drug-eluting stents were used more frequently than bare-metal stents in these patients. Coronary artery bypass grafting was more frequently performed in patients with NSTEMI/UA (5.9% vs. 2.1% in STEMI).
Early invasive strategy in patients with NSTEMI/UA.
| Strategy | Patients (n=4038) |
|---|---|
| Percutaneous coronary intervention, % | 39.6 |
| Conservative | 17.1 |
| Early | 10.8 |
| Urgent | 3.0 |
| Other | 8.7 |
| Type of stent, % | |
| Bare-metal stent | 13.7 |
| Drug-eluting stent | 25.3 |
| Number of stents, % | |
| 1 | 22.4 |
| 2 | 7.1 |
| 3 | 1.7 |
| >3 | 0.1 |
NSTEMI, non-ST-segment elevation myocardial infarction; UA, unstable angina.
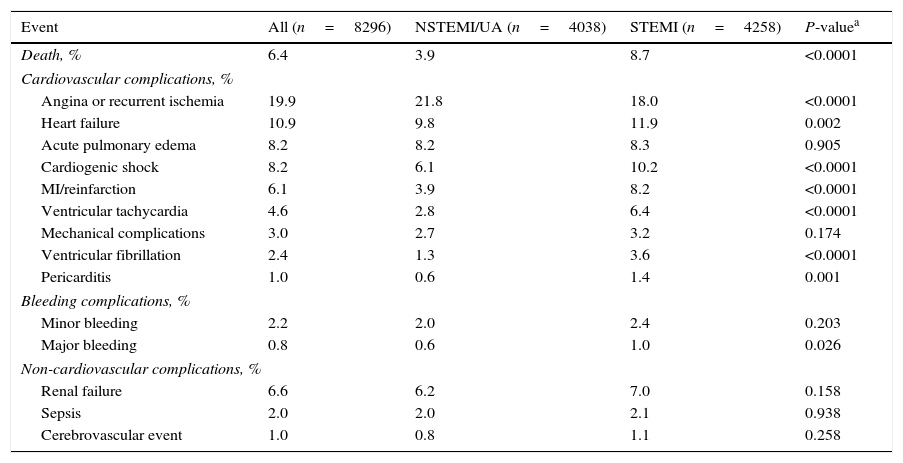
The overall hospital death rate was 6.4%, and was lower in the NSTEMI/UA population (3.9% vs. 8.7%, p<0.0001) (Table 7). The rates of heart failure, cardiogenic shock, myocardial infarction or reinfarction, ventricular arrhythmias, pericarditis, and major bleeding were higher in the STEMI population. Angina or recurrent ischemia was more frequent in patients with NSTEMI/UA (p<0.0001). The incidences of renal failure, sepsis, and cerebrovascular events were similar.
In-hospital major cardiovascular adverse events, adverse events, and death.
| Event | All (n=8296) | NSTEMI/UA (n=4038) | STEMI (n=4258) | P-valuea |
|---|---|---|---|---|
| Death, % | 6.4 | 3.9 | 8.7 | <0.0001 |
| Cardiovascular complications, % | ||||
| Angina or recurrent ischemia | 19.9 | 21.8 | 18.0 | <0.0001 |
| Heart failure | 10.9 | 9.8 | 11.9 | 0.002 |
| Acute pulmonary edema | 8.2 | 8.2 | 8.3 | 0.905 |
| Cardiogenic shock | 8.2 | 6.1 | 10.2 | <0.0001 |
| MI/reinfarction | 6.1 | 3.9 | 8.2 | <0.0001 |
| Ventricular tachycardia | 4.6 | 2.8 | 6.4 | <0.0001 |
| Mechanical complications | 3.0 | 2.7 | 3.2 | 0.174 |
| Ventricular fibrillation | 2.4 | 1.3 | 3.6 | <0.0001 |
| Pericarditis | 1.0 | 0.6 | 1.4 | 0.001 |
| Bleeding complications, % | ||||
| Minor bleeding | 2.2 | 2.0 | 2.4 | 0.203 |
| Major bleeding | 0.8 | 0.6 | 1.0 | 0.026 |
| Non-cardiovascular complications, % | ||||
| Renal failure | 6.6 | 6.2 | 7.0 | 0.158 |
| Sepsis | 2.0 | 2.0 | 2.1 | 0.938 |
| Cerebrovascular event | 1.0 | 0.8 | 1.1 | 0.258 |
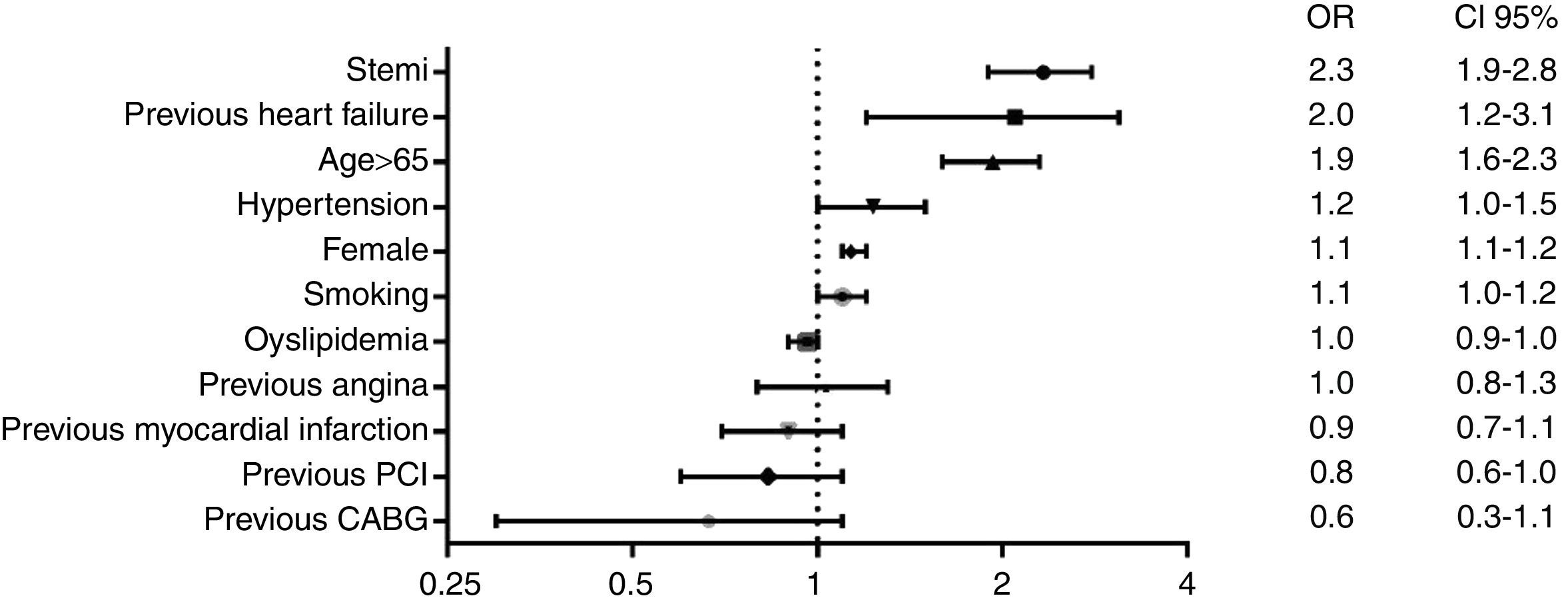
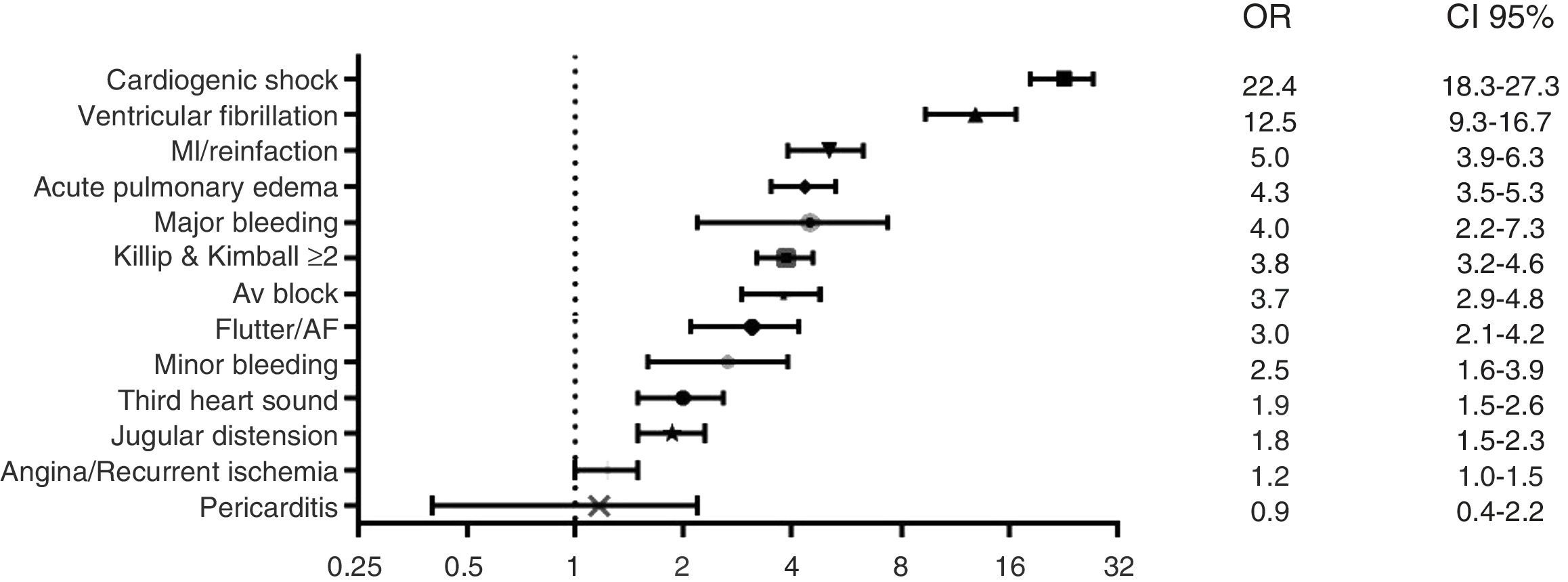
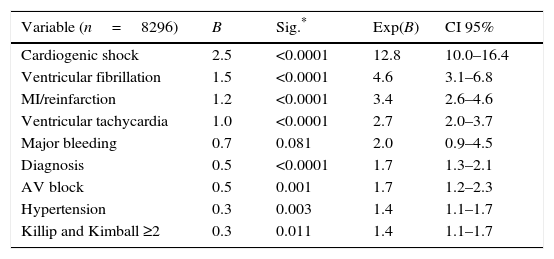
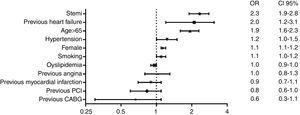
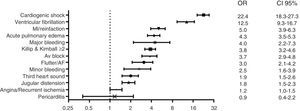
Independent predictors of hospital mortality are shown in Table 8. The strongest predictors of hospital death, based on risk factors and cardiovascular history, were STEMI diagnosis, left ventricular dysfunction, and age >65 years (Fig. 1). The strongest predictor of death in terms of clinical data and complications was any expression of left ventricular dysfunction (Fig. 2). Ventricular and supraventricular arrhythmias, myocardial infarction or reinfarction, acute pulmonary edema, and major bleeding also had close associations with death.
Multivariate analysis to early mortality.
| Variable (n=8296) | B | Sig.* | Exp(B) | CI 95% |
|---|---|---|---|---|
| Cardiogenic shock | 2.5 | <0.0001 | 12.8 | 10.0–16.4 |
| Ventricular fibrillation | 1.5 | <0.0001 | 4.6 | 3.1–6.8 |
| MI/reinfarction | 1.2 | <0.0001 | 3.4 | 2.6–4.6 |
| Ventricular tachycardia | 1.0 | <0.0001 | 2.7 | 2.0–3.7 |
| Major bleeding | 0.7 | 0.081 | 2.0 | 0.9–4.5 |
| Diagnosis | 0.5 | <0.0001 | 1.7 | 1.3–2.1 |
| AV block | 0.5 | 0.001 | 1.7 | 1.2–2.3 |
| Hypertension | 0.3 | 0.003 | 1.4 | 1.1–1.7 |
| Killip and Kimball ≥2 | 0.3 | 0.011 | 1.4 | 1.1–1.7 |
The RENASICA series of registries has accumulated the largest database of information on ACS patients in Latin America, providing important and reliable evidence of the quality of ACS care in Mexico.1,2,5 The data obtained over a 15-year period provide important insights into the therapeutic approaches used, patient outcomes, and the uptake of new treatments into clinical practice in this middle-income country.1,2,5 The results reported here, from RENASICA III, show new evidence on the epidemiologic characteristics and clinical presentation of ACS patients, therapeutic trends, and hospital mortality in an era of therapeutic transition.
Lessons learned from RENASICA IRENASICA I included 4353 ACS patients with or without ST elevation.1 This registry highlighted practices and therapeutic approaches in the transition from the 20th to the 21st century. Non-ST-elevation ACS was a common cause of hospital admission and cardiac biomarkers were not available. In non-ST-elevation ACS, age >65 years, ST depression, necrosis, and extensive coronary disease detected on angiography were risk markers of mortality. Ischemic chest pain, dyspnea, and diaphoresis were closely associated with a diagnosis of STEMI. Less than half of the patients received reperfusion therapy despite the availability of appropriate facilities in 90% of hospitals. Standard antiplatelet and antithrombin treatments were used in 70% of patients; low-molecular-weight heparin and glycoprotein IIb/IIIa receptor blockers were used fewer patients. The RENASICA I registry provided valuable information for Mexican health authorities to better apply health resources for the management of ACS in the 21st century.1
Lessons learned from RENASICA IIThe second RENASICA registry included 8098 patients with final diagnosis of ACS. This registry had a higher ratio of STEMI to NSTEMI/UA (1.3:1) compared with RENASICA I (35% vs. 65%), attributed to the availability of mechanical reperfusion facilities in participating hospitals, as well as a high prevalence of diabetes in patients with STEMI. The average length of hospital stay was 8.1 days, independent of the final diagnosis. The incidence of diabetes (42%) was slightly lower than that in RENASICA I (50%). This metabolic disorder was a strong predictor of hospital mortality in patients with NSTEMI/UA. Tests to assess patients’ glucometabolic state were not done, so the true prevalence of diabetes, impaired glucose tolerance, and insulin resistance are likely to be higher than indicated, and the rate of obesity was not determined.2 The clinical presentation of ACS was similar to that observed in RENASICA I and use of troponin was low. Optimal pharmacological treatment (e.g. glycoprotein IIb/IIIa inhibitors and statins) was lower than expected in both groups of ACS patients. The rate of pharmacological thrombolysis decreased from 50% in RENASICA I to 37% in RENASICA II, with streptokinase being the most commonly used drug. The rate of PCI was 15%. Consequently, a considerable proportion of patients with STEMI received no form of reperfusion therapy. Left ventricular dysfunction (Appendix B) was the most important hospital complication and the strongest predictor of death, which occurred with a frequency of 10% in STEMI and 4% in NSTEMI/UA.2
RENASICA IIIThe profile of patients in RENASICA III in terms of age and sex is similar to that in RENASICA II,2 suggesting that the high incidence of ACS persists in this high-productivity social group and in male patients. Of note, the rate of tobacco smoking decreased from 60% in RENASICA I1 and 64% in RENASICA II2 to 53.3% in RENASICA III, signifying that the health policy toward reducing rates of tobacco consumption has had some effect. Conversely, the prevalence of traditional risk factors such as hypertension, diabetes, and hypercholesterolemia increased, and the rate of diabetes remained unchanged, at nearly 50%. The proportion of men and the prevalence of hypertension in RENASICA III (73.8% and 62.1%, respectively) are similar to those reported in the GRACE registry (62–72% and 50–65%, respectively),8 the National Registry of Myocardial infarction (NRMI),9–11 and the Euro Heart Survey (64–72% and 52–64%, respectively).12 However, the prevalence of diabetes is higher than that reported in GRACE (21–27%),8 ACCESS (33%),4 INTERHEART (7.2%),13 and KERALA (37.6%).14 The Indian CREATE registry found that diabetes prevalence was related to socioeconomic status, being lowest amongst the poor (18.7%) and highest among the most affluent (40.9%).15 It is apparent that strategies for primary prevention of diabetes and ischemic heart disease are mandatory in this Mexican population.
Clinical presentation and duration of hospitalizationIn terms of clinical presentation, ACS with or without ST elevation, high-clinical suspicion of ACS, and risk stratification were conducted through a clinical process that included historic risk factors, chest pain with ischemic profile, and electrocardiographic findings. Considering the low proportion of ischemic equivalents, clinical suspicion does not seem to be a problem in the emergency departments of community and tertiary hospitals. In view of the low rates of use of cardiac biomarkers, a significant number of high-risk patients may not have been identified early. In both groups of ACS patients, the high proportion with left ventricular dysfunction could be attributed to several mechanisms, including extensive and severe coronary artery disease, ischemia time, metabolic instability, and acute inflammatory state. Biomarkers of left ventricular dysfunction were also underused (BNP in 28% and NT-proBNP in 22.9% of the overall population), so it is possible that a significant proportion of “clinically stable” patients with left ventricular dysfunction without clinical expression were omitted from the study. The continuing underuse of biomarkers1,2 remains as an important problem in Mexico, and highlights the need to identify and establish an appropriate risk model tailored toward middle-income countries. Also encourage health authorities to provide access to these biomarkers.
The present data show that the length of hospitalization for an ACS has decreased slightly in Mexico (9.1 days in RENASICA II2 and 7.1±7.8 days in RENASICA III), but exceeds that reported in the NRMI registries (4.3 days).9–11
Pharmacological therapiesThe optimal treatment for ACS continues to evolve, on the basis of robust evidence emerging from randomized trials and recommendations from cardiac societies.16 Although well-conducted studies have been undertaken, their results are open to interpretation; furthermore, they are conducted within the confines of a selected population and may not therefore be applicable to the broad spectrum of patients treated in clinical practice. Furthermore, because of resource limitations new drugs may not be available in all countries and settings. Consequently, good clinical indicators that reflect our “true” practices need to be identified. In RENASICA III, use of in-hospital and discharge treatments showed improvements when compared with the findings from RENASICA II.2 Most of the patients received dual antiplatelet therapy comprising aspirin plus a second-generation thienopyridine. In spite of the advantages of the new antiplatelet drugs in ACS,17,18 prasugrel and ticagrelor were infrequently used, most likely due to the low rate of PCI, lack of local availability, high costs, and absence of strong evidence in medically treated patients with non-ST elevation ACS.
AnticoagulationIn the acute phase, anticoagulation remains the cornerstone of treatment for ST-elevation and non-ST-elevation ACS. In our study, a large proportion of patients received heparin, most commonly enoxaparin, which is easy to administer and has a better anticoagulation profile (improved coronary patency, greater prevention of reocclusion, low incidence of bleeding complications and heparin-induced thrombocytopenia compared with unfractionated heparin). However, in spite of the advantages of heparin therapy, a sizable proportion of patients (17.3%) did not receive any anticoagulation therapy. Reasons for the failure to administer heparin include risk of bleeding complications or perception of a limited benefit in patients outside the pharmacological or mechanical reperfusion window.
Reperfusion therapyPCI remains a key therapy for patients with symptomatic coronary artery disease, primarily those with acute myocardial infarction. PCI has garnered tremendous attention in the past 15 years, with issues relating to the risks and benefits of drug-eluting stents and the use of adjunctive antithrombotic therapies.19 The present data from Mexico, spanning nearly two decades,1–3 show that pharmacological reperfusion remains the most frequently used form of reperfusion in STEMI patients. Primary PCI in STEMI and early cardiac catheterization (within 48–72h) in NSTEMI/UA were infrequent. When compared with the earlier RENASICA registries,1,2 the most notable finding from RENASICA III was the transition in STEMI patients from first-generation to third-generation thrombolytic therapy and from unfractionated heparin to low-molecular weight heparin.
In terms of ischemia time, delays from first medical contact to electrocardiogram or to starting a specific therapy were within acceptable ranges, whereas delays to reperfusion exceeded the current recommendations.16 While quality-improvement initiatives that target both physicians and patients should help to reduce these delays, a national program is far from being a reality; therefore it is important to reinforce the need for regional programs in order to improve the care of ACS patients. One such program is currently in place at the Cardiology National Institute “Ignacio Chavez” in Mexico City. In this program, staff in the emergency departments of community hospitals have been trained to administer pharmacological thrombolysis. Electrocardiograms that are difficult to interpret are sent for evaluation, and recommendations on the best treatment are provided. This program intent evaluating the efficacy and safety of a pharmaco-invasive strategy.
Hospital outcomesThe hospital mortality rate in RENASICA III was 6.4% (8.7% in STEMI and 3.9% in NSTEMI/UA), which shows a reassuring decrease from RENASICA II (10% in STEMI, 4% in NSTEMI/UA).2 However, it is higher than the 30-day mortality reported in ACCESS (3.6% all ACS; 5.0% in STEMI, 2.4% in NSTE-ACS)4 and hospital mortality in GRACE (4.6% in STEMI, 2.2% in NSTE-ACS).20 The GRACE registry also demonstrated a statistically significant decline in hospital death over the course of the study (from 8.4% to 4.6%, p<0.001, for STEMI; and from 2.9% to 2.2%, p=0.02, for NSTE-ACS), which corresponded with increasing use of medications and primary PCI and decreasing use of pharmacological reperfusion.20 The rate of bleeding complications in RENASICA III was low and can be attributed to the low rates of use of PCI, glycoprotein IIb/IIIa inhibitors, and new antiplatelets and oral anticoagulants.
The most important hospital complication in our study was the expression of left ventricular dysfunction (Appendix B). In the multivariable analysis, as observed previously,1,2 cardiogenic shock was the strongest predictor of death, as well as myocardial infarction or reinfarction. All of these major adverse cardiovascular events are closely related to prolonged ischemic times and low rate of PCI. Although bleeding complications and ventricular arrhythmias were scarce, both were related with hospital mortality.
LimitationsRENASICA III was not a population-based epidemiological study; therefore, some bias may have been introduced with respect to the selection of participating centers. Other limitations include the restricted number of visits to monitor data accuracy. Patients who died early, before admission to hospital or while in the emergency department, were excluded. The inclusion of community hospitals may explain the low rate of PCI.
ConclusionThe results from RENASICA III establish the urgent need to develop effective regional programs to improve rates of reperfusion therapy, to increase the ratio of PCI to thrombolysis, and to reduce ischemia times. Through the achievement of these targets, we can ensure a better quality of life for many Mexican patients presenting with an ACS.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
FundingAn unrestricted grant was provided by Sanofi.
Conflict of interestThe authors declare no conflict of interest.
Sophie Rushton-Smith, PhD (Medlink Healthcare Communications Ltd), provided editorial support.
Rafael Barraza Félix, Luis Alberto Cervantes Chávez, Vania Quisbert Vattuone, Centro Médico Nacional “La Raza”, IMSS, México, D. F. (1413); Anabel García Sosa, Juan Gerardo Campos Cassio, Aarón Jasso Guido, Esteban Luis Reyes Cerezo, Hospital de Cardiologia 34, IMSS, Monterrey, Nuevo León (1188); Rodolfo Parra Michel, Héctor Raúl Barajas Campa, Juan Ángel Campos Aguilar, Jaime Chávez Michel, Luis Alfredo Pelayo Jiménez, Silvano Vela Alarcón, Centro Médico Nacional de Occidente, IMSS, Guadalajara, Jalisco (985). Alexandra Arias Mendoza, Alfredo Altamirano Castillo, Amada Alvarez San Gabriel, Francisco Azar Manzur, José Luis Briseño De la Cruz, Gustavo Rojas Velasco, Instituto Nacional de Cardiología “Ignacio Chávez”, México, D. F. (951); Belinda González Díaz, Oracio González Ortiz, Iván Alfonso González Rosas, Ismael Hernández Santamaría, Hospital Juárez de México, México, D. F. (658); Ambrosio Cruz Díaz, Hospital Central de Alta Especialidad Sur, PEMEX, México, D.F. (342); Rocío Camacho Casillas, Antonio García Espino, UMAE Hospital de Especialidades No. 71, IMSS, Torreón, Coahuila (315); José Luis Leiva Pons, Hospital Central “Dr. Ignacio Morones Prieto”, San Luis Potosí, San Luis Potosí (230); Amanda M. Castelán Ojeda, UMAE Hospital de Especialidades No. 2, IMSS, Ciudad Obregón, Sonora (221); Juan Carlos Osnaya Martínez, Sergio Salas Padilla Hospital Regional “Lic. Adolfo López Mateos”, ISSSTE, México, D. F. (184); Alfredo Comparan Núñez, Hospital Regional No. 1, IMSS, Tijuana, Baja California (169); Lidia E. Betancourt Hernández, UMAE Hospital de Especialidades No. 14, IMSS, Veracruz, Veracruz (135); Sergio González Romero, Miguel Ángel Luna Calvo, Juan Carlos Núñez Fragoso, Hospital General de Durango, Durango, Durango (127); Raúl Alberto Rivas Lira, Hospital Central del Norte, PEMEX, México, D.F. (122); Manuel Alfonso Baños González, Hospital Regional de Alta Especialidad “Dr. Juan Grauss”, Villahermosa, Tabasco (112); Rodolfo de Jesús Castaño Guerra, Roberto López Rosas, Hospital General de México, México, D.F. (82); Anabella C. Delgado Sánchez, Sergio Luna Ramírez, Oscar Samuel Medina Torres, UMAE Hospital de Especialidades No 1 Bajío, León, Guanajuato (67); Norberto Matadamas Hernández, Hospital General de Acapulco, ISSSTE, Acapulco, Guerrero (45); Guillermo Llamas Esperón, Rolando Chávez Martínez, Elizabeth Espinoza Garza, Monserrat González Limón, Ana Patricia Grajales Alonso, Miguel Ángel Ramos Guzmán, Martha Vacio Olguín, Alberto Zamora Muciño-Arroyo, Cardiológica Aguascalientes, Aguascalientes, Aguascalientes (42); Marco Antonio Alcocer Gamba, Pablo Jaime Arias Fajardo, Karla Verónica Cárdenas Mejía, Eliodoro Castro Montes, Enrique García Hernández, Salvador León González, Abel Linares Rodríguez, Wendy Muñoz Rosales, José Ernesto Pombo Bartelt, Instituto de Corazón de Querétaro, Querétaro, Querétaro (37); Laura Román Herrera, Guadalupe René Ruiz Avila, Hospital General de Zona No.6, IMSS, Ciudad Juárez, Chihuahua (36); Leocadio Muñoz Beltrán, Hospital Ángeles, Ciudad Juárez, Chihuahua (34); Jesús Zúñiga Sedano, Luis Virgen Carrillo, Hospital Ángeles del Carmen, Guadalajara, Jalisco (34); Jorge H. Jiménez Orozco, Unidad de Medicina Física “Magdalena de las Salinas”, IMSS, México, D. F. (34); Salvador Facundo Bazaldua, Hospital General Victoria, Ciudad Victoria, Tamaulipas (32); Manuel Odín De los Ríos Ibarra, Alberto Zenón Baños Velasco, Hospital General de Culiacán, Culiacán, Sinaloa (32); Ángel Leovigildo Alberto Delgado, María del Rocío Blázquez Cruz, Luis Enrique Berumen Domínguez, Alberto Cortez Benítez, Luis Manuel Páez Lizárraga, Hospital Central Militar, México, D.F. (27); Víctor Armando Varguez Argüelles, Hospital Regional de Puebla, ISSSTE, Puebla, Puebla (27); Manuel de los Reyes Barrera Bustillos, Clínica Mérida, Mérida, Yucatán (26); Norberto Matadamas Hernández, Hospital General de Acapulco, Acapulco, Guerrero (25); José Fabián Hernández Díaz, Hospital Regional de Alta Especialidad de Oaxaca; Oaxaca, Oaxaca (25); Ma. Isabel Sánchez Ramírez, Hospital General La Paz, ISSSTE, La Paz, Baja California Sur (25); Francisco Javier Redding Escalante, Star Médica “Lomas Verdes”, Lomas Verdes, Estado de México (25); José Luis González Peña, Hospital Regional Reynosa, PEMEX, Reynosa, Tamaulipas (24); Ramón José Cué Carpio, Instituto Mexicano de Trasplantes, Cuernavaca, Morelos (24); Raúl Isaac Márquez, Hospital General de Zona No.3, IMSS, Cancún, Quintana Roo (22); Federico Rodríguez Weber, Hospital Ángeles del Pedregal, México, D.F. (21); Oswaldo Lagunas Uriarte, Hospital Civil de Culiacán, Culiacán, Sinaloa (20); Raúl Teniente Valente, Hospital Regional de Alta Especialidad del Bajío, León, Guanajuato (20); Juan Carlos Núñez Fragoso, Hospital Regional No. 1, IMSS, Durango, Durango (20); Hazel González Castro, Juan Carlos Pérez Alva, Hospital General de Puebla, Puebla, Puebla (19); Carlos A. Arean Martínez, Hospital General “Dr. Miguel Silva”, Morelia, Michoacán (18); Miguel E. Beltrán Gámez, ISSSTECALI, ISSSTE, Mexicali, Baja California (18); Pánfilo Mauricio Saucedo, Hospital General de Zacatecas, Zacatecas, Zacatecas (17); Vladimir Ruiz Ronquillo, Hospital General de Zona No.18, IMSS; Playa del Carmen, Quintana Roo (15); Agustín Vallecillo Gómez, Hospital Regional Salamanca, PEMEX, Salamanca, Guanajuato (15); Reynaldo Magaña Magaña, Hospital General de Uruapan, Uruapan, Michoacán (14); Jaime Barragan Luna, Hospital Regional Poza Rica, PEMEX, Poza Rica, Veracruz (14); Jorge G. Chávez Páez, Amerimed, Puerto Vallarta, Jalisco (13); Ma. Isabel Sánchez Ramírez, Hospital General con Especialidades “Juan María de Salvatierra”, La Paz, Baja California Sur (12); José Luis González Peña, Hospital “Christus Muguerza”, Reynosa, Tamaulipas (11); Jorge G. Chávez Páez, Hospital Cornerstone, Puerto Vallarta, Jalisco (11); Alfredo Comparan Núñez, Hospital Florence, Tijuana, Baja California (11); Jesús Manuel Acosta Grajeda, Hospital General de Zona No.1, IMSS, Chihuahua, Chihuahua (11); Luis Delgado Leal, Centenario Hospital “Miguel Hidalgo”, Aguascalientes, Aguascalientes (11); Orlando Henne Otero, Hospital Ángeles de Villahermosa, Villahermosa, Tabasco (10); Víctor Páramo Bautista, Hospital General “Adolfo Prieto”, Taxco, Guerrero (10); César Gerardo Martínez Hernández, Hospital General de Zona No.50, IMSS, San Luis Potosí, San Luis Potosí (10); José Manuel Enciso Muñoz, Pánfilo Mauricio Saucedo, Hospital “San Agustín”, Zacatecas, Zacatecas (10); Alberto Zenón Baños Velasco, Hospital Ángeles de Culiacán, Culiacán, Sinaloa (9); Francisco Guerrero Martínez, Hospital General de León, León, Guanajuato (9); Mario Morales Medina, Policlínica de Especialidades, Tuxtla, Chiapas (9); Luis Sánchez Trujillo, Hospital Metropolitano, Monterrey, Nuevo León (7); Roberto Domínguez López, Centro de Especialidades Médicas “Dr. Rafael Lucio”, Xalapa, Veracruz (6); Julio Iván Farjat Ruiz, Sergio Alonso Villarreal Umaña, Centro Médico “Las Américas”, Mérida, Yucatán (6); José Luis Novelo Del Valle, Hospital General de Zona No.1, IMSS, Campeche, Campeche (6); Rosa Elena Limas Rodríguez, Hospital General, ISSSTE, Tijuana, Baja California (6); Ivette Y. Alferez Jiménez, Hospital Ángeles de Lindavista, México, D.F. (5); Melissa Jiménez Madrigal, Centro de diagnóstico y tratamiento Cardiológico, Mexicali, Baja California (5); Víctor Páramo Bautista, Clínica de Especialidades Taxco, Taxco, Guerrero (5); Jorge G. Chávez Páez, Hospital CMQ, Puerto Vallarta, Jalisco (5); Alfredo Comparan Núñez, Hospital del Prado, Tijuana, Baja California (5); José Luis Novelo Del Valle, Hospital General de Especialidades INDESALUD, Campeche, Campeche (5).
Myocardial infarction clinical classification Uncomplicated: normal blood pressure without left ventricular dysfunction signs, EF >40%, brain natriuretic peptide type-B (BNP) <100pg/dL, or both5. Complicated with left ventricular dysfunction without clinical manifestations: normal blood pressure or lower limits, no third left ventricular heart sound, EF <40%, or both, BNP >100pg/dL, or both5. Complicated with ventricular dysfunction with clinical manifestations: acute pulmonary edema or pre-cardiogenic shock suspicion (borderline blood pressure, hypotension, or both), clinical expression of – renin-angiotensin-aldosterone vasopressin system activity, oliguria, delayed capillary refill, lower lobes crackling rales, EF <30%, BNP >600pg/dL5.
I: without left ventricular dysfunction signs; II: left ventricular third heart sound, basal crackling rales, or both; III: acute pulmonary edema IV: cardiogenic shock.5
Pulmonary edema: increased work of breathing with or without severe desaturation (saturation <90%), left ventricular gallop or tachycardia, crackling rales or wheezes >50% in lung fields. Chest X-ray with interstitial, alveolar, or mixed pulmonary edema.5
Cardiogenic shock: (1) systolic blood pressure <90mmHg without support of vasoactive substances or 100mmHg with vasopressors use; (2) clinical, radiographic expression of pulmonary venocapillary hypertension, or both; (3) signs of peripheral vascular hypoperfusion; (4) metabolic acidosis; (5) cardiac index <2.2L/min/m2; (6) pulmonary capillary pressure >18mmHg; and (7) arteriovenous oxygen difference >5.5ml/dL.5
A list of the RENASICA III investigators is provided in Appendix A.
Clinicaltrials.gov: NCT02141750.