Myocardial calcification is an unusual entity. The first report appeared in 1919 when Scholz1 observed a calcified heart in an X-ray film. A heart may develop calcifications in different locations such as valves, arteries, endocardium, pericardium and conduction tissue.2,3 Degenerative and inflammatory mechanisms have been postulated as possible explanations for these calcifications. Intra-myocardial calcification is extremely rare and has been associated with pathological entities such as chronic renal failure, particularly if treated with hemodialysis, and secondary hyperparathyroidism.4
It has also been associated to previous myocardial damage such as that due to myocarditis,5,6 cardiac trauma, scarring after cardiac surgery7 or calcium deposits in the area of a post-infarction aneurysm.8 There are reports based on nonscientific observations that refer to other potential causes such as tuberculomas, radiation or the toxic effect of vasoactive amines.9–11 In this article we report the case of a female patient who did not present any of the previously referred conditions, but developed singularly distributed severe calcification within the left ventricular myocardial tissue, which led to significant restriction of diastolic filling. She underwent cardiac transplantation and the explanted heart revealed interesting findings.
At the time of this report, the patient was 56 years old, with a female born, and a resident of Mexico City, from a middle income socio-economic level. She had no relevant family, traumatic or infectious disease history.
She was admitted to our hospital in 2007 with a 2-year history of dyspnea on strenuous exertion that progressed to dyspnea on mild exertion. Physical examination only revealed the presence of an S4.
The chest X-ray film showed widespread radio-opaque areas within the left ventricle, surrounding it in a spiral pattern.
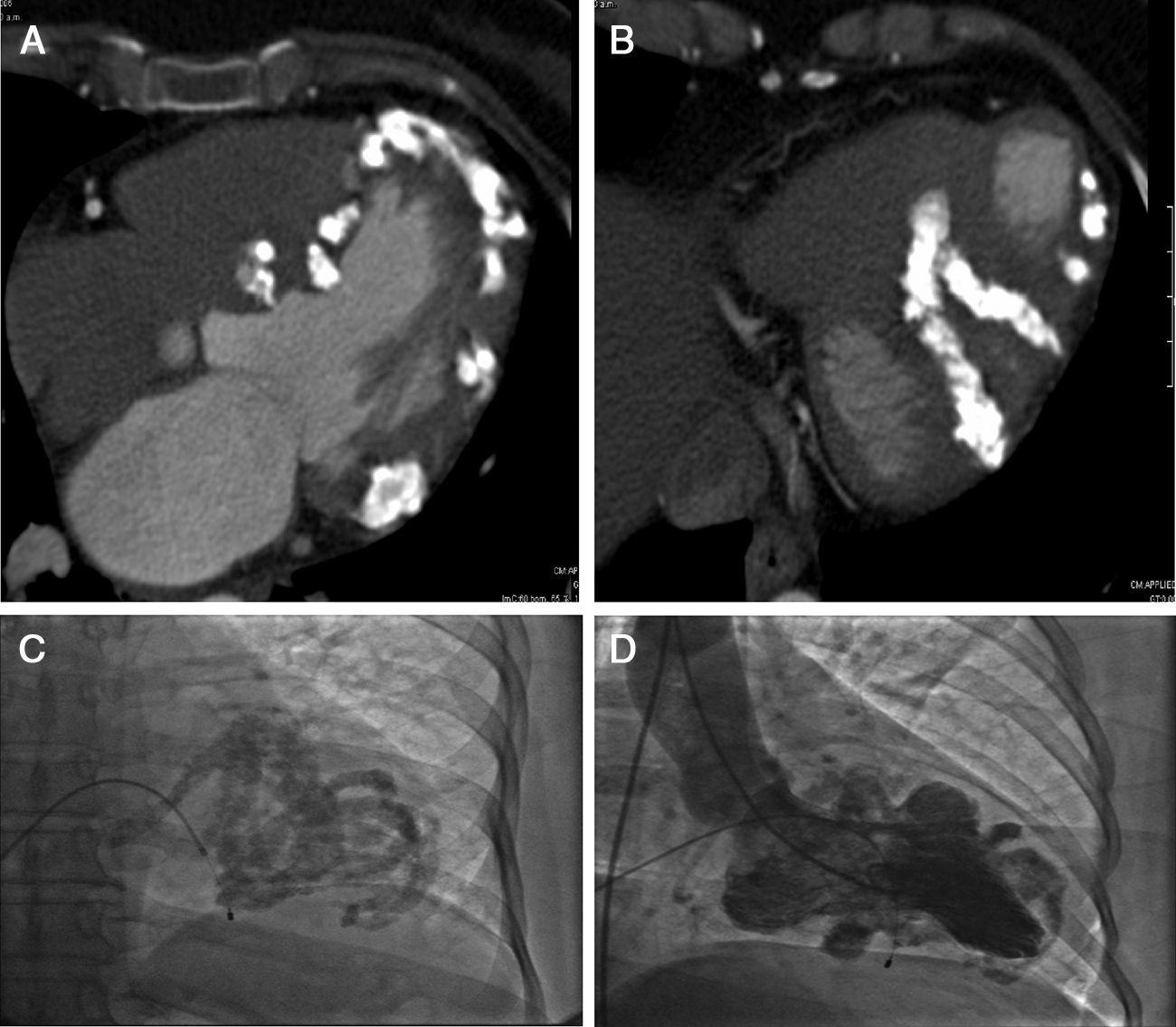
The CT scan of the four chambers at the papillary muscle level showed calcium infiltrations of the myocardium (Fig. 1A). Myocardial calcium infiltration was evident (B), when observed from the inferior wall of the left ventricle (B).
Heart CT scan showing four chambers at the papillary muscle level and the presence of calcium infiltrating the myocardium (A). And seen from the inferior wall of the left ventricle, myocardial calcium infiltration is evident (B). Cardiac catheterization showed extensive calcification distributed in bands and with the respected areas (calcium free) protruding between the calcium bands during ventricular diastole (C and D).
This same study excluded mediastinal great vessel or pulmonary parenchymal involvement. More calcifications in other organ systems were intentionally sought but not found.
Cardiac catheterization showed extensive calcification distributed in bands and with the respective areas (calcium free) protruding between the calcium bands during ventricular diastole. (Fig. 1C and D) Coronary arteries were normal and the hemodynamic curves showed a restrictive filling pattern.
An 18FDG-PET scan did not show any metabolic abnormalities suggesting increased glycolytic activity throughout the body.
Systemic, autoimmune, infectious, rheumatic and metabolic processes relating to the detected calcifications were duly excluded.
A myocardial biopsy obtained from the left ventricle revealed cellular hypertrophy, areas with amphiphilic material with a patchy distribution between muscle fibers and negative staining, thus except for the deposition of mucoid substances, calcium or amyloid. H and E staining showed no inflammatory infiltrates. PAS, Gram and Grocott staining were also negative. Neoplastic cells were not observed.
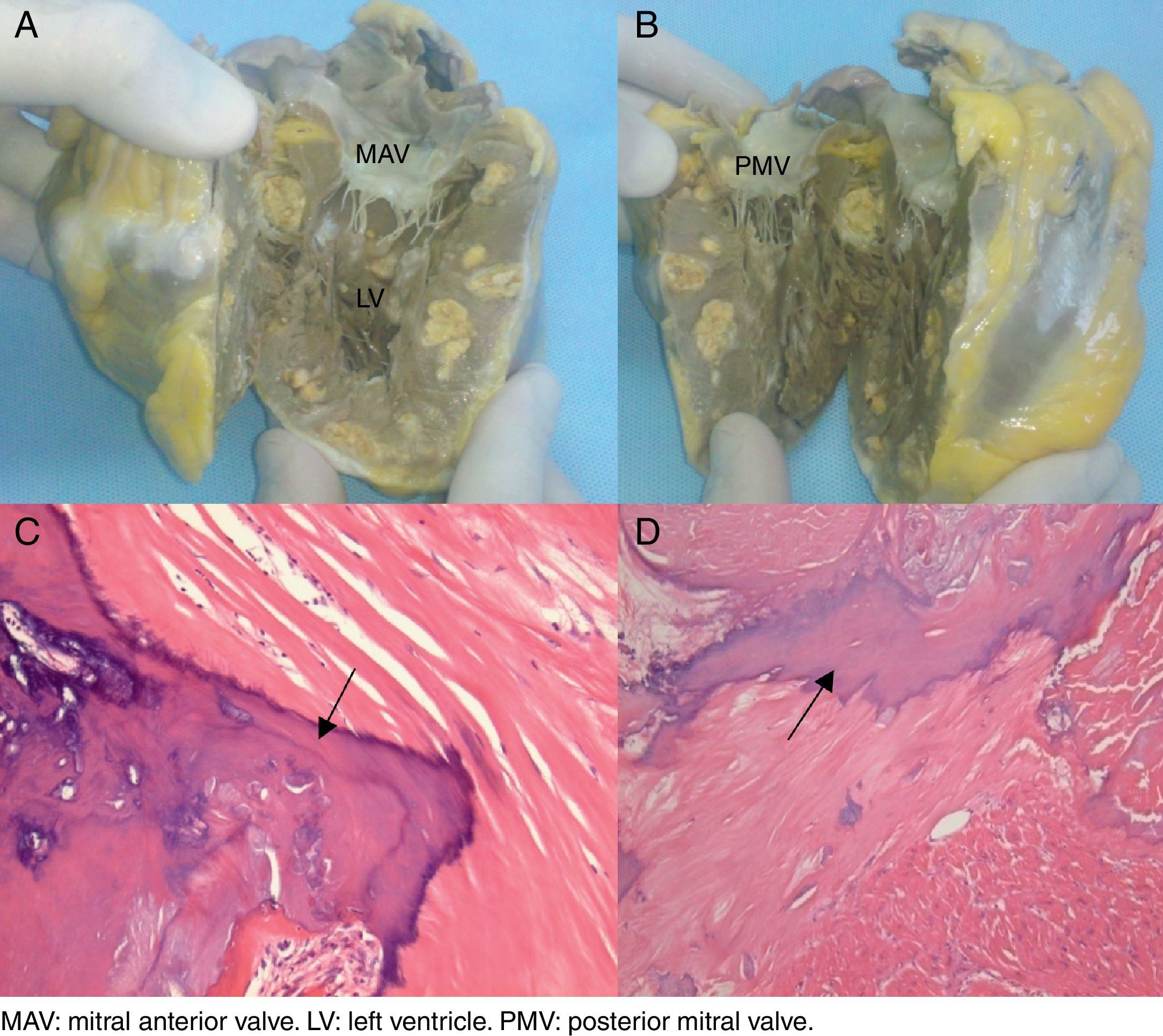
The patient continued to deteriorate clinically, developed dysnea and fainting spells with minimal exertion that required cardiac transplantation with a favorable outcome. The explanted heart was studied in detail as shown in Fig. 2: macroscopic Fig. 2A and B of the explanted heart in a coronal section showed multiple intra-myocardial calcifications. Microphotographs 2C and D (10× and 40×, respectively) stained with hematoxylin and eosin. C: a bony spicule (arrow) replaces residual cardiac muscle. D: a laminated spicule (arrow) is seen on the left and residual cardiac muscle on the right. Consequently, tissue areas were replaced by spongy bone trabeculae with bone marrow in their interior; no interstitial fibrosis or microorganisms were observed.
Macroscopic images of the explanted heart in a coronal section showing multiple intra-myocardial calcifications (A and B). Microphotographs (10× and 40×, respectively) of Hematoxylin and Eosin staining (C and D). (C) A bony spicule (arrow) replaces residual cardiac muscle. (D) A laminated spicule (arrow) is seen on the left and residual cardiac muscle on the right.
Two well-known mechanisms explain myocardial calcification: metastatic calcification and dystrophic calcification. In the first case, abnormalities in calcium and phosphorus metabolism are present and thus, calcifications are not exclusive to the heart but can be present in other organs such as the skin, corneas, lung, liver, spleen and kidneys.2 This type of calcification is commonly associated with renal failure and destructive bone lesions due to hyperparathyroidism.4
In dystrophic calcification, the proposed mechanisms suggest that calcium is deposited in the heart due to a decrease in carbon dioxide production resulting from a “slowed down” metabolism that leads to tissue alkalinization and decreased calcium solubility;8 this may occur in cases of myocardial hemorrhage, fibrosis or necrosis, so there generally is a positive history of myocardial damage. Dystrophy may develop over a period of a few days, months and even years after the myocardial damage.12
In the present case, metastatic calcification was excluded since no evidence of calcium/phosphorus metabolism abnormalities was present and systemic pathological entities were not diagnosed (immune, rheumatic or infectious). In terms of dystrophic calcification, no previous myocardial damage was detected.
A similar case of this type of calcification was reported by El-Bial et al.,13 in an 80-year-old woman who developed severe myocardial calcifications over a 4-year period. Their etiology could not be established either.
We were able to analyze the patient's explanted heart and corroborate the presence of idiopathic myocardial ossification upon observing spongy bone segments with bone marrow in their interior. We believe this report encourages the study of intra-myocardial calcification. Even though we do not understand its etiology, we have a clear idea of the myocardial ossification's behavior that had not been previously studied in depth. The patient's clinical course was favorable and she is currently asymptomatic.