Introduction
Treatment of patients with previous coronary bypass graft (CABG) and significant obstructive disease in the saphenous vein graft (SVG) is a major therapeutic challenge.1,2
Obstructive lesions of the venous graft become apparent in 50 to 75% of patients at 10 years after surgery and only 40% of venous grafts are disease-free.3 Recurrent symptoms are secondary to progression of atherosclerosis in both native coronary vessels and grafts, becoming a common clinical manifestation. There are two therapeutic options available for patients requiring repeat revascularization (bypass) after surgery: repeating CABG or percutaneous intervention.1,2 A new revascularization surgery is associated with a higher risk of major complications (> 12% mortality), lesser improvement of angina, and reduced patency of the venous grafts.1,2 In this group of patients, disease progression in the native coronary vessels is 5% per year during the first 10 years.3 Percutaneous coronary intervention (PCI) is recommended only if feasible, mainly in patients with co-morbidities, left ventricular dysfunction, lack of available saphenous veins, and in the elderly. SVG stenting is an attractive alternative in patients without PCI option in the native vessels.4-6 A greater interval between surgery and SVG PCI reduces the technical success and is associated with a higher rate of early complications and reduced long-term patency.7
The goal of our study was to determine the rate of in-hospital and long-term major adverse cardiovascular events (MACE) in patients with previous CABG and stents implanted in native coronary vessels or in saphenous vein grafts.
Material and method
Patients
The study included patients older than 18 years, with a history of CABG performed more than 3 months before and with one or more "de novo" lesions in native coronary vessels or in a saphenous vein graft, and meeting clinical criteria for stable or unstable angina, or having documented silent ischemia. Angiographicaly, we included lesions > 50% and < 100%, determined by visual estimation, and larger than 2-mm in diameter. Exclusion criteria were: patients with acute myocardial infarct, SVG age of less than 3 months, total SVG occlusion, severe renal failure (creatinine > 3 mg/dl) or known allergy to the medication.
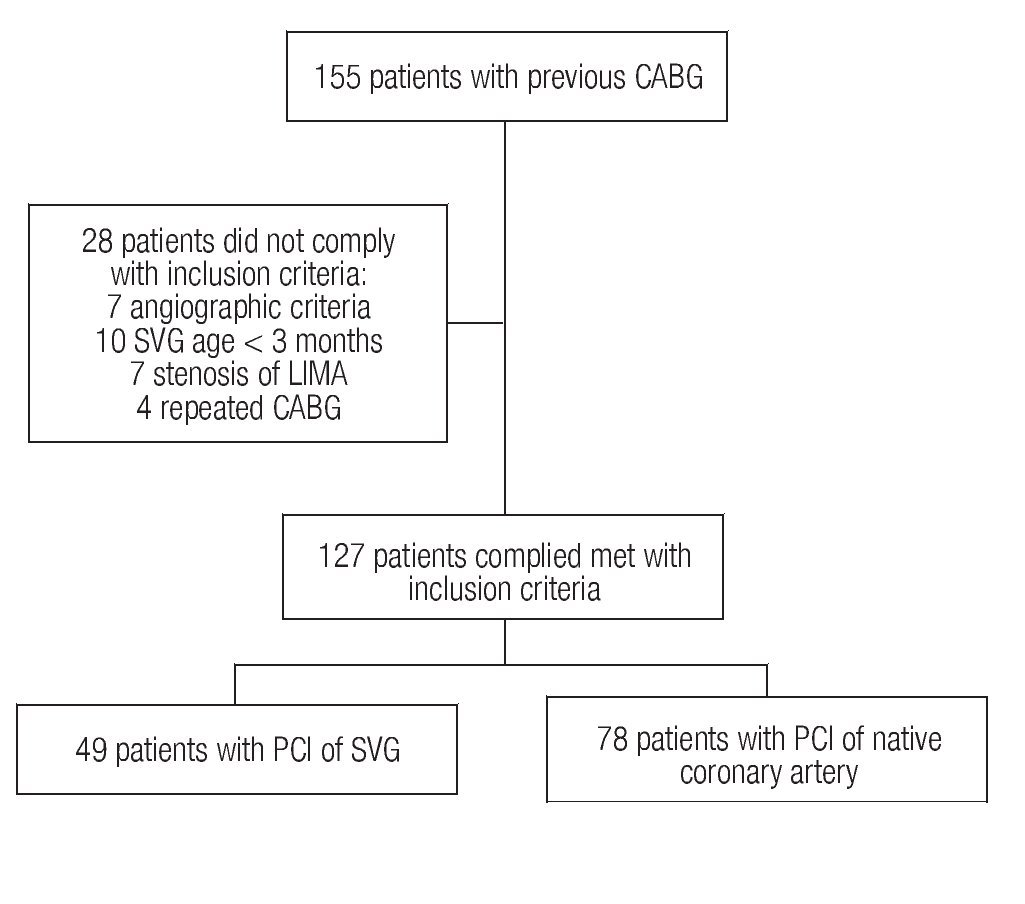
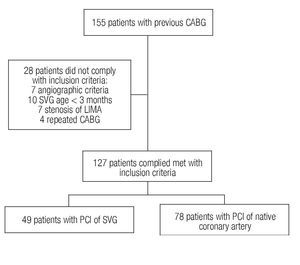
From January 1998 to December 2007, 155 PCIs were performed in patients with a history of coronary revascularization surgery. Seven patients were excluded based on angiographic criteria (total occlusion, intra-stent restenosis, or combined treatment), as well as 21 more due to age of the venous graft < 3 months (n = 10), stenosis of the internal mammary artery (n = 7), and four patients with repeated surgical treatment. Of the 127 patients fulfilling the inclusion criteria, 49 were subjected to percutaneous intervention of the saphenous vein (Group 1) and 78 to PCI of the native vessels (Group 2). Figure 1 describes patients' assignment.
Figure 1.Patients' assignment to the groups.
Intervention procedure
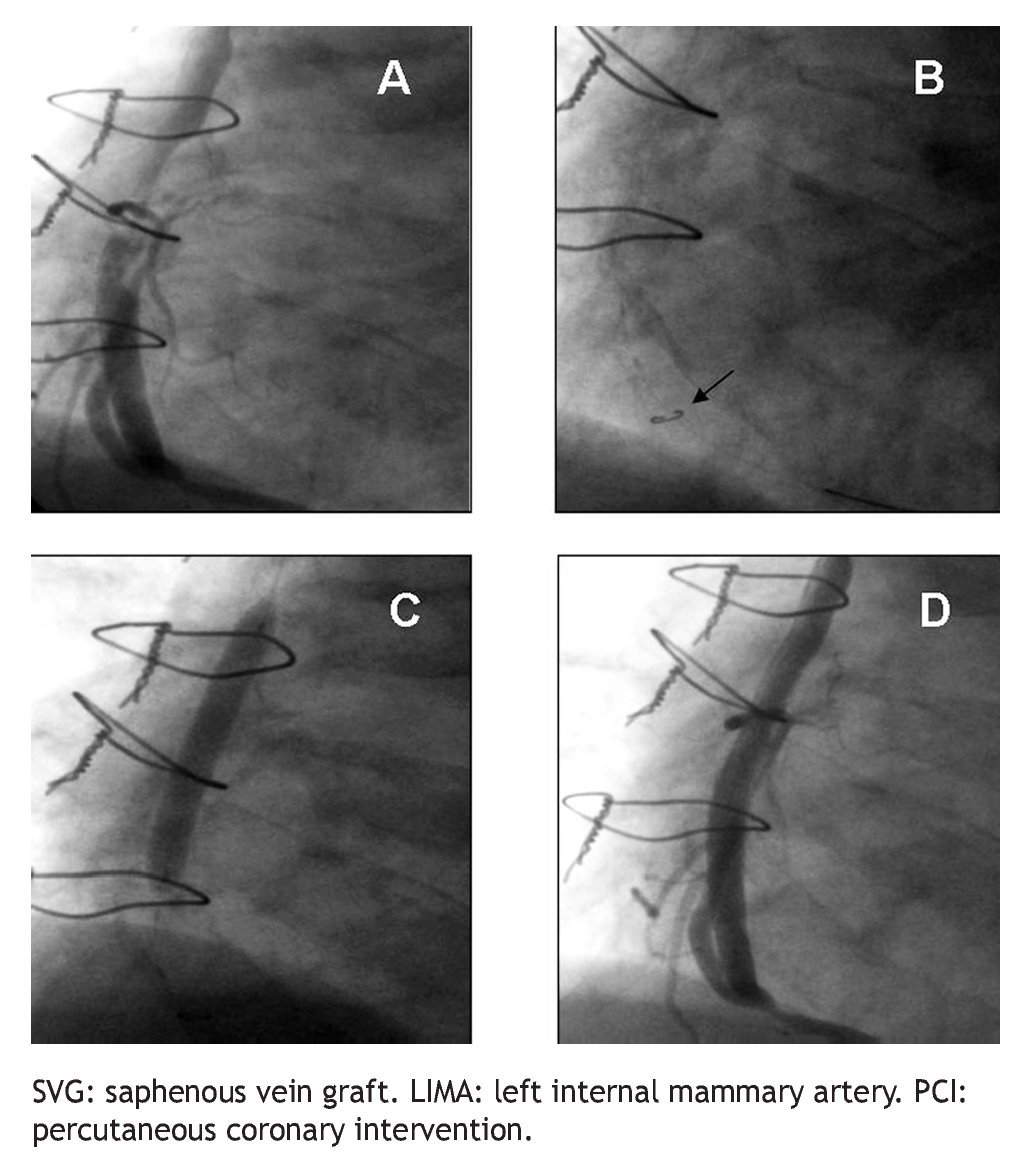
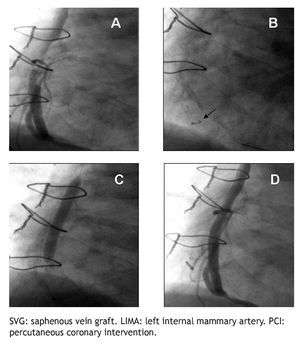
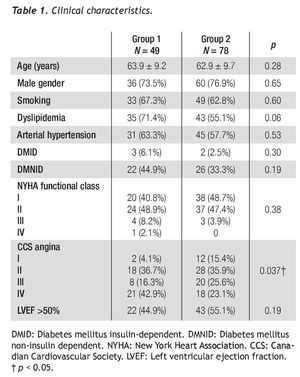
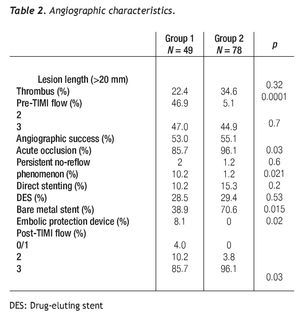
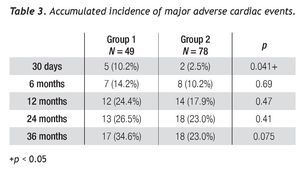
All PCIs were performed according to standard techniques. All patients received 300 to 600 mg of clopidogrel or ticlopidine (250 mg) and aspirin (100 mg) before the procedure; aspirin was continued indefinitely and clopidogrel (75 mg) for at least 3 months after the implantation of bare-metal stents and during 9 months after drug-eluting stent (DES) placement. Non-fractionated heparin was administered during the procedure to obtain an activated clotting time > 250 s. The operator decided the type of stent, the use of a distal protection device, the direct stenting technique, as well as the administration of glycoprotein IIb/IIIa inhibitors (Figure 2).
Figure 2 A.An eccentric lesion can be observed, with a thrombus in the middle segment of the saphenous vein graft to the posterior descending artery. B. Distal protection device (arrow). C. Drug-eluting stent implantation. D. Control angiogram showing and adequate stent position, TIMI 3 flow, and without no-reflow phenomenon or residual lesion.
End-points and definitions
The primary end-point of the study was to compare the incidence of in-hospital MACE as well as during the 3-year follow-up between patients subjected to PCI of native coronary vessels and those subjected to PCI of SVG. Secondary objectives were to analyze global long-term MACE-free survival, and without the need of target vessel revascularization (TVR).
Angiographic success was defined as a < 10% residual stenosis, TIMI 3 flow, absence of complex dissection or of a major thrombus. Clinical success was defined as the absence of major complications after the technical success. MACE was defined as the composite of cardiovascular death, myocardial infarct (MI), and TVR. Q wave MI was defined as the presence of new pathological Q waves in two or more contiguous leads and a creatinine kinase (CK-MB) rise > 3x the reference values. Non Q wave MI was considered when a higher than 3-fold CK-MB elevation was documented in the absence of new pathological Q waves in the ECG. Cardiac death was considered when it occurred as a consequence of MI, arrhythmias, or cardiac failure. TVR corresponded to a new intervention of a treated vessel, including previously treated lesions. No-reflow phenomenon was defined as the presence of TIMI 0/1 flow in the absence of a major thrombus, a complex dissection, spasm, or a significant residual lesion.
Statistical analysis
Data were compiled in a database. Continuous variables were expressed as percentage ± standard deviation (SD) and were assessed by non-paired t test. Categorical variables are described as proportions and were assessed by either the Chi square test or Fisher's exact test. The two-way p value was considered statistically significant at < 0.05. Logistic regression method was used to analyze the effect of the different variables on patient outcome. The included variables were smoking, arterial hypertension, dyslipidemia, statins use, left ventricular ejection fraction, angina according to the Canadian Society of Cardiology (CSC), NYHA functional class, use of direct stenting, no-reflow phenomenon, presence of definite thrombus, and lesion length. Correlation of long-term outcome was analyzed with Cox's proportional survival method, and the correlation of multiple variables with the long-term in-hospital MACE incidence was expressed as odds ratio (OR). The Kaplan-Meier method was used to estimate the MACE-free and TVR-free survival, compared with the log-rank test. All analyses were performed with the SPSS (version 13.0) software.
Results
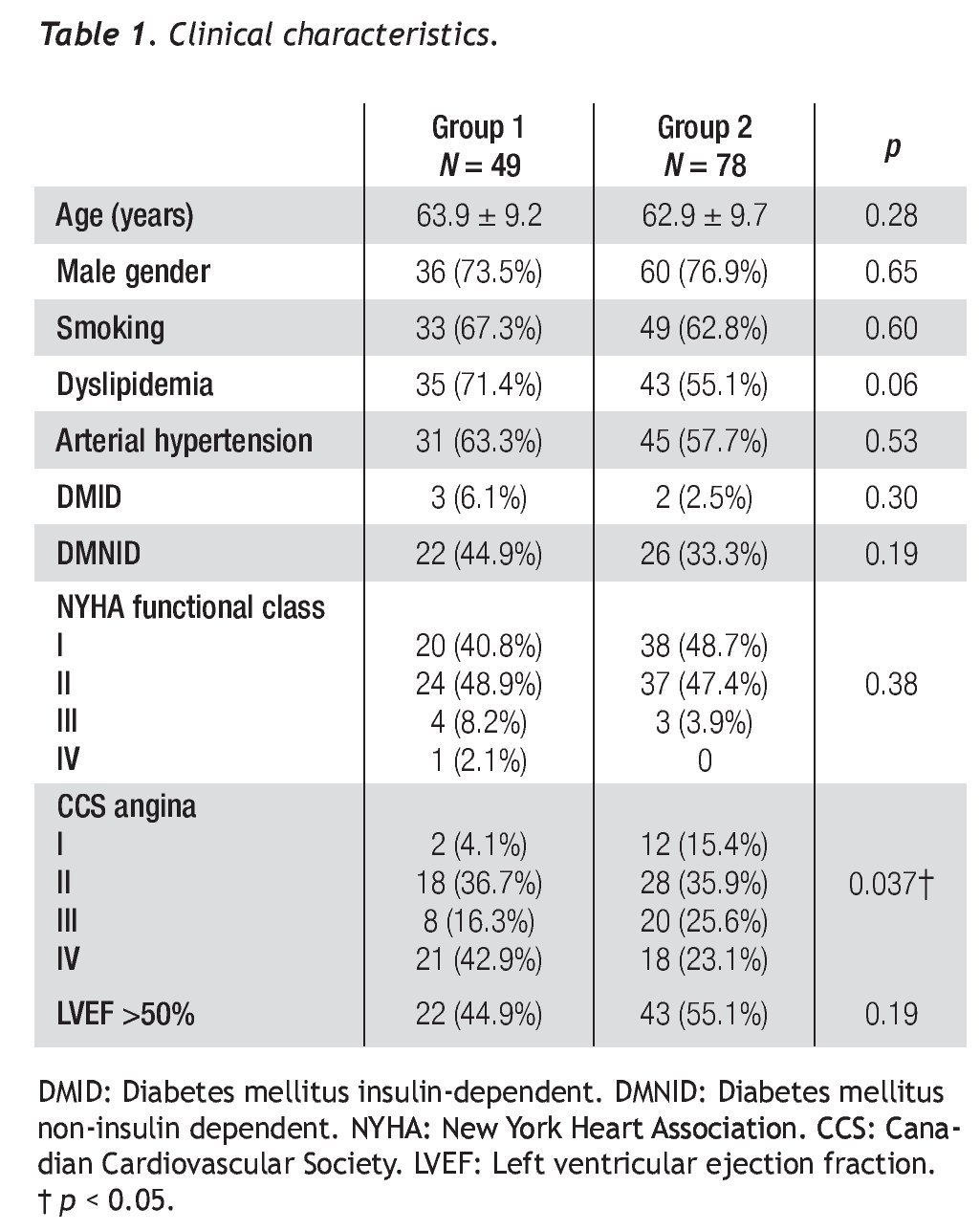
The clinical characteristics of both groups are presented in Table 1. Average age was 63.9 ± 9.2 years for group 1 and 62.9 ± 9.7 years for group 2 (p = 0.28). We observed no differences in risk factors, such as diabetes mellitus, arterial hypertension, smoking, and dyslipidemia between both groups. The ejection fraction and NYHA functional class were similar in both groups (p = 0.19 and p = 0.38). Angina class III/IV and CCS were more frequent in group 1 than in group 2 (59.2% vs. 48.7%, p = 0.026), and the time elapsed from CABG to PCI was greater in group 1 (8.7 ± 5.6 years vs. 5.7 ± 4.1 years, p = 0.001).
Characteristics of the procedure and end-points
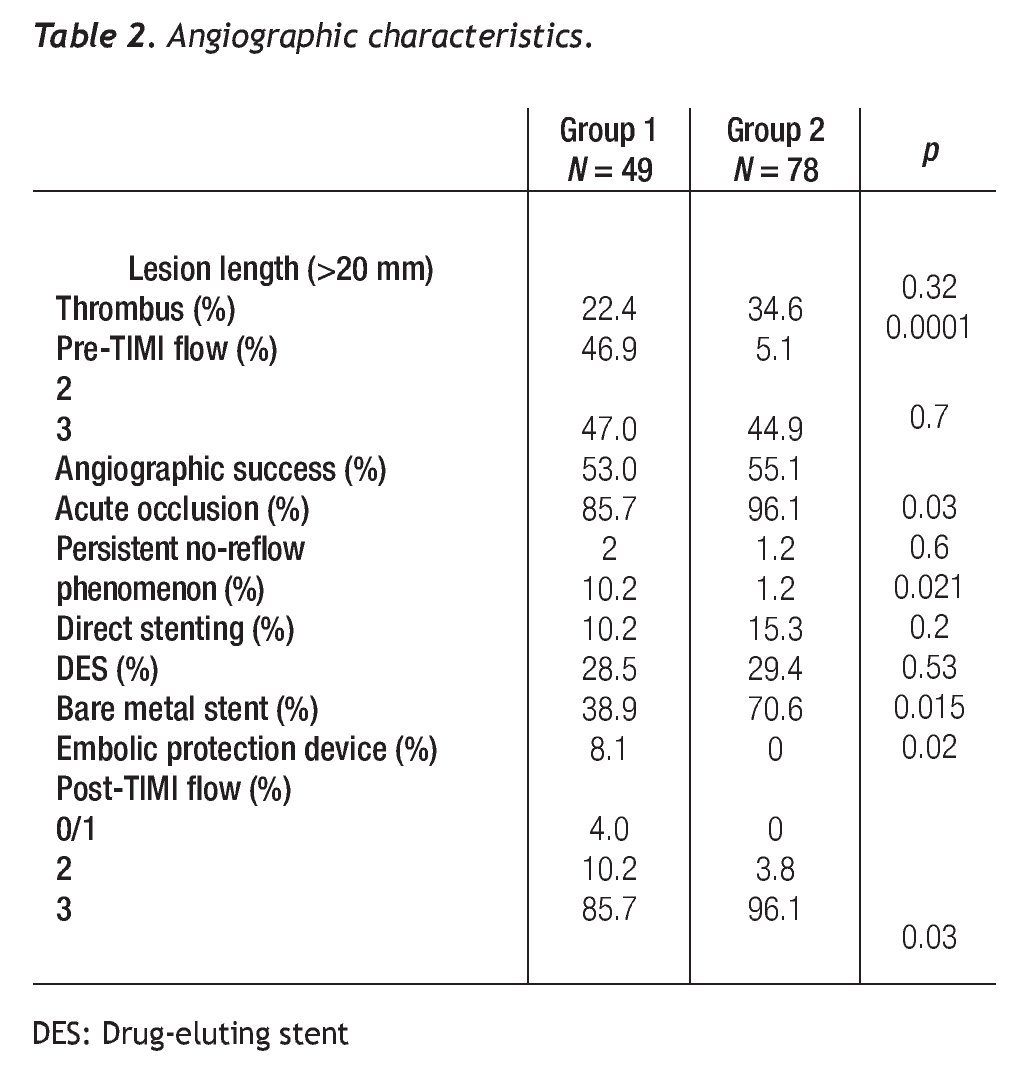
Angiographic success was better in group 2 (85.7% vs. 96.1%, p = 0.03), without differences in the rate of acute occlusion between both groups. IIb/IIIa inhibitors were used in 42.8% of patients with SVG intervention and in 11.5% of the native coronary artery intervention group. The number of treated lesions was 1.02 ± 0.24 for group 1 and of 1.47 ± 0.65 for group 2 (p=0.33). The average length of the lesion was similar in both groups (p = 0.32). The left anterior descending artery was treated in 55.9% of group-2 patients; of these, 70.4% had proximal segment involvement; in this group, a protected left main was treated percutaneously in 18 patients. In group 1, seven stents were covered with expandable polytetrafluoroethylene (PTFE) and nine with bovine pericardium, No differences existed between the groups in the number of lesions treated with drug-eluting stents (DES) and those with direct stenting. A distal protection device was used in 8.1% of group-1 patients (Table 2).
No-reflow phenomenon. The incidence of persistent no-reflow was higher in group 1 (10.2% vs. 1.2%, p = 0.021). The estimated risk of the no-reflow phenomenon when performing PCI of a SVG was OR = 11.0 [(95% IC, 2.32-52.14), p = 0.0001]. IIb/IIIa inhibitors showed no benefit in the SVG stenting group. No-reflow phenomenon was not observed when direct stenting was performed or in whom the distal protection device was used. In the population as a whole, the no-reflow phenomenon was an independent predictor of MACE at 36 months (OR 5.63, 95% IC 1.32 - 23.92, p = 0.019).
Clinical outcome
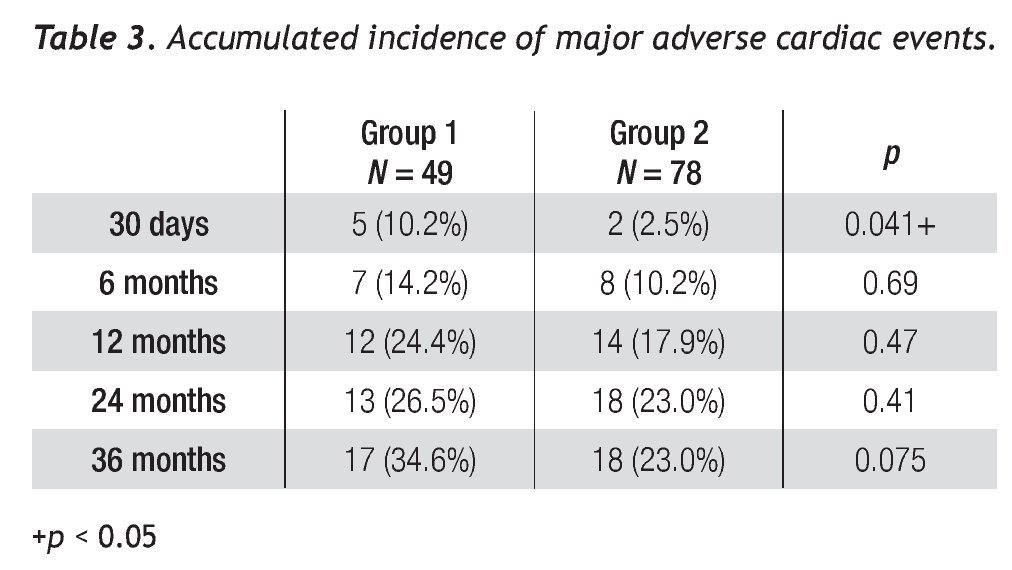
Average follow-up was 27.5 ± 22.2 and 33.6 ± 25.4 months for saphenous vein and native coronary vessel grafts, respectively. The accumulated MACE incidence is shown in Table 3; a difference was observed between groups at 1 month follow-up (10.2% vs. 2.5%, p = 0.041).
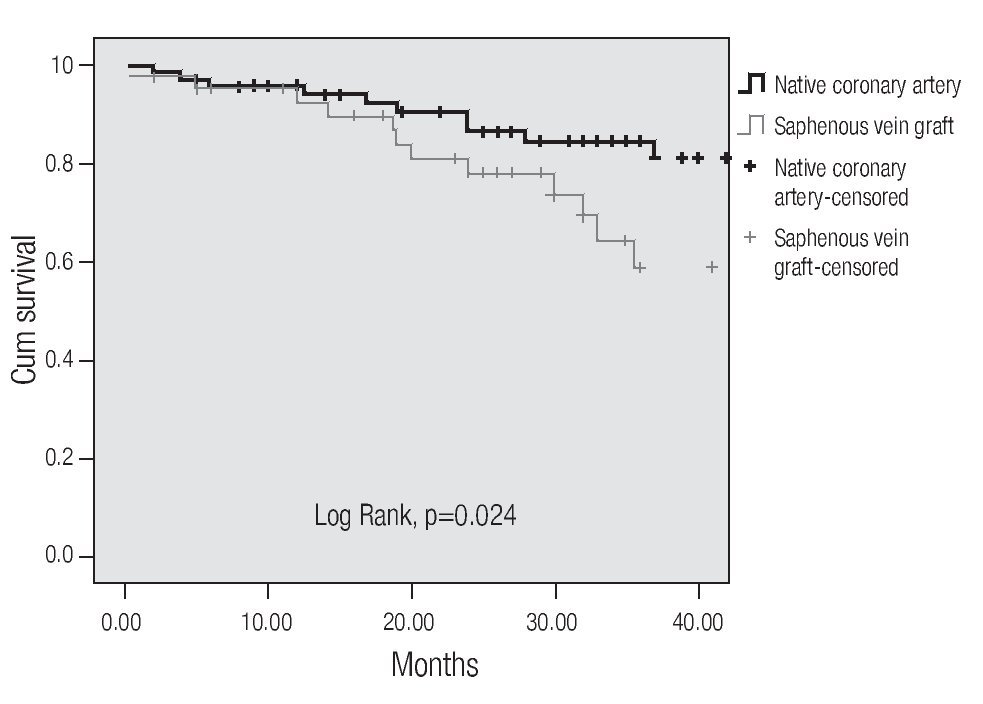
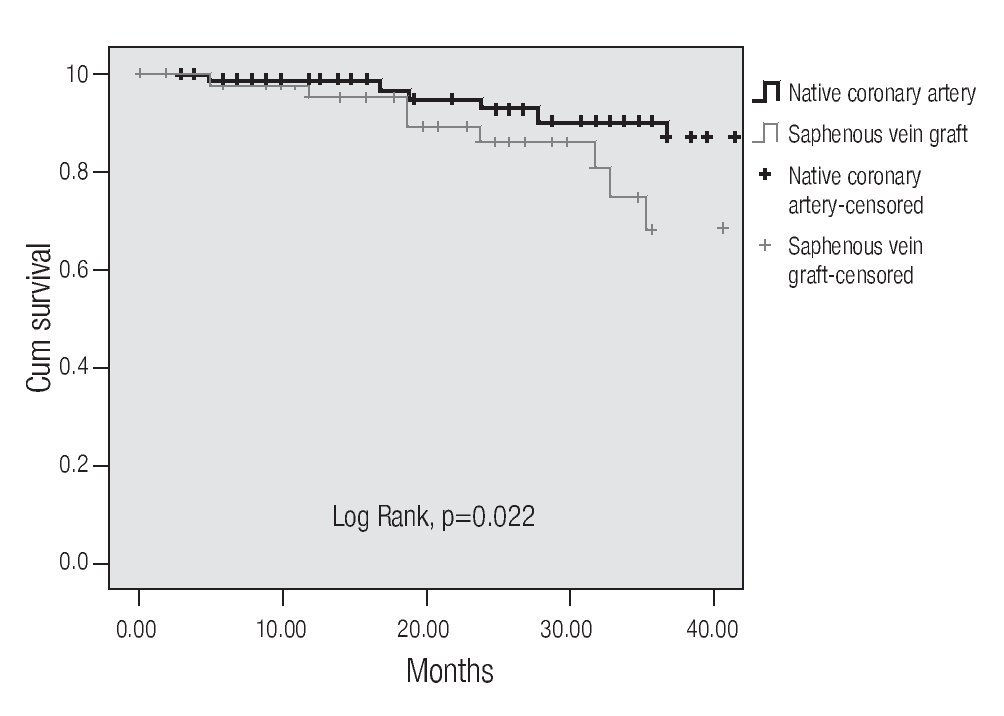
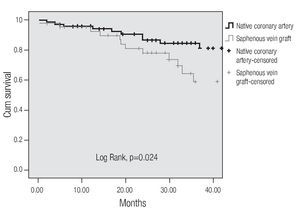
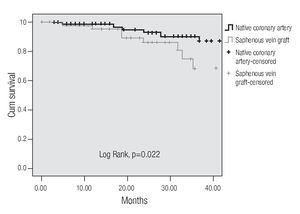
A tendency towards a lower MACE incidence was observed in group 2 at 3 years (34.6% vs. 23.0%, p = 0.075). The use of bare-metal stents or DES did not modify the incidence of accumulated MACE. The MACE-free survival by the Kaplan-Meier analysis was 65.0% in the SVG group and 89.1% in the native vessels group (p = 0.024). TVR-free survival was 74.8% and 92.8%, respectively (p = 0.022; Figures 3 and 4). Risk estimation for TVR with the use of DES revealed a reducing-effect tendency, with OR 0.28 (IC 95%, 0.06-1.31, p = 0.09). The TVR-free survival at 36 months in the whole population with the use of DES was 94.1%, compared to 89.8% with bare metal stents (p = 0.64).
Figure 3.MACE-free survival.
Figure 4.Free survival of a target vessel revascularization.
Discussion
Treatment of recurrent ischemia in patients with previous CABG is still a therapeutic challenge. It is feasible to repeat surgical revascularization, but this is associated with a higher morbidity and mortality.1,2 The variables associated with long-term mortality, as described in different studies, are: low left ventricular ejection fraction, congestive heart failure, advanced age, diabetes mellitus, and a longer time interval between CABG and PCI.3 ACC/ AHA guidelines indicate that PCI is a reasonable therapeutic option for patients with recurrent ischemia and diseased SVG or for patients with incapacitating angina secondary to new stenosis in the native coronary artery (Class IIa, level of evidence B). 5-7
The characteristics of a diseased SVG are soft, friable, and voluminous plaques, with a high risk of distal embolization and of periprocedural myocardial infarction, in contrast to the fibrocollagenous content and calcification encountered in native coronary arteries.4 In a report of 1056 consecutive patients with venous grafts, 15% had a > 5x CK-MB rise, leading to a higher mortality rate at one year as compared to patients with normal CK-MB levels (11.7% vs. 4.8%) and corresponds to the most powerful predictor for late mortality.8 In general, PCI of SVGs has been associated to less favorable results as compared with percutaneous intervention of native coronary vessels, with high rates of cardiovascular events, mainly MI.8-11 Our primary end-point —a composite of death, MI, and repeated revascularization— was significantly lower during the first 30 days for the native artery PCI group (10.2% vs. 2.5%, p = 0.041).
Increment in the MACE rate, mainly of MI, has been related with distal embolism during the procedure, caused by debris or friable material released from the venous graft that leads to alterations in coronary flow, expressed as slow flow or the no-reflow phenomenon. Although the use of glycoprotein IIb/IIIa inhibitors has increased the safety of PCI, these drugs have not had a significant impact in reducing distal embolism during venous graft interventions. The accumulated data from 627 patients (32% abciximab and 31% eptifibatide) in five studies with venous graft intervention failed to demonstrate any clinical efficacy of IIb/IIIa inhibitors in this subtype of patients.12 The rate of IIb/IIIa inhibitors used in our study was lower, even in those patients with native artery intervention, and did not prevent the development of no-reflow phenomenon in the group with venous grafts.
The most successful approach to reduce distal embolism is the use of protection devices that trap released embolic debris or thrombi during the intervention, using a distal occluding balloon, a filter, or a reverse flow system. The PercuSurge® system (Medtronic AVE, Sunnyvale, CA) was used in the SAFER (Saphenous vein graft angioplasty free of emboli randomized trial) study performed in 406 patients and compared with 395 patients in whom that device was not used. In the PercuSurge® group, a 42% reduction in the relative risk for MACE was obtained at 30 days (9.6% vs. 16%, p = 0.004).13 Other published studies have shown a similar benefit with the distal occluding balloon.14-16
In our study, the low rate in the use of these devices (8.1%) could explain the higher frequency of persistent no-reflow phenomenon in the SVG group, and that this phenomenon is a powerful predictor of MACE at 36 months in our studied population.
Current studies suggest the use of direct stenting for PCI of saphenous vein bypass in patients with significant obstructive disease. In a recently published registry, the clinical events at 30 days were similar when comparing the direct stenting technique with that using distal embolic protection devices in SVG.17 A 28% reduction has been reported in the revascularization rate at one year with the use of direct stenting.18 These results must still be validated in a randomized study.
In our population, we used this technique in a low percentage of cases (10.2% vs. 15.3%, p = 0.2) at a similar rate in both groups.
The use of PTFE graft stents and the rheolytic thrombectomy have not provided a reduction in the rate of MACE in patients with PCI of SVG, when compared with conventional angioplasty.19-22
The long-term benefit of DES in SVG compared with conventional stents has not been completely defined yet. Vermeersch et al, in a randomized study, found a reduction in the restenosis rate (13.6% vs. 32.6%, p= 0.031) and of TVR (4.3% vs. 24.5%, p = 0.005) at 6 months.23 Some published series comparing the evolution of DES and bare metal stents at 1 and 2 years have demonstrated similar MACE and TVR rates with both types of stents.24-28 The presence of a thrombus in the venous graft and the length of the stent (> 30 mm) have been shown to be independent predictors of MACE during the early phase and at 1 year.25,29 In our population as a whole, these variables were not independent predictors of MACE at 3 years of follow-up.
Bansal et al, in a series with an average follow-up of 33 months, found a MACE rate of 49% for PCI in SVG and a 33% restenosis rate. These authors did not find statistical differences when the DES were compared with bare metal stents in either the MACE rate (50% vs. 46%, p = 0.63) or binary restenosis (30% vs. 35%, p = 0.60).30
In our study, the MACE-free and TVR-free survival at 36 months was higher in the PCI of native vessels group than in the PCI of venous grafts group. The use of DES did not modify the TRV rate during the 3 years of follow-up in the population as a whole.
Limitations
Our study is retrospective, and therefore has the limitations of this type of design. We consider that the use of direct stenting and DES in a similar proportion in both groups validate the analyses and does not affect the results. The reduced use of distal embolic protection devices during SVG intervention (8.1% vs. 0%, p = 0.02) could influence the MACE rate found in this group. All of these devices were used during the last two years of the study, that is, after the publication of the updated ACC/AHA guidelines for percutaneous coronary intervention in 2005, when the recommendation to use distal embolic protection devices in diseased saphenous vein grafts was included for the first time (Class I, evidence level B).7
Conclusion
In this study, we compare the results of intervening venous grafts versus native arteries in patients with previous coronary artery bypass surgery. Results show a higher MACE rate during the in-hospital phase for group 1 (venous grafts), and a better MACE-free and TVR-free survival for native vessel PCI. To get definite conclusions, it will be necessary to perform a randomized prospective study using distal embolic protection devices, as well as direct stenting and DES in both groups.
Corresponding author:
Guering Eid-Lidt,
Departamento de Hemodinámica. Instituto Nacional de Cardiología Ignacio Chávez. Juan Badiano #1, Colonia Sección XVI, Delegación Tlalpan, Distrito Federal, México, CP 14080.
Telephone: 555573 2911 Ext. 1235 1236, Fax: 55557 3099.
E mail:guering@yahoo.com
Received: May 12,
2009; accepted: August 28, 2009.