The Framingham Coronary Heart Disease Risk Score is an important clinical tool. The aim of this cross-sectional study was to compare plasma homocysteine levels and polymorphism 677CT MTHFR with this score to determine the utility of these new biomarkers in clinical practice.
MethodsPlasma homocysteine levels determined by chemiluminescence and polymorphism 677CT MTHFR, detected by PCR-RFLP, were compared with Framingham coronary risk score in a cross-sectional survey on 68 men and 165 women.
ResultsCoronary heart disease risk augmented with an increase in the quartile of plasma homocysteine. In the 2nd, 3rd and 4th quartile of plasma homocysteine, men showed significantly (P<0.001) higher risk than women. For the highest quartile of plasma homocysteine, OR of high-risk (10-year risk≥20%) compared with the lowest quartile was 17.45 (95% CI: 5.79–52.01). Frequencies of CT and TT genotype and T allele were not over-represented in the individuals with score≥10%. The higher plasma homocysteine concentrations in individuals with score≥10% with respect to those with low risk (P<0.005 and P<0.001) were not due to the presence of T allele. The T allele (CT+TT genotypes) of the MTHFR C677T polymorphism was not significantly associated with an increased risk of coronary disease (OR=1.09, 95% CI=0.50–2.39, P=0.844).
ConclusionsThe present study demonstrated an association between plasma homocysteine levels and the severity of coronary heart disease estimated with the Framingham coronary risk score, and this association appeared to be independent on the genotype of MTHFR. We postulate that plasma homocysteine is effective enough, considered even in isolation.
La puntuación del riesgo coronario de Framingham es una importante herramienta clínica. El objetivo del presente estudio transversal fue comparar los niveles plasmáticos de homocisteína plasmática y el polimorfismo 677CT de la MTHFR con esta herramienta para determinar la utilidad de estos nuevos biomarcadores en la práctica clínica.
MétodosLos niveles de homocisteína plasmática determinados por quimioluminiscencia y el polimorfismo 677CT MTHFR por PCR-RFLP fueron comparados con la puntuación del riesgo coronario de Framingham en un estudio transversal sobre 68 hombres y 165 mujeres.
ResultadosEl riesgo de enfermedad coronaria aumentó con el incremento en los cuartiles de homocisteína plasmática. En el segundo, tercero y cuarto cuartil de homocisteína plasmática los hombres mostraron significativamente (p<0.001) mayor riesgo que las mujeres. Para el cuartil más alto de homocisteína plasmática, la OR de riesgo alto (riesgo a 10 años≥20%) comparado con el cuartil más bajo fue 17,45 (IC 95%: 5,79-52,01; p<0.001). Las frecuencias de los genotipos CT y TT y del alelo T no estuvieron aumentados en los individuos con una puntuación≥10%. Las mayores concentraciones de homocisteína plasmática en los individuos con una puntuación≥10% respecto a los de bajo riesgo (p<0.005 y p<0.001) no se debieron a la presencia del alelo T. El alelo T (genotipos CT+TT) del polimorfismo MTHFR C677T no estuvo significativamente asociado con mayor riesgo de enfermedad coronaria (OR=1.09, IC 95%=0.50–2.39, p=0.844).
ConclusionesEl presente estudio mostró una asociación entre los niveles de homocisteína plasmática y la severidad de la enfermedad coronaria estimada con el algoritmo de puntuación de riesgo coronario de Framingham y esta asociación resultó ser independiente del genotipo de MTHFR. Postulamos que la homocisteína plasmática es lo suficientemente eficaz, estudiada incluso aisladamente.
Cardiovascular diseases (CVD) are some of the leading causes of death. The Framingham Coronary Heart Disease Risk Score (FCRS), modified by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) guideline, based on the predictive capacities of the cardiovascular risk factors including sex, age, high blood pressure, total cholesterol (TC) level, high density lipoprotein cholesterol (HDL-C) level, diabetes mellitus, and cigarette smoking, has been adopted as a standard approach to the prediction of risk of coronary heart disease (CHD).1,2 This score is an important clinical tool although not all persons at high CHD risk are identified by the FCRS. In order to improve CHD risk prediction, other cardiovascular risk markers have been evaluated. Among those, plasma homocysteine (tHcy) is recognized as a risk factor3 independent of conventional risk factors such as diabetes mellitus, smoking and hyperlipidemia.4 However, few data are available comparing tHcy levels with calculated FCRS.5,6 Homocysteine is re-methylated to methionine by the enzyme methionine synthase that requires 5-methyltetrahydrofolate (5-MTHF) as methyl donor and vitamin B12 as cofactor. This donor is synthesized by the enzyme methylenetetrahydrofolate reductase (MTHFR) from 5,10-methylene-tetrahydrofolate.7 Reduction in its activity can be caused as a result of genetic and non-genetic factors.8 There are 24 genetic polymorphisms associated with this gene and four (203G>A, 677C>T, 1298A>C and 1793G>A) have been studied in relation to different pathologies.9 Within these SNPs, 677C>T, resulting in the replacement of alanine for valine, produces a thermolabile enzyme with decreased MTHFR specific activity. As a result, homozygous 677TT has 30% of residual activity and heterozygous 677CT has 70% when compared to 677CC variant,10,11 resulting in an enhanced plasma level of tHcy and low of folate.11 Several studies suggest that this SNP is a genetic risk factor for vascular disease;8,12 however others could not confirm this association.13,14 Various meta-analysis studies arrived at different conclusions about the association of an increase in tHcy levels with risks of vascular diseases in patients with MTHFR mutation.15,16 To determine whether these new biomarkers are useful in clinical practice, in this study we examined the association between tHcy levels and polymorphism 677CT MTHFR with the FCRS at population level.
MethodsWe carried out a cross-sectional study comprising 68 men and 165 women aged 18–87 years, randomly selected in three health centers from Buenos Aires City, belonging to the University Hospital of Universidad Abierta Interamericana. They were all physically active. The exclusion criteria were pregnancy, use of any type of (multi-)vitamin tablet in the preceding year and younger than 18 years. Written informed consent was obtained from all participants before the study began. The hospital local ethics committee approved the study protocol in accordance with the Declaration of Helsinki as revised in 1983. A questionnaire with clinical data was completed by all subjects on the day of enrollment. Participants were classified as hypertensive if they were taking antihypertensive medication. The risk factors of vascular disease recorded in this study were hypertension (≥140/90mmHg), diabetes (glycemia≥6.96mmol/L), hyperlipidemia (HDL-C<1.04mmol/L for men and <1.30 for women, and TC>5.20mmol/L).17 A self-reported questionnaire was used to collect the subjects’ data including their past medical history, smoking status, and the prescribed drugs.
Blood collectionAll blood samples were collected after an overnight fast (>10h) by venipuncture into an EDTA containing tube. A separated aliquot was kept for DNA extraction. Plasma samples were obtained by double centrifugation at room temperature for 15min at 2000×g. The plasma aliquots were immediately frozen at −80°C until use.
Laboratory measurementsBiochemical measurements, including glycemia, TC, and HDL-C, were carried out using the Metrolab 2300 auto-analyzer. Plasma tHcy was determined by chemiluminescence in a Bayer Corp. ADVIA Centaur apparatus (Leverkusen, Germany).
MTHFR mutation analysis. DNA extractionGenomic DNA was isolated from nucleated blood cells using the FlexiGene DNA Kit QIAGEN® GmbH, (Hilden, Germany) following the manual's instructions. DNA samples were kept at −80°C until analyzed.
PCR-RFLP analysisThe 677C>T MTHFR gene mutation was detected by the polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) method according to Frosst et al.11 Briefly, about 50–80ng DNA samples were amplified in a final volume of 25μL containing 1×PCR buffer with 2.0mmol/L MgCl2, 0.75 unit GoTaq DNA Polymerase (Promega) 200μmol/L dNTP, and 0.5μmol/L of each primer (5′-TGAAGGAGAAGGTGTCTGCGGGA-3′ and 5′-AGGACGGTGCGGTGAGAGTG-3′). PCR was performed in an Eppendorf Mastercycler Personal thermocycler (Germany), and the profile consisted of an initial melting step of 5min at 94°C; followed by 45 cycles of 40s at 94°C, 50s at 62°C, and 40s at 72°C; and a final elongation step of 7min at 72°C. The restriction enzyme Hinf I (Promega, UK) was used to distinguish the 677C>T polymorphism, since the polymorphic allele presents a Hinf I restriction site.
The 677CC variant had a single band representing the entire 198-bp fragment, and the heterozygous genotype 677CT resulted in three fragments of 198, 175 and 23bp, while the homozygous 677TT for the MTHFR mutation results in 2 fragments of 175 and 23bp. Finally the products of the Hinf I digestion were electrophoresed on a 3% agarose gel. To ensure quality control, genotyping was performed blindly, and random samples were tested twice by different persons. The participants were categorized as homozygous (CC), heterozygous (CT), or homozygous (TT) for the thermolabile variant.
Calculation of 10-year CHD riskA 10-year CHD risk was calculated using FCRS modified by the NCEP ATP III guideline that uses major independent risk factors including cigarette smoking, blood pressure, TC, HDL-C, age and type-2 diabetes.1,2 Individual risk factor scores were assigned on the basis of age, TC, systolic blood pressure, HDL-C, and smoking status, and were summed to determine the 10-year absolute risk. The absolute risk percentage in 10 years was calculated, which can be classified as low risk (<10%), intermediate risk (10–20%) and high risk>20%).
Statistical analysisFor continuous variables, values were presented as mean±standard error (SE) or median (inter-quartile range), as appropriate. Differences between the examined quantitative parameters were compared by the Mann–Whitney U-test. Categorical data were expressed as the number of subjects (percentage). The study subjects were grouped into quartiles according to the circulating levels of tHcy. Kruskal–Wallis test was used to test the difference in the 10-year CHD risk among quartiles of biomarkers, followed by the post hoc Bonferroni test for multiple comparisons. Multiple logistic regression analysis was performed to determine the odds ratio (OR) of high-risk for CAD (10-year risk≥10%) in each quartile of biomarkers. Deviation of the genotype distribution from the Hardy–Weinberg equilibrium was determined by χ2 test/Fisher's exact test. All tests were 2-tailed. Statistical analyses were performed using the SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
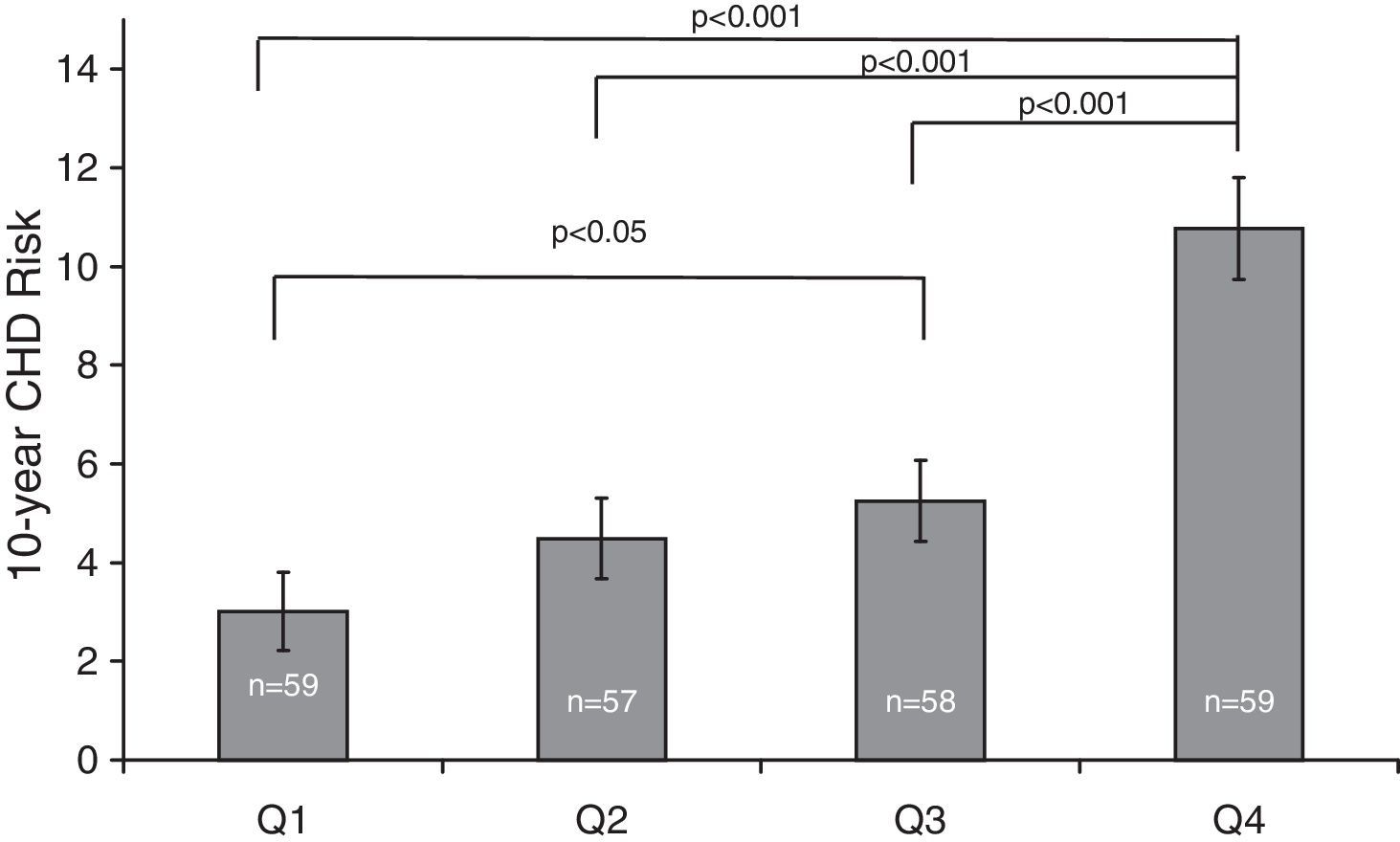
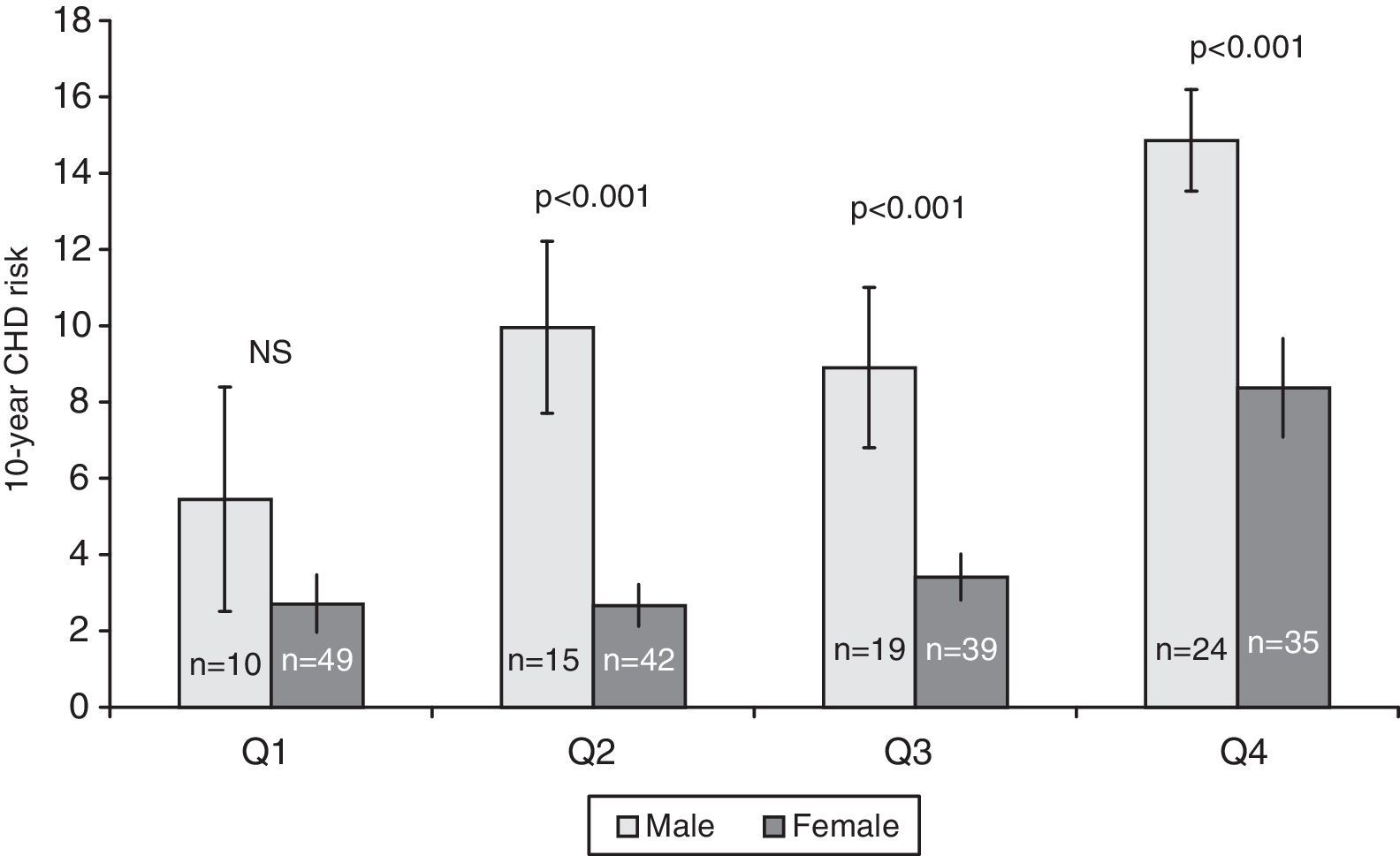
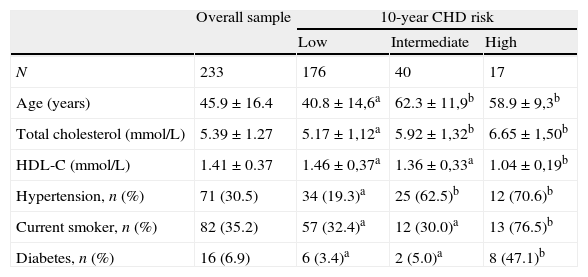
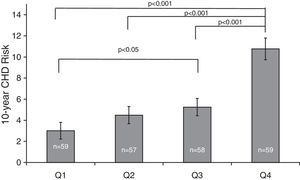
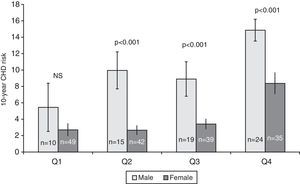
ResultsThe population sample was composed of 65 men and 168 women aged between 18 and 87 years. Hypertension was present in 71 (30.5%) subjects, cigarette smokers 82 (35.2%) and diabetics 16 (6.7%) (Table 1). The prevalence of genotypes determined in the overall sample population of this study were: CC 43.4%, CT 47.2% and TT 9.4%. The frequencies of C and T allele were 0.67 and 0.33, respectively, and this polymorphism was in Hardy–Weinberg equilibrium (χ2=1.041, df=1, P=0.308). These frequencies are consistent with other studies of this variant.15,18Table 1 shows the distribution of the variables’ average values according to the seriousness of CHD risk score. Individuals with the lowest CHD risk score had the youngest age, the lowest concentrations of TC, highest concentrations of HDL-C, and lowest percentage of hypertensive, cigarettes smokers, and diabetics. The 10-year CHD risk augmented with an increase in the quartile of tHcy (P<0.001) (Fig. 1). The 10-year CHD risk in the 4th quartile of tHcy was significantly higher than those in the other three quartiles (P<0.001). Gender differences were also found in the 10-year CHD risk for levels of tHcy. Fig. 2 shows that in 2nd, 3rd and 4th quartiles of tHcy, men exhibited significantly (P<0.001) more risk than women.
10-year CHD risk characteristics of subjects in the overall sample and stratified by risk levels.
| Overall sample | 10-year CHD risk | |||
| Low | Intermediate | High | ||
| N | 233 | 176 | 40 | 17 |
| Age (years) | 45.9±16.4 | 40.8±14,6a | 62.3±11,9b | 58.9±9,3b |
| Total cholesterol (mmol/L) | 5.39±1.27 | 5.17±1,12a | 5.92±1,32b | 6.65±1,50b |
| HDL-C (mmol/L) | 1.41±0.37 | 1.46±0,37a | 1.36±0,33a | 1.04±0,19b |
| Hypertension, n (%) | 71 (30.5) | 34 (19.3)a | 25 (62.5)b | 12 (70.6)b |
| Current smoker, n (%) | 82 (35.2) | 57 (32.4)a | 12 (30.0)a | 13 (76.5)b |
| Diabetes, n (%) | 16 (6.9) | 6 (3.4)a | 2 (5.0)a | 8 (47.1)b |
Age, total cholesterol and HDL-C expressed as mean±SD.
Different letters indicate significant differences (p<0.05).
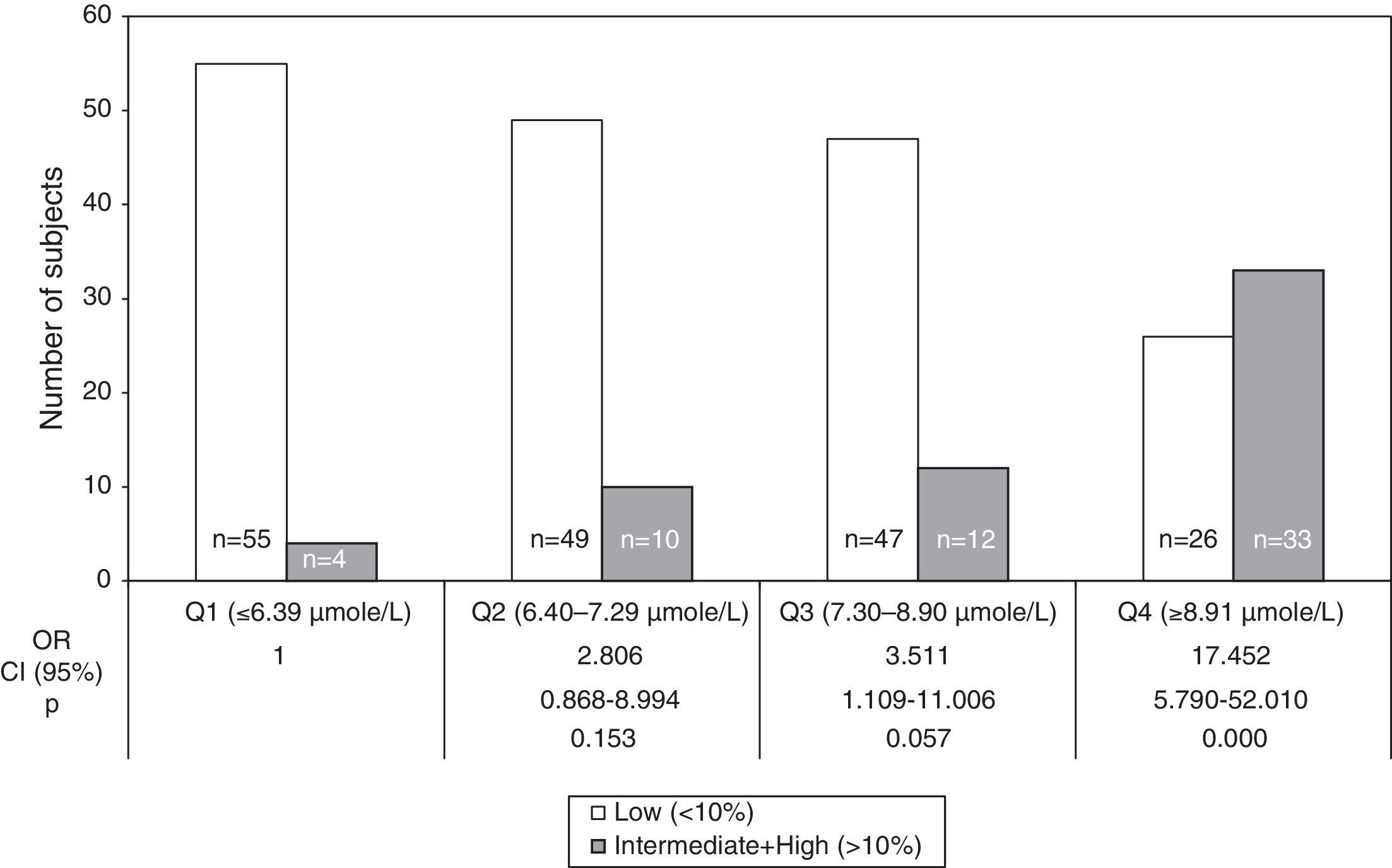
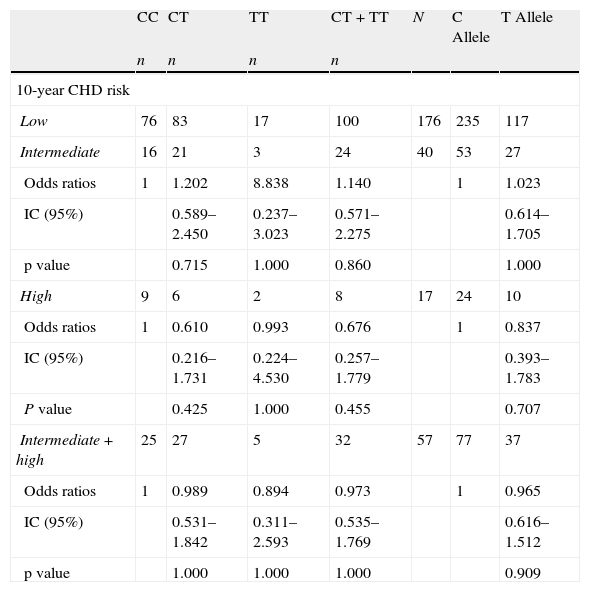
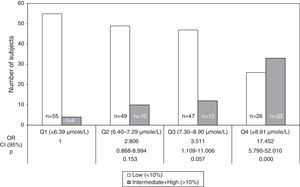
The OR for 10-year CHD risk≥10% in 2nd (6.40–7.29μmol/L), 3rd (7.30–8.90μmol/L) and 4th (>8.91μmol/L) quartiles of tHcy compared to 1st quartile (<6.39μmol/L) were 2.81 (95% CI: 0.87–8.99; NS), 3.51 (95% CI: 1.11–11.01; NS) and 17.45 (95% CI: 5.79–52.01; P<0.001), respectively (Fig. 3). The tHcy, dichotomized with cut off point 11μmol/L, was also calculated. This cut off point was chosen because it was proposed in previous work19,20 in population samples from the same city as this work. The 10-year CHD risk≥10% increased more than 11-fold (OR=11.67, P<0.001) with respect to tHcy lower than or equal to 11μmol/L, when tHcy was higher than 11μmol/L (data not shown). Comparison of the intermediate and high risk groups against the low risk group showed that the frequency of CT and TT genotype and T allele were not over-represented in the individuals with FRS≥10% (Table 2), even if the risk of CHD was compared within the subjects with the CC genotype and the combined number of subjects with T allele (CT heterozygotes and TT homozygotes).
Distribution of genotypes and allele frequencies of the MTHFR 677CT polymorphism in low, intermediate and high risk groups.
| CC | CT | TT | CT+TT | N | C Allele | T Allele | |
| n | n | n | n | ||||
| 10-year CHD risk | |||||||
| Low | 76 | 83 | 17 | 100 | 176 | 235 | 117 |
| Intermediate | 16 | 21 | 3 | 24 | 40 | 53 | 27 |
| Odds ratios | 1 | 1.202 | 8.838 | 1.140 | 1 | 1.023 | |
| IC (95%) | 0.589–2.450 | 0.237–3.023 | 0.571–2.275 | 0.614–1.705 | |||
| p value | 0.715 | 1.000 | 0.860 | 1.000 | |||
| High | 9 | 6 | 2 | 8 | 17 | 24 | 10 |
| Odds ratios | 1 | 0.610 | 0.993 | 0.676 | 1 | 0.837 | |
| IC (95%) | 0.216–1.731 | 0.224–4.530 | 0.257–1.779 | 0.393–1.783 | |||
| P value | 0.425 | 1.000 | 0.455 | 0.707 | |||
| Intermediate+high | 25 | 27 | 5 | 32 | 57 | 77 | 37 |
| Odds ratios | 1 | 0.989 | 0.894 | 0.973 | 1 | 0.965 | |
| IC (95%) | 0.531–1.842 | 0.311–2.593 | 0.535–1.769 | 0.616–1.512 | |||
| p value | 1.000 | 1.000 | 1.000 | 0.909 | |||
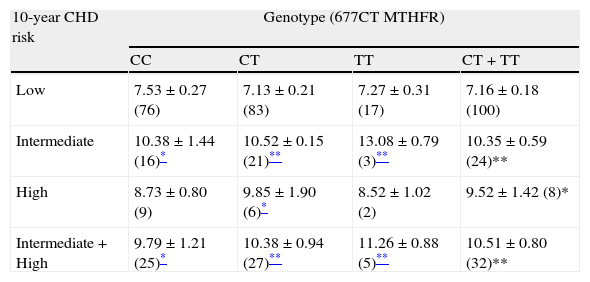
Table 3 shows plasma concentrations of tHcy analyzed by genotypes in the low, intermediate and high risk of CHD. The higher tHcy concentrations observed in the individuals with FRS≥10% (intermediate, high and intermediate plus high) with respect to those with low risk (P<0.005 and P<0.001) were not due to the presence of the T allele.
Homocysteinemia in risk groups classified by genotypes of polymorphism 677CT MTHFR.
| 10-year CHD risk | Genotype (677CT MTHFR) | |||
| CC | CT | TT | CT+TT | |
| Low | 7.53±0.27 (76) | 7.13±0.21 (83) | 7.27±0.31 (17) | 7.16±0.18 (100) |
| Intermediate | 10.38±1.44 (16)* | 10.52±0.15 (21)** | 13.08±0.79 (3)** | 10.35±0.59 (24)** |
| High | 8.73±0.80 (9) | 9.85±1.90 (6)* | 8.52±1.02 (2) | 9.52±1.42 (8)* |
| Intermediate+High | 9.79±1.21 (25)* | 10.38±0.94 (27)** | 11.26±0.88 (5)** | 10.51±0.80 (32)** |
Homocysteinemia in μmolar±SE. Number of subjects in parenthesis.
The present study also showed that in subjects in the 4th quartile of tHcy, the presence of the T allele (CT+TT genotypes) of the MTHFR C677T polymorphism was not significantly associated with an increased risk of CHD (FRS>10%) (OR=1.09, 95% CI=0.50–2.39, P=0.844) (data not shown).
DiscussionSeveral risk calculators are available to adopt preventive interventions of CVD, including the ATP III and traditional FCRS.1 This simplified tool categorizes adults into 3 risk categories, low (<10% over 10 years), intermediate (10–20%), and high (>20% risk), on the basis of age, sex, systolic blood pressure, serum TC level, HDL-C level, cigarette smoking and diabetes history.1
Homocysteine is of epidemiologic interest as a potential risk factor. The strong association between raised concentrations of tHcy and CHD has been recognized since 1969.21 This relationship between tHcy level and risk for cardiovascular events was supported in a large number of cohort studies22 and has been repeatedly described by various populations.3 Ganji and Kafai23 in a meta-analysis of a subset of studies reported that each 5-μmol/L increase in tHcy level confers an approximately 9% increase in the risk for CHD events and is independent from traditional CHD risk factors.
In order to have an estimate of the power of hyperhomocysteinemia as a risk factor for CHD, in the present study we compared plasma tHcy levels with the universally accepted FCRS.
Our data on factors related with the FCRS (Table 1) indicated that CHD risk score increased with age and TC and decreased with HDL-C, and was related to hypertension, diabetes and smoking.
In this study, the average tHcy level was significantly higher in subjects with FCRS>10% than in persons with lower FCRS and a highly significant correlation between the tHcy levels and the FCRS was observed (Fig. 1). When both sexes were studied separately, in the three highest quartiles of tHcy, men showed significantly more risk than women (Fig. 2), reinforcing the predictive power of the tHcy in isolation. Gender difference in tHcy can be explained considering that sex hormones have effects on methionine metabolism, the level of creatinine is higher in men, the musculoskeletal system of men is usually more developed and has a larger volume and mass compared to women, and plasma level of folic acid and vitamin B12 is different between men and women.24 Besides, the highest quartile of tHcy showed a significantly higher OR of high-risk for CHD (10-year risk≥10%), which was also observed when serum tHcy was dichotomized with the cut off point 11μmol/L (data not shown).
These cross-sectional data indicating that plasma concentration of tHcy is significantly associated with calculated FCRS are in close agreement with those of recent studies, which used NECP ATP III guideline.25–27 For decades the FCRS has been used to predict the 10 year risk of developing coronary heart disease, the search of a more accurate measure of cardiovascular disease risk is very relevant. It is known that cardiovascular disease is not accurately predicted by classic risk factors such as those included in the FCRS, separately. By contrast, as we found in this investigation, a single measurement of tHcy accurately identifies those at high risk of cardiovascular disease. These results suggest that risk identification for primary prevention of cardiovascular disease can be based on plasma concentrations of tHcy in isolation.
Montalescot et al.28 demonstrated that the presence of hypertension, one of the risk factors considered within the FCRS, and hyperhomocysteinemia were associated with more severe coronary atherosclerosis. Nevertheless, other studies found no correlation between tHcy levels and the extent and severity of CHD.29–31
The development of CHD is believed to be largely under genetic control. In previous studies we demonstrated that MTHFR 677CT mutation may contribute to hypertension or affect the development of hypertension through hyperhomocysteinemia,20 and we also found an association between this polymorphism and tHcy with hypertension, which is manifested with age in women. We concluded that the differences observed in sex and age could be the result of endocrine activity, since also the hormonal status may modify the phenotypic expression of this genetic variant.25,32 In this context, in the present work, we also wanted to determine whether these variables are associated with the FCRS. In our population, this polymorphism did not correlate with the risk of CHD calculated by the FCRS. We also could observe that the higher tHcy levels in persons with FCRS≥10% were independent to the T allele. We concluded that this MTHFR C677T polymorphism was not associated with the risk of CHD calculated with the FCRS.
Our results are in accord with many investigations that failed to detect an association of the MTHFR gene variation with the presence of CHD.13,25,33–35 Although other studies showed the relationship of the MTHFR gene polymorphism with the extent of CHD.8,36,37 These discrepancies could, at least in part, be explained by gene interactions and life-style which were also postulated to be associated with CHD38–41 and might interfere to a different degree with the link between the MTHFR 677CT gene variation and the risk of CHD. Genetic factors are not the only cause of an increase in the plasma tHcy. Many other demographic and lifestyle factors, such as age, gender, folate intake through food, smoking, coffee and alcohol consumption, use of certain medications, and several diseases can affect the tHcy concentration.25
Although it has been shown that TT homozygotes have higher tHcy levels than the other MTHFR C677T genotypes,11 Jacques et al.42 demonstrated that these differences disappeared when individuals had folate levels greater than 15.4nmol/l, suggesting that MTHFR C677T TT homozygotes require folate supplementation to regulate plasma tHcy concentrations.
A potential weakness in our study was the lack of folate determination. This was caused by the fact that it is not currently available as a procedure in our laboratories. Therefore, we do not have direct evidence that the TT genotype was also associated with reduced plasma levels of folate.
The relationship between plasma tHcy and other mutations in the MTHFR gene such as the A1298C in our studied group remains to be addressed and in fact, it is in progress. Also, our results must be analyzed considering that Argentina is a country with a heterogeneous population, which results in cultural, socioeconomic and ethnic diversity, particularly in the metropolitan area. It is important to note that the frequency of the MTHFR 677T allele is known to vary substantially among different ethnic populations.43 Finally, in our study, the following limitations must be considered. First, the sample size may limit the power of testing a significant association between MTHFR 677TT and FCRS. However, the associations that have reached statistical significance in this context of limited sample size may be thought as valid and reproducible in larger samples. In the future, large prospective studies are needed in order to confirm our findings and analyze the possible benefits of prevention and treatment.
ConclusionsOur findings showed an association between tHcy levels and the severity of CHD estimated with the FCRS algorithm, and this association appeared to be independent on the genotype of MTHFR. Since the phenotypic expression of the T allele is highly dependent on many other variables, it is reasonable that in average its presence does not correlate with FCRS. We postulate that determination of plasma homocysteine is effective enough as predictor of risk of CHD, considered even in isolation.
FundingThis work was supported by a grant from the Universidad Abierta Interamericana (UAI).
Conflict of interestThe authors declare having no conflict of interest.