Spontaneous coronary artery dissection (SCAD) is a rare, deadly cause of myocardial infarction in the general population, especially during the peripartum period. Multivessel SCAD is an extremely rare condition, little reported in the medical literature.1 Hormonal changes and hemodynamic stress are some of the factors that have been implicated in the etiology of this condition in pregnant women, which can persist for up to six months after delivery. However, the exact pathophysiological process leading to SCAD has not yet been elucidated. The spectrum of clinical presentation ranges from mild symptoms to cardiac arrest.
The localization of the dissection and the number of vessels involved determine the prognosis and treatment, which varies from conservative medical treatment to percutaneous intervention or surgery.2
We present a case of SCAD in a young woman ninety days after her second delivery, with No-ST elevation myocardial infarction (NSTEMI) and cardiogenic shock. The coronary angiography revealed SCAD of all the coronary arteries tree for which she underwent coronary artery bypass graft surgery (CABG) supported at the beginning with an intra-aortic balloon pump.
Case reportThis 34-year-old woman had a medical history of hypertension, hypothyroidism and renal fibromuscular dysplasia (FMD) treated with angioplasty and stent 5 years before. The patient denied any illicit drug use, smoking or excessive alcohol consumption. She was transferred to our hospital for further evaluation after being hospitalized one day before with chest pain, dyspnea and hypotension.
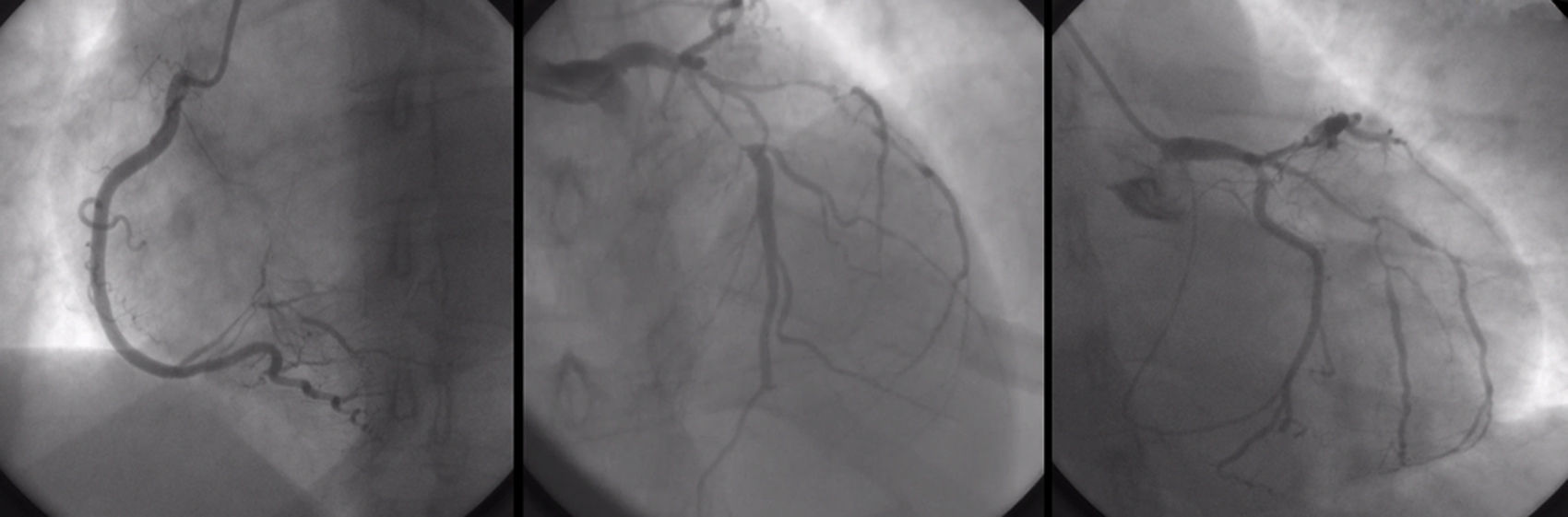
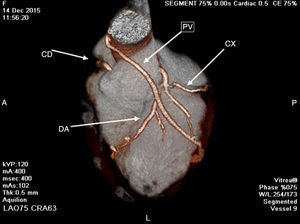
In the emergency department, the electrocardiogram showed sinus rhythm and negative T wave of the antero-lateral wall (D1 AVL and V1-V6) and abnormal cardiac enzymes. She was under inotropic agents. A bedside echo demonstrated global myocardial wall hypokinesis with a left ventricular ejection fraction (LVEF) of 30%. Cardiac catheterization and coronary angiography was performed, which showed non-occlusive coronary artery type I dissection of the left main and the three other coronary arteries with considerable involvement of their proximal to mid portions (Fig. 1).
Coronary angiography, showing non-occlusive coronary artery type I dissection of the left main and the three other coronary arteries with considerable involvement of their proximal to mid portions. LEFT: right coronary artery; it is atrio-ventricular branch with proximal-mid dissection. MIDDLE-RIGHT: left coronary artery; left main, the obtuse marginal branch of the left circumflex and left anterior descending artery with proximal-mid dissection.
Because of the large amount of myocardium at risk and the clinical instability, we elected to refer the patient for coronary artery bypass grafting (CABG), because a complete revascularization had to be performed in order to offer our patient a favorable outcome. An intraaortic balloon pump was inserted and the CABG was immediately performed. She received left internal mammary artery (LIMA) to the proximal left anterior descending artery (LAD), and two saphenous vein grafts (SVG) to the obtuse marginal branch of the left circumflex (LCX) and to the ramus intermedius. There were no procedural or in-hospital complications. The patient was discharged from hospital 7 days after CABG. A full panel of tests, including erythrocyte sedimentation rate, C-reactive protein level, complement level, anti-nuclear antibody test, rheumatoid factor level, perinuclear anti-neutrophil cytoplasmic antibody and centrally accentuated anti-neutrophil cytoplasmic antibody tests, were found to be normal.
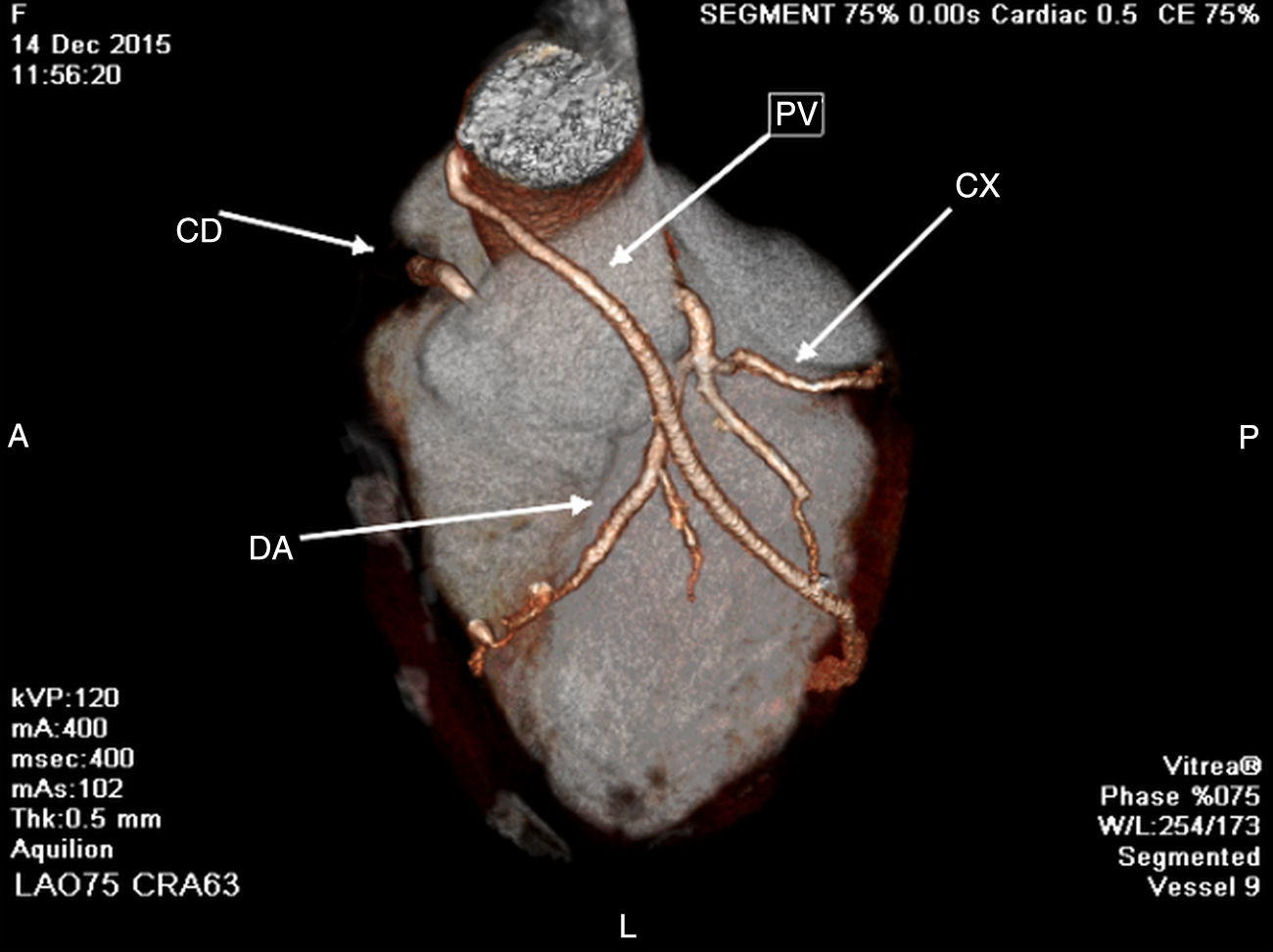
During a 2-year follow-up period, the patient was free of chest pain and her exercise test was negative and the LVEF by echocardiography normal. A Single Photon Emission Computed Tomography (SPECT) shows LVEF normal and nonischemic areas one year after the event. A 64 multislice computed tomography coronary angiography performed 2 years after the event shows LVEF normal, 0 calcium score, non-obstructive lesion in native coronary arteries, poor growth of LIMA and patency of the SVG to the obtuse marginal branch (Fig. 2).
DiscussionSCAD is a rare, deadly entity; the overall incidence in coronary angiographies is around 0.2%. It is difficult to ascertain the true prevalence as it is often under-diagnosed and has presentations varying from mild chest pains to SCD. The presence of AMI under the age of 40 years is uncommon, especially in women, accounting for approximately 0.7% of all AMI cases. Furthermore, the prevalence of AMI during pregnancy and puerperium reported by various studies ranges from 2.8 to 10 cases per 100,000 deliveries.3 27% of AMI cases during pregnancy and postpartum are associated with SCAD compared to ≥1% in the general population. The true prevalence during pregnancy and the following days is also not known with accuracy.1 SCAD has been observed in women in peripartum or with multiple prior pregnancies, and thus a significant association with pregnancy has been postulated.4
There are two proposed mechanisms for the formation of intramural hematomas (IMH) with SCAD. The first involves an intimal tear resulting in blood from the endoluminal space entering the intimal space, creating a false lumen filled with blood. The second is thought to be rupture of the vasa vasorum, which is a network of small arterioles within the walls of arteries supplying blood to the walls. When this rupture occurs, blood can pool within the intramural space, creating a false lumen filled with hematoma.
In most cases, the insult occurs a few weeks after delivery, with an average time-delay of about 1 month.5 In addition, hormonal changes during pregnancy are thought to alter normal elastic fibers and impair collagen synthesis and mucopolysaccharide content, causing weakened media. Progesterone is thought to be the culprit hormone and estrogen, on the other hand, creates a hypercoagulable state. The weakened arterial walls at risk of dissection with a prothrombotic state increase the risk of false lumen creation and thrombosis. Eosinophilic infiltrates in arterial adventitia have also been observed in autopsies of peripartum women. It is believed that these eosinophilic granules cause breakdown of the medial–adventitial layer via lytic substances, predisposing the artery to dissection. However, it is unclear if the eosinophilic granules cause SCAD or are a result of SCAD. Interestingly, SCAD in women often occurs during late pregnancy or in the post-partum period.4,6 Hemodynamic changes during late pregnancy can precipitate SCAD.
Saw et al. show a strong association between SCAD and FMD, a condition that also predominantly affects women.7 Our patient had had this pathology affecting both renal arteries treated by stenting a few years earlier. This is a non-inflammatory, non-atherosclerotic disorder of the arterial vasculature that leads to arterial stenosis, occlusion, aneurysm or dissection. It can involve any small to medium-sized arterial beds, especially the renal and internal carotid arteries. The etiology of FMD is unknown, but hormonal influences have been proposed and a small proportion may be genetically inherited. Since 90% of cases affect women,8 sex hormones were thought to influence the development of FMD, with some similarities to SCAD.
One of the most dangerous aspects of SCAD involves its clinical presentation. In recent retrospective studies, chest pain was the presenting symptom in the majority of SCAD cases,5,9,10 as in our patient. Ventricular arrhythmia occurred in 8–14% of patients.9 In another series, all cases of SCAD presented with troponin-positive ACS, with 26% presenting with ST-elevation MI and 3.6% with ventricular arrhythmia.6 Two older studies showed a higher proportion presenting with ST-elevation MI (80–84%), while non-ST elevation MI cases were 8–16% and 4% presented with unstable angina.9 Case series have also noted that patients may have severe left ventricular dysfunction (LVD), which often has subsequent full recovery.11 However, left ventricular (LV) recovery may occur with standard forms of MI, but whether there may be more marked LV recovery with SCAD, as postulated by some, is yet unknown. Our patient had LVD and recovered normal function a few months after surgery.
The optimal treatment for patients with SCAD in the peripartum period has not been clearly determined and there is no consensus on guidelines for recommendations. This may be the result of the rarity of the disease.
The selection of treatment strategy depends upon the clinical manifestation, location and extent of dissection and the amount of ischemic myocardium at risk. Options include medical therapy, PCI and coronary artery bypass surgery.2 Conservative medical therapy is a reasonable first option in mid- or distal single vessel dissection with a lumen diameter limitation <50% and TIMI grade 2 or 3 flow in the affected coronary artery.2
However, some studies have demonstrated that early intervention with either PCI or coronary artery bypass graft (CABG) following the diagnosis of SCAD leads to a better outcome.12 Thus, PCI is the therapy of choice in single vessel disease, especially proximal dissection, in which there are ongoing symptoms and persistent restriction of coronary blood flow (TIMI grade 0 or 1 flow).
CABG is the treatment of choice in multiple vessel disease, especially where there is left main stem involvement with ongoing ischemia, refractory to medical or interventional therapy, and when there is hemodynamic instability. Likewise, in one series, early results of coronary surgery appeared favorable, but 11 of 15 bypass grafts undergoing late angiographic assessment were occluded.
One recent retrospective study indicates that the risk of emergency CABG is disturbingly high among patients with SCAD treated with PCI, including those with normal flow. In contrast, conservative management was associated with favorable outcomes.
A collection of case reports from 1980 to 2000 showed SCAD mortality rates ranging from 0% to 7%.5 More recent studies reported lower in-hospital mortality rates ranging from 1% to 5%.2 One-year mortality rates after discharge were similar, at 1–4%.2 Female SCAD patients potentially have a poorer prognosis. In particular, the subgroup of patients with postpartum SCAD appear to have the worst prognosis. The mortality rate in AMI due to SCAD in pregnant and postpartum women is relatively high, ranging from 38% to 66%.
Many patients require subsequent hospital visits for recurrent chest pains and repeat coronary angiograms. Event-free rates at 1 year range from 74% to 96%.2,6 Our patient had a spontaneous hospital visit for recurrent chest pains, one year after the event and a perfusion stress test was performed, which was normal without ischemia.
As far as the location of SCAD is concerned, a review of pregnancy-associated SCAD case reports shows that it generally involves the LAD solely or in concomitance with the LMCA and the RCA or the LCX (2). To the best of our knowledge there are only three cases in the current literature reporting SCAD involving the LMCA, the LAD and the LCX arteries simultaneously in the peripartum period1 but none like our case that involved all the left and right coronary tree. Moreover, our case has other features that make it unique, which is that, in addition to the compromise of the entire coronary tree, she presented with cardiogenic shock, required intra-aortic balloon pump and had to be subjected to emergency surgery due to the severe myocardial compromise and seriousness of her condition at that time. She was discharged in 7 days without complications, and monitoring the recovery of left ventricular function, the absence of residual ischemia and survival beyond 24 months was evident.
Patients should be monitored for any symptoms of recurrent ischemia. Stress testing with nuclear perfusion imaging is preferred over CGA as a means of surveillance. Possible late complications include progression of the dissection and formation of pseudoaneurysms.
ConclusionsSCAD is a rare cause of AMI, commonly associated with pregnancy and puerperium and in this context has a very high mortality rate. The possibility of dissection should always be considered when any woman presents with chest pain and/or AMI during pregnancy or puerperium in order to treat it quickly and avoid complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.