The aim of this study is to determine the incidence, associated factors, and 30-day mortality of patients with heart failure (HF) after ST elevation myocardial infarction (STEMI) in Peru.
MethodsObservational, cohort, multicentre study was conducted at the national level on patients enrolled in the Peruvian registry of STEMI, excluding patients with a history of HF. A comparison was made with the epidemiological characteristics, treatment, and 30 day-outcome of patients with (Group 1) and without (Group 2) heart failure after infarction.
ResultsOf the 388 patients studied, 48.7% had symptoms of HF, or a left ventricular ejection fraction <40% after infarction (Group 1). Age>75 years, anterior wall infarction, and the absence of electrocardiographic signs of reperfusion were the factors related to a higher incidence of HF. The hospital mortality in Group 1 was 20.6%, and the independent factors related to higher mortality were age>75 years, and the absence of electrocardiographic signs of reperfusion.
ConclusionsHeart failure complicates almost 50% of patients with STEMI, and is associated with higher hospital and 30-day mortality. Age greater than 75 years and the absence of negative T waves in the post-reperfusion ECG are independent factors for a higher incidence of HF and 30-day mortality.
Se desea saber la incidencia, los factores asociados y la mortalidad a 30 días de los pacientes con insuficiencia cardiaca (IC) postinfarto de miocardio con elevación del segmento ST (IMCEST) en Perú.
MétodosEstudio observacional, de cohortes, multicéntrico a nivel nacional, de pacientes enrolados en el registro peruano de IMCEST, excluyendo los pacientes con antecedente de IC. Se compararon las características epidemiológicas, tratamiento y evolución a 30 días de los pacientes con (grupo 1) y sin (grupo 2) IC postinfarto.
ResultadosDe 388 pacientes se encontró un 48.7% con síntomas de IC o fracción de eyección de ventrículo izquierdo <40% postinfarto (grupo 1). La edad >75 años, el infarto de pared anterior y la ausencia de signos electrocardiográficos de reperfusión fueron los factores relacionados a mayor incidencia de IC. La mortalidad intrahospitalaria en el grupo 1 fue del 20.6% y los factores independientes relacionados a mayor mortalidad fueron la edad >75 años y la ausencia de signos electrocardiográficos de reperfusión.
ConclusionesLa IC complica casi al 50% de pacientes con IMCEST y está asociada a mayor mortalidad intrahospitalaria y a 30 días. La edad >75 años y la ausencia de ondas T negativas en el electrocardiograma posreperfusión son factores independientes de mayor incidencia de IC y de mortalidad a 30 días.
Heart failure (HF) is a clinical entity produced by several etiological agents, being the coronary heart disease the most frequent cause worldwide (70%).1 Symptomatic HF that complicates myocardial infarction (MI) occurs in approximately 15–25% of patients2–6 and is associated with an in-hospital mortality range of 15–40%.2–5
The prognosis after a MI will depend on the degree of left ventricular systolic dysfunction (LVSD) with or without symptomatic HF, the presence of recurrent ischemia and the degree of progression of coronary disease;7 being the LVSD the strongest predictor of mortality.8 This dysfunction has as substrate in the acute phase: the loss of myocardial cells and ventricular remodeling, besides the myocardial “stunning” and valve dysfunction.8
The Killip score has been used to predict higher mortality after infarction 9,10 and its simple applicability at the time of admission of the patient with MI makes this score an important prognostic tool.
Due to the high morbidity and mortality of HF, we want to evaluate its incidence in the context of ST-segment elevation myocardial infarction (STEMI), its epidemiological characteristics and factors associated with its presentation, as well as identify predictors of in-hospital mortality.
MethodsThe PERSTEMI registry 11 is a prospective, multicenter, observational registry of patients with STEMI, where a total of 396 patients were enrolled from February 2016 to February 2017 in the most important cities of Peru. For this report, patients with a history of heart failure prior to admission were excluded.
Patients were classified according to the presence (group 1), or absence (group 2) of heart failure (HF) which was defined by:
- (a)
Symptoms and signs compatible with heart failure according to Framingham criteria12 during hospitalization, or;
- (b)
Killip Classification >1 on admission or during hospitalization, or;
- (c)
Left ventricular ejection fraction (LVEF) less than or equal to 40% during hospitalization.
Data were collected about the current clinical history, cardiovascular past history and risk factors, as well as electrocardiogram (ECG) characteristics, in-hospital management, reperfusion therapy, adverse events during hospitalization and medication at hospital discharge in both groups.
The LVEF was evaluated by transthoracic echocardiography between 24 and 48h after admission; the method used for its assessment was the Simpson biplane method. Follow-up was done by telephone or by evaluating the records in the clinical history.
Statistic analysisQualitative variables are presented in frequencies and percentages and continuous variables as medians and interquartile range, except age (mean and standard deviation). The comparison between the groups was carried out with the chi-square test and student's t test for discrete and continuous variables, respectively. A value of p<0.05 was considered statistically significant. Multiple logistic regression analysis was performed for the evaluation of variables associated with a greater possibility of developing heart failure due to MI and for the evaluation of in-hospital mortality in the group with HF. The 30-day survival in both study groups was evaluated by Cox regression and plotted with Kaplan Meyer curves. All information was tabulated in Stata 14.0 program.
ResultsA total of 388 patients were evaluated. One hundred and eighty-nine patients (48.7%) met the pre-established criteria for heart failure after MI (group 1) and 199 patients (51.3%) without HF entered to group 2.
One hundred patients (26.03%) had HF symptoms during hospitalization, 130 patients (33.5%) arrived to emergency wards with symptoms of HF (KK II to IV) and 117 patients (31.03%) had an echocardiographic report with LVEF <40%.
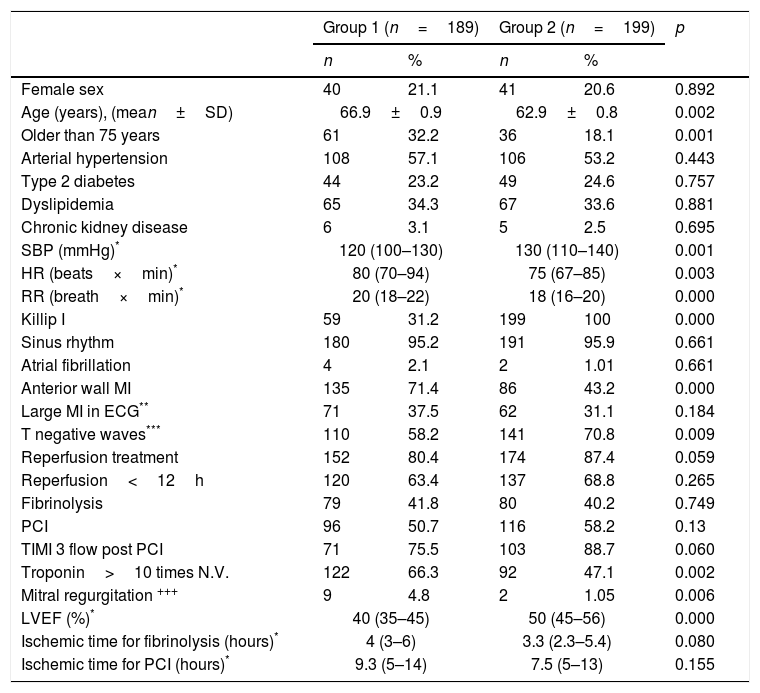
There were no differences according to sex, or the presence of traditional coronary risk factors (hypertension, diabetes mellitus, dyslipidemia, renal failure) between the two groups (Table 1); but the average age was significantly higher in the population with HF (66.9 versus 62.9 years, p=0.002).
Characteristics of the patients.
| Group 1 (n=189) | Group 2 (n=199) | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Female sex | 40 | 21.1 | 41 | 20.6 | 0.892 |
| Age (years), (mean±SD) | 66.9±0.9 | 62.9±0.8 | 0.002 | ||
| Older than 75 years | 61 | 32.2 | 36 | 18.1 | 0.001 |
| Arterial hypertension | 108 | 57.1 | 106 | 53.2 | 0.443 |
| Type 2 diabetes | 44 | 23.2 | 49 | 24.6 | 0.757 |
| Dyslipidemia | 65 | 34.3 | 67 | 33.6 | 0.881 |
| Chronic kidney disease | 6 | 3.1 | 5 | 2.5 | 0.695 |
| SBP (mmHg)* | 120 (100–130) | 130 (110–140) | 0.001 | ||
| HR (beats×min)* | 80 (70–94) | 75 (67–85) | 0.003 | ||
| RR (breath×min)* | 20 (18–22) | 18 (16–20) | 0.000 | ||
| Killip I | 59 | 31.2 | 199 | 100 | 0.000 |
| Sinus rhythm | 180 | 95.2 | 191 | 95.9 | 0.661 |
| Atrial fibrillation | 4 | 2.1 | 2 | 1.01 | 0.661 |
| Anterior wall MI | 135 | 71.4 | 86 | 43.2 | 0.000 |
| Large MI in ECG** | 71 | 37.5 | 62 | 31.1 | 0.184 |
| T negative waves*** | 110 | 58.2 | 141 | 70.8 | 0.009 |
| Reperfusion treatment | 152 | 80.4 | 174 | 87.4 | 0.059 |
| Reperfusion<12h | 120 | 63.4 | 137 | 68.8 | 0.265 |
| Fibrinolysis | 79 | 41.8 | 80 | 40.2 | 0.749 |
| PCI | 96 | 50.7 | 116 | 58.2 | 0.13 |
| TIMI 3 flow post PCI | 71 | 75.5 | 103 | 88.7 | 0.060 |
| Troponin>10 times N.V. | 122 | 66.3 | 92 | 47.1 | 0.002 |
| Mitral regurgitation +++ | 9 | 4.8 | 2 | 1.05 | 0.006 |
| LVEF (%)* | 40 (35–45) | 50 (45–56) | 0.000 | ||
| Ischemic time for fibrinolysis (hours)* | 4 (3–6) | 3.3 (2.3–5.4) | 0.080 | ||
| Ischemic time for PCI (hours)* | 9.3 (5–14) | 7.5 (5–13) | 0.155 | ||
ECG: electrocardiogram, HR: heart rate, LVEF: left ventricular ejection fraction, N.V.: normal value, PCI: percutaneous coronary intervention, RR: respiratory rate, SAP: systolic blood pressure.
In the group 1, 120 patients (63.4%) had LVEF≤40%, and 36.6% of cases with symptomatic HF had LVEF>40%. It was also found that 31 patients (16.4%) had only LVEF≤40% without symptoms (left ventricular asymptomatic dysfunction). Six patients (3.2%) of group 1 had right ventricle infarction.
A 31.2% of patients in the group 1 were admitted to emergency without symptoms of HF (Killip I), on this group 23.7% were older than 75 years and 74.5% had anterior location's MI (OR 3.8, CI: 2–7.3, p=0.000).
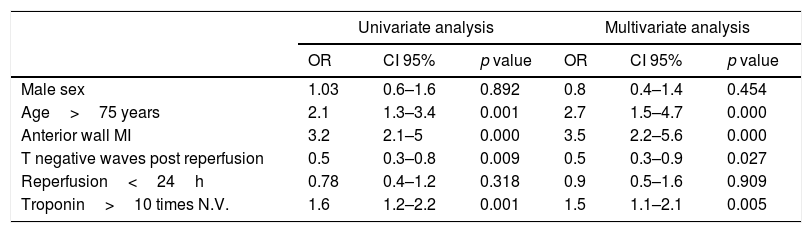
A high percentage of patients in group 1 were older than 75 years, the infarction involved the anterior wall of the left ventricle, had higher troponin values and did not have negative T waves after reperfusion in the EKG compared with patients without HF (Tables 1 and 2).
Uni and multivariate logistic regression analysis of heart failure predictors post STEMI.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | CI 95% | p value | OR | CI 95% | p value | |
| Male sex | 1.03 | 0.6–1.6 | 0.892 | 0.8 | 0.4–1.4 | 0.454 |
| Age>75 years | 2.1 | 1.3–3.4 | 0.001 | 2.7 | 1.5–4.7 | 0.000 |
| Anterior wall MI | 3.2 | 2.1–5 | 0.000 | 3.5 | 2.2–5.6 | 0.000 |
| T negative waves post reperfusion | 0.5 | 0.3–0.8 | 0.009 | 0.5 | 0.3–0.9 | 0.027 |
| Reperfusion<24h | 0.78 | 0.4–1.2 | 0.318 | 0.9 | 0.5–1.6 | 0.909 |
| Troponin>10 times N.V. | 1.6 | 1.2–2.2 | 0.001 | 1.5 | 1.1–2.1 | 0.005 |
MI: myocardial infarction, N.V.: normal value.
The median LVEF of group 1 was 40% (IQR 35–45%) and in group 2 was 50% (IQR 45–56%) (p=0.000), more cases of severe mitral regurgitation were observed in group 1 (4.8% versus 1.05%, p=0.006).
A 50.7% of patients of group 1 and 58.2% of group 2 had coronary angiograms for reperfusion treatment of STEMI (p=0.138). Among patients who underwent coronary angiography, there was a tendency to lower TIMI 3 coronary flow post intervention in group 1 than in group 2 (75.5% versus 88.7%, p=0.060). We did not find differences in the time of ischemia to reperfusion, or in the reperfusion strategy (fibrinolysis versus angioplasty) between the 2 groups. Pharmaco-invasive strategy was used in 25 patients in group 1 (26.04%) and in 26 patients of group 2 (22.41%).
In patients undergoing PCI (primary or pharmaco-invasive) we found that the anterior descending coronary artery was the infarct related artery (IRA) in 73.9% of patients in group 1 and in 42.2% in group 2; the right coronary artery was the IRA in 14.5% of patients in group 1 and 49.1% in group 2 (p=0.000). More than one coronary artery with severe obstruction was found in 55.8% of patients in group 1 and 53.4% in group 2 (p=0.734). Another PCI besides of the IRA, were done in 33.7% of cases in group 1 and 35.5% of cases in group 2 (p=0.809) mainly deferred before discharge from the hospital (65% in group 1 and 90% in group 2). Drug eluting stent was used in 62.5% of cases in group 1 and 58.6% in group 2 (p=0.701).
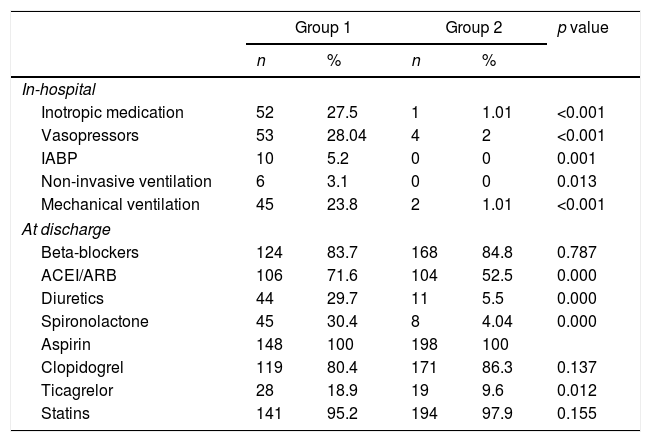
Regarding the in-hospital treatment, there was a greater percentage of inotropic use, invasive ventilation and intra-aortic balloon counterpulsation (IABP) in group 1 (Table 3), as well as greater use of angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blocker (ARBs), diuretics and spironolactone at discharge, there was no difference in the percentage of use of beta blockers. In patients with LVEF<40%, beta blockers were used in 85% of cases, ACEI/ARBs in 82.9% and spironolactone in 45.4%.
In-hospital treatment and discharge medication in both study groups.
| Group 1 | Group 2 | p value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| In-hospital | |||||
| Inotropic medication | 52 | 27.5 | 1 | 1.01 | <0.001 |
| Vasopressors | 53 | 28.04 | 4 | 2 | <0.001 |
| IABP | 10 | 5.2 | 0 | 0 | 0.001 |
| Non-invasive ventilation | 6 | 3.1 | 0 | 0 | 0.013 |
| Mechanical ventilation | 45 | 23.8 | 2 | 1.01 | <0.001 |
| At discharge | |||||
| Beta-blockers | 124 | 83.7 | 168 | 84.8 | 0.787 |
| ACEI/ARB | 106 | 71.6 | 104 | 52.5 | 0.000 |
| Diuretics | 44 | 29.7 | 11 | 5.5 | 0.000 |
| Spironolactone | 45 | 30.4 | 8 | 4.04 | 0.000 |
| Aspirin | 148 | 100 | 198 | 100 | |
| Clopidogrel | 119 | 80.4 | 171 | 86.3 | 0.137 |
| Ticagrelor | 28 | 18.9 | 19 | 9.6 | 0.012 |
| Statins | 141 | 95.2 | 194 | 97.9 | 0.155 |
ACEI: angiotensin-converting enzyme inhibitors, ARB: angiotensin receptor blocker, IABP: intra-aortic balloon counterpulsation.
Hospital stay was slightly longer in group 1 (7 days, IQR: 4–12 days) than in group 2 (6 days, IQR: 5–8 days) p=0.000.
In-hospital cardiovascular mortality was 20.6% in the group 1 (39 patients) and 0.5% in group 2 (p=0.000). Mortality in patients with symptomatic HF was 33% versus 2.1% in asymptomatic patients (p<0.0001). The most frequent cause of death in group 1 was cardiogenic shock (74.3%) followed by arrhythmic death (15.4%). In patients with cardiogenic shock: 16% was due to mechanical complication and in 73.8% of cases the EKG post reperfusion showed persistent T positive waves in infarct related leads reflecting non myocardial cell reperfusion in spite of good angiographic results.
The rate of re-infarction, ventricular tachycardia or ventricular fibrillation and severe pericardial effusion was higher in group 1 than in group 2 (4.2% versus 0.5%, p=0.015, 12% versus 1.5%, p=0.000 and 6.8% versus 1.5%, p=0.008 respectively).
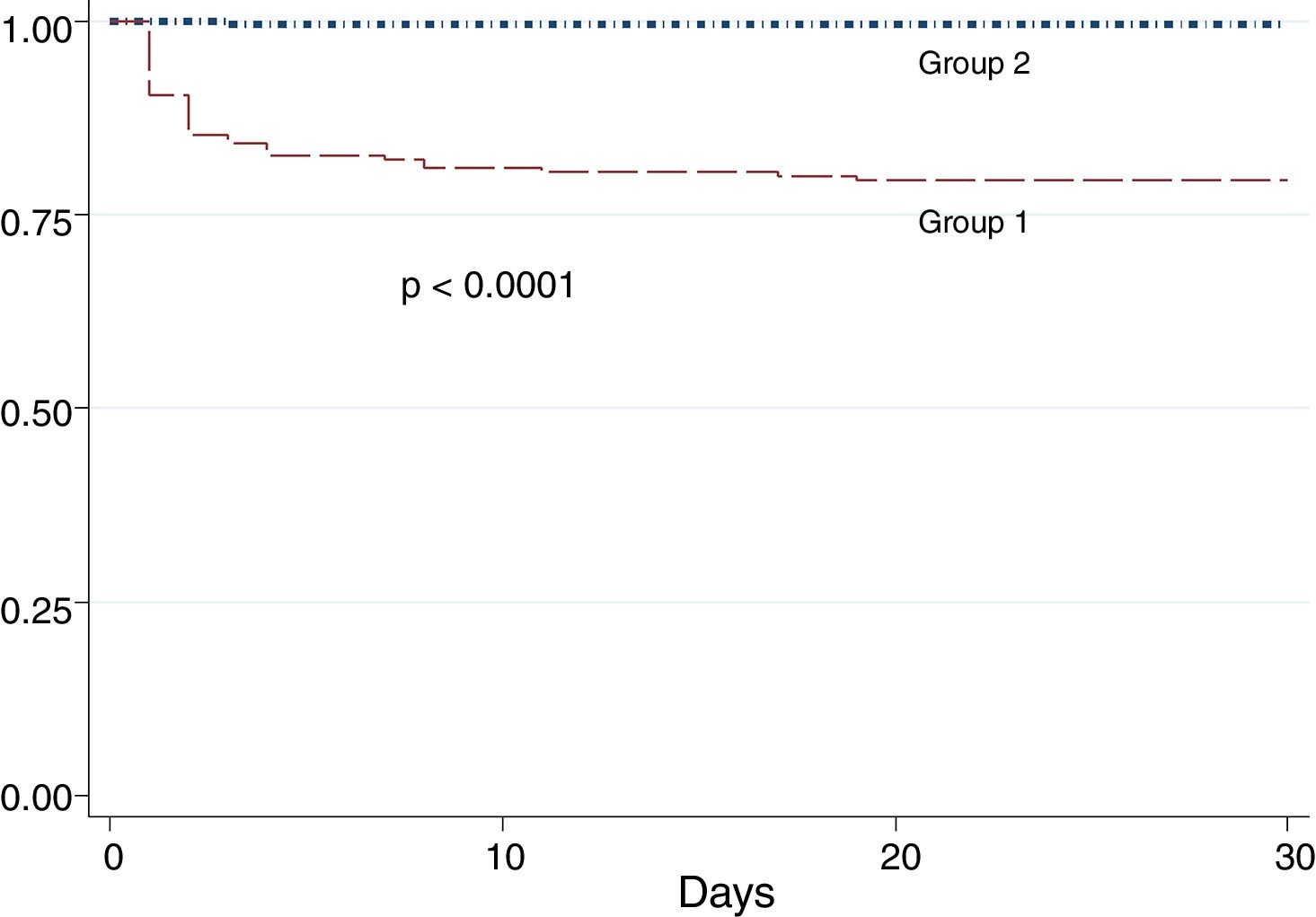
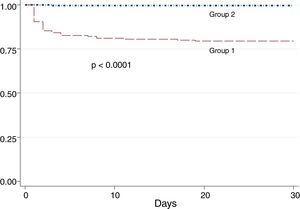
Patients of group 1 had more in-hospital mortality (OR: 1.45; 95% CI: 1.15–1.82, p<0.001) and worst 30-day survival (Fig. 1).
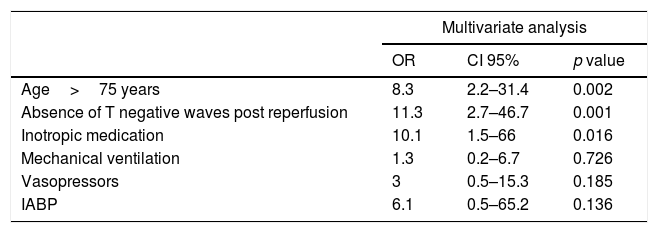
The factors related to higher mortality in patients of group 1 in the first 30 days from admission are shown in Table 4.
Predictors of in-hospital and 30-day mortality in group 1.
| Multivariate analysis | |||
|---|---|---|---|
| OR | CI 95% | p value | |
| Age>75 years | 8.3 | 2.2–31.4 | 0.002 |
| Absence of T negative waves post reperfusion | 11.3 | 2.7–46.7 | 0.001 |
| Inotropic medication | 10.1 | 1.5–66 | 0.016 |
| Mechanical ventilation | 1.3 | 0.2–6.7 | 0.726 |
| Vasopressors | 3 | 0.5–15.3 | 0.185 |
| IABP | 6.1 | 0.5–65.2 | 0.136 |
IABP: intra-aortic balloon counterpulsation.
According to Killip classification on admission, in-hospital mortality was 3.1, 17.8, 27.2 and 61.1% in Killip I, II, III and IV respectively.
The univariate analysis of therapies administered in the intensive care unit, showed that only the use of noradrenaline was related to higher mortality in group 1 (OR 6.2, CI: 1.3–28, p=0.018), although it is true that the need for invasive mechanical ventilation, IABP and inotropic use was associated with higher mortality, these associations were not statistically significant (OR 2.2, CI: 0.5–9.4, p=0.281, OR 6, CI: 0.6–59.7, p=0.124 and OR 4.3, CI: 0.8–21.9, p=0.079 respectively).
DiscussionIn this study, we found an incidence of HF post STEMI of 48.7%, higher than that found by the FAST-MI researchers; who with the same criteria for defining the group with HF, found an incidence of 37.5%,13 this difference may due in part to the inclusion of patients with non ST-segment elevation MI in the French study (with a lower incidence of HF in this sub-group patients). In addition, in our study, a largest number of patients were treated in national referral centers, which probably increased the number of more ill patients, with symptomatic HF since admission (68.8% versus 60.9% in the French study).13
HF and left ventricular systolic dysfunction (LVEF less than or equal to 40%) should not be considered as synonyms14 because many patients with LVSD may be asymptomatic (asymptomatic left ventricular dysfunction); in our study, this percentage reached 16.4% in group 1. Similarly, 30–50% of patients with HF do not have LVSD15,16; but in both cases the morbidity and mortality are increased.17 In our study, up to 36.5% of patients presented HF symptoms with preserved LVEF (>40%).
Unlike some registries, where the female sex was more prone to develop HF in the context of a MI,18,19 we did not find difference in the incidence of HF according to sex (OR 1.03 95%, CI 0.6–1.6, p=0.892).
Regarding the incidence of symptomatic HF in the first 28 days after MI, some authors have found values of up to 22.4% of cases, more frequent in the elderly, women, diabetics and hypertensive patients; its presence was associated with a longer hospital stay and a higher mortality rate at 1-year and 10 years (4% year and 24.6% 10 years).20
Rich et al. found that HF during myocardial infarction was present in 33% of people younger than 70 years old and in 56% of those older than 70 years.19 Similar to our results where the highest incidence of HF was in population older than 75 years.
Steg et al. found that 46.2% of HF patients had an anterior wall infarction, compared with 33.6% in those without HF (p<0.0001), as well as more Q waves on ECG, left bundle branch block and atrial fibrillation10; they also found that advanced age, high heart rate, diabetes and previous diuretic use were independent predictors of HF.10 We also found that the anterior wall MI was related to more HF. Although there was a trend for higher incidence of HF in large infarcts (extensive anterior, infero-postero-lateral), it was not significant (unlike the definition of large infarction by troponin value >10 times the normal range).
The use of ACEI and beta-blockers early in the context of MI showed a decrease in mortality.21–25 In PRIAMHO II study 55.9% of patients received beta-blockers and 45.1% ACEI at discharge, but in patients with HF (Killip>1 or LVEF<40%) only 25.4 received both drugs at discharge and had a mortality rate of 57.4% per year.26 We found that the percentages of use of both drugs are above 70% in patients of group 1; this may be due to the fact that patients were treated in national teaching hospitals with a high rate of use of medication according to international guidelines. Some national reports,27 observed that beta-blockers use in patients with HF at the time of decompensation is only 31.7%, which leads us to infer a discontinuation of its use after the MI.
The use of aldosterone inhibitors together with ACEI prevents left ventricular remodeling.28,29 López de Sá et al.,30 found that it was prescribed in 54.8% of patients with the indication for its use and it was found associated with greater survival at 30 days compared to untreated patients (88.3% and 77.7% respectively). We found that the use of spironolactone in group 1 reached only 30%, although in patients with LVEF<40% it was used in up to 45% of cases.
Many reports since last century, correlates post MI heart failure with higher 1-year mortality, reaching up to 40% (HF group) versus 9% (without HF).19,31 The in-hospital mortality found by Steg et al.,10 was 12% in patients with HF versus 2.9% without HF (OR 4.6, 95% CI: 3.8–5.4) lower than our study (20.6%). The higher mortality found by us, may be due to the higher average age and the lack of adequate reperfusion of the myocardium, evidenced by the lower percentage of negative T waves in the electrocardiogram after the application of reperfusion therapy.32
The Killip score at admission is a well-known predictor of short and long-term risk10,33; Vicent et al., found that in-hospital mortality increased according to the Killip class (1.5; 3.7; 16.7 and 36.7% for KK I, II, III and IV, respectively)32 similar to our study, although in cases with Killip IV our mortality was notoriously higher (61.1 versus 36.7%).
The use of inotropes in patients with acute heart failure has been related to higher mortality in the ADHERE study34; in the multivariate analysis, in our study, it was associated with higher in-hospital mortality, although not statistically significant, unlike the use of noradrenaline, which indicates that our patients could be in more severe states of HF (cardiogenic shock) with consequent higher mortality.
The importance of adequate and timely myocardial reperfusion is vital to reduce mortality and the development of HF; Comparing with the SwedeHeart registry35 that found reperfusion rates greater than 80%, our reperfusion rate in the registry was only 67% in the first 12h of the infarction,11 (successful in less than 50% of fibrinolysis and up to 80% of primary PCI) which explains the high percentage of patients in group 1. We did not find differences in ischemic time to reperfusion in both groups as long as the reperfusion has been done in the first 12h of symptoms onset.
LimitationsThe present study has several limitations: being a registry, causality associations are not as strong as one would expect from a randomized study. A high percentage of patients were enrolled in the capital city and in teaching hospitals, which may include a bias in the degree of severity of the disease and the management of patients, so it could be unrepresentative of the reality of the country.
ConclusionsHeart failure complicates almost 50% of patients with STEMI and is associated with higher in-hospital and 30-days mortality. Age greater than 75 years, anterior wall MI, troponin more than 10 times the normal value and the absence of negative T waves in the post-reperfusion ECG are independent factors for higher incidence of HF. Also age>75 years and the absence of negative T waves are independent predictors for in-hospital and 30-day mortality.
FundingNone declared.
Conflict of interestThe authors declare no conflict of interest.
The PERSTEMI investigators: Alejandro Vega, Patricia Ríos, Roberto Baltodano, Fernando Villanueva, Alexander Montesinos, Jorge Martos, John Zevallos, David Miranda, Jorge Gutiérrez, José Carasas, Alexander Pecho, Sandra Negrón, Henry Anchante, Nassip Llerena, Germán Yábar, Javier Chumbe, Sara Ramírez, Marco Lazo, Jorge Sotomayor y Marco López.