There are several excellent alternatives to warfarin on the horizon for atrial fibrillation. Results from the trials, as well as pharmacokinetic data from the edoxaban studies, suggest that dose selection, based on pharmacokinetic and pharmacodynamic properties, is a critical component in the development of novel anticoagulants. Greater flexibility in dosing with edoxaban and the opportunity for dose adjustment throughout the ENGAGE AF-TIMI 48 trial may be advantageous in the competitive field of novel oral anticoagulants.
Existen excelentes alternativas al tratamiento con warfarina en el campo de la fibrilación auricular. Los resultados de los ensayos con RE-LY, ROCKET-AF y ARISTOTLE, así como los datos fármaco cinéticos obtenidos en estudios con edoxaban, sugieren que la selección de la dosis, basada en propiedades fármaco cinéticas y fármaco dinámicas, es un componente crítico en el desarrollo de nuevos anticoagulantes. Una mayor flexibilidad en establecer la dosis de edoxaban y la oportunidad de ajustar la dosis mediante ENGAGE AF-TIMI 48, puede constituir una ventaja en el campo de los nuevos anticoagulantes orales.
One of the biggest challenges with any novel oral anticoagulant is finding the therapeutic range for which the risk does not outweigh the benefit. With warfarin, the optimal INR range for most indications is 2.0-3.0. If the INR is <1.8 in patients with atrial fibrillation, the risk of ischemic stroke is increased, while INRs much above 3.0 are associated with an increased risk of intracranial bleeding.1
When developing a novel oral anticoagulant, there are several ideal properties that would be desirable. Once-daily vs twice-daily dosing is preferable to maximize compliance. Additionally, minimal food-drug and drug-drug interactions would simplify dosing. A drug with a predictable anticoagulant effect eliminates the need for coagulation monitoring. A drug with extra renal clearance enables patients with mild to moderate renal disease to take the anticoagulant safely. A rapid onset of action eliminates the need for bridging anticoagulant therapy (e.g., heparin), and a rapid offset in action simplifies management in case of bleeding or the need for an invasive procedure. It is also advantageous to have an antidote available to reverse the anticoagulation effect in case of emergencies. Clearly, selecting the right dose and regimen is a critical part of the development of an anticoagulant.
There are currently 5 novel oral anticoagulants in late stage clinical development: dabigatran, rivaroxaban, apixaban, edoxaban and betrixaban.2–4 Each of these drugs is a Factor Xa inhibitor, except for dabigatran, which is a Factor IIa inhibitor. These drugs share some pharmacokinetic (PK) properties. They all have a rapid onset of action and most are substrates of the P-gp transporter. However, there are several PK differences between these drugs, most importantly related to dosing. The bioavailability of the drugs ranges from 7% (dabigatran) to 80% (rivaroxaban). Betrixaban has minimal renal clearance (< 5%), while dabigatran has 80% renal elimination. Lastly, there is variability in the half-lives ranging from 8-10hours (edoxaban) to 19-20hours (betrixaban).
It is also important to consider PK data in specific subgroups of patients with AF. PK data in patients treated with dabigatran show that a reduction in creatinine clearance results in higher plasma concentration of dabigatran, which is associated with a prolongation of the aPTT.5 PK models used in clinical trial simulations with edoxaban showed a similar effect.6 In addition, edoxaban levels are increased in patients treated concomitantly with strong P-gp inhibitors such as verapamil, quinidine and dronedarone, and in patients of low body weight (≤ 60 Kg). These differences in pharmacokinetic properties are important considerations when selecting the dose(s) of a novel oral anticoagulant.
Phase II dose-ranging studies have been conducted with dabigatran, rivaroxaban, apixaban, edoxaban, and betrixaban to evaluate safety and explore possible efficacy trends across various doses. The phase II studies helped guide the selection of dose(s) to be studied in phase III. Dedicated phase II trials with dabigatran and edoxaban were performed in patients with atrial fibrillation (as well as in other patient populations) while no similar phase II dose-ranging studies in AF have been published with rivaroxaban or apixaban. In the dabigatran AF phase II study,5 more frequent bleeding was observed in the highest dose studied (300mg BID), while thromboembolic events were only observed in the lowest dose studied (50mg BID). Based on these data, a decision was made to move forward with the intermediate doses of 150mg BID and 110mg BID in the phase III AF trial. In the edoxaban phase II AF trial,7 no significant differences in efficacy rates were shown in any of the treatment groups. Significantly higher rates of bleeding were observed in the 30mg BID and 60mg BID doses while similar rates of bleeding compared to warfarin were seen with the 30mg QD and 60mg QD doses; therefore these latter 2 doses were chosen to be studied in the phase III trial.
Large phase III studies in atrial fibrillation have been completed for 3 drugs (dabigatran, rivaroxaban, apixaban). Of these 3 studies, only the RE-LY trial8 with dabigatran explored more than one dose of the novel anticoagulant. The higher dose (150mg BID) of dabigatran was shown to reduce stroke and had a similar rate of bleeding as warfarin. The lower dose (110mg BID) of dabigatran had a similar rate of stroke but reduced bleeding in comparison to warfarin. The ROCKET-AF trial9 studied rivaroxaban 20mg once-daily vs warfarin, with dose adjustment to 15mg in patients with a creatinine clearance of 30 to 49mL/min. The on-treatment analysis of rivaroxaban showed a reduction of stroke compared to warfarin and a similar rate of bleeding. The ARISTOTLE trial10 studied 5mg apixaban taken twice-daily. Only 5% of patients received a one-time dose reduction at randomization to 2.5mg twice-daily because they met 2 or more of the following criteria: ≥ 80 years old, body weight < 60 Kg, or a serum creatinine level ≥1.5mg/dl. Apixaban reduced hemorrhagic stroke compared to warfarin but did not affect ischemic stroke. Apixaban reduced all-cause mortality 3.5% vs 3.9% per year compared to warfarin (p=0.047). In addition, a lower rate of major bleeding was also observed with apixaban compared to warfarin. A substantial decrease (33%-70% relative) in intracranial hemorrhage was observed in all 3 drugs compared to warfarin.
Among the 5 novel oral anticoagulants, the development of edoxaban is unique in that a large phase IIb dose-ranging study7 was conducted, and multiple doses are being evaluated in phase III.3 In the phase IIb study,7 4 doses of edoxaban (30mg QD, 60mg QD, 30mg BID, 60mg BID) were compared to warfarin. The expected pattern of dose-related bleeding with edoxaban was observed (i.e., the higher the dose, the greater the bleeding). Of note, less bleeding was observed in the 30mg QD dose group compared to warfarin, while the 60mg QD dose and warfarin had similar rates of bleeding. However, the most intriguing finding was that less bleeding occurred with once-daily dosing of 60mg (7.3%) compared to 30mg twice-daily dosing (12.7%), despite the fact that the exact same total daily dose (60mg) was given. The two once-daily doses of 30mg QD and 60mg QD edoxaban are now being compared to warfarin in 21,105 patients enrolled in the double-blind, randomized phase III trial ENGAGE AF-TIMI 48.3
In detailed pharmacokinetic analyses of edoxaban, trough levels corresponded best with the rate of bleeding.6 Since once-daily dosing has lower trough values, this helps to explain the occurrence of less bleeding with the once-daily doses compared to twice-daily doses studied. This observation of less bleeding with once-daily regimens may seem counterintuitive, as the prevailing thought had been that peak levels of antithrombotic drugs might better predict bleeding. However, this finding regarding the superior safety profile of once-daily dosing of a factor Xa inhibitor (that achieve lower trough levels than twice-daily dosing) was further supported by the results of a dose-ranging phase II study with darexaban, known as RUBY-1.11 Six dose regimens of darexaban were studied, including three once-daily doses (10mg QD, 30mg QD, 60mg QD) and 3 comparable twice-daily doses (5mg BID, 15mg BID, 30mg BID). In all 3 comparisons of once-daily vs twice-daily dosing of the same total daily dose, there was a similar pattern of a lower rate of bleeding with the once-daily dose.11
Several factors are known to affect drug concentration such as decreased renal function, low body weight and concomitant medications that interfere with the metabolism of a drug. The ENGAGE AF-TIMI 48 study takes these factors into consideration and mandates a 50% edoxaban/placebo dose reduction if the creatinine clearance (CrCl) is below 50mL/min, weight is below 60kg, or there is concomitant use of a strong P-gp inhibitor (verapamil, quinidine, dronedarone).3,7,12 Whereas the ROCKET-AF and ARISTOTLE trials only allowed a dose reduction at the time of randomization, ENGAGE AF-TIMI 48 also mandates dynamic dose adjustment (up or down) if any of these factors change after randomization. Of note, the RE-LY trial did not incorporate any dose reduction in their trial design.
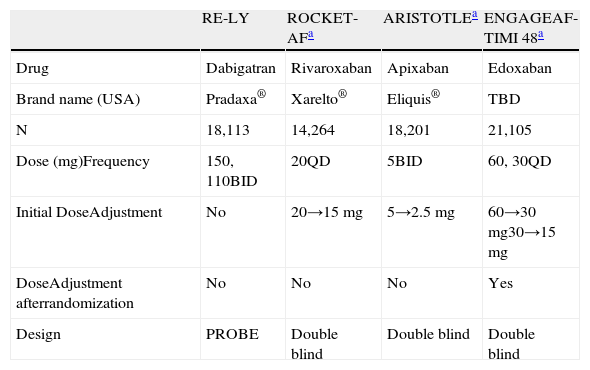
There are several other important differences in the way novel anticoagulants have been studied in each of the 4 large phase III trials (Table 1). The RE-LY trial was an open-label design while ROCKET-AF, ARISTOTLE and ENGAGE AF-TIMI 48 utilized a double-blind study design. In addition, whereas ROCKET-AF and ENGAGE AF-TIMI 48 studied once-daily doses, the RE-LY and ARISTOTLE trials studied twice-daily regimens. Two of the 4 trials, RE-LY and ENGAGE AF-TIMI 48, studied 2 different doses, however the dose differential in RE-LY was modest (110mg vs 150mg) compared to the 2-fold dose differential in ENGAGE AF-TIMI 48. Three of the 4 studies included a dose adjustment based on renal clearance, with ENGAGE AF-TIMI 48 being the only one of the 3 mandating dose adjustments post-randomization. Since the ENGAGE AF-TIMI 48 trial is randomizing to 2 dose levels of edoxaban, the additional reduced dose from 30mg to 15mg QD (in the case that a subject randomized to the low-dose regimen requires dose reduction due one of the factors described above) introduces a third dose group and creates a 4-fold differential from the lowest (15mg) to the highest (60mg) dose being evaluated.
Phase III AF Trials – Dose Comparisons.
| RE-LY | ROCKET-AFa | ARISTOTLEa | ENGAGEAF-TIMI 48a | |
| Drug | Dabigatran | Rivaroxaban | Apixaban | Edoxaban |
| Brand name (USA) | Pradaxa® | Xarelto® | Eliquis® | TBD |
| N | 18,113 | 14,264 | 18,201 | 21,105 |
| Dose (mg)Frequency | 150, 110BID | 20QD | 5BID | 60, 30QD |
| Initial DoseAdjustment | No | 20→15mg | 5→2.5 mg | 60→30 mg30→15 mg |
| DoseAdjustment afterrandomization | No | No | No | Yes |
| Design | PROBE | Double blind | Double blind | Double blind |
PROBE: prospective, randomized, open-label, blinded endpoint evaluation.
There are several excellent alternatives to warfarin on the horizon for atrial fibrillation. Results from the RE-LY, ROCKET-AF and ARISTOTLE trials, as well as pharmacokinetic data from the edoxaban studies, suggest that dose selection, based on pharmacokinetic and pharmacodynamic properties, is a critical component in the development of novel anticoagulants. Greater flexibility in dosing with edoxaban and the opportunity for dose adjustment throughout the ENGAGE AF-TIMI 48 trial may be advantageous in the competitive field of novel oral anticoagulants.