Cardiovascular diseases (CVD) are the most important cause of mortality in Latin America, while peripheral arterial disease (PAD) is the third leading cause of atherosclerotic cardiovascular morbidity.
ObjectiveTo establish the prevalence of PAD and the distribution of traditional CVD risk factors in a population from the Department of Cauca, Colombia.
MethodsA cross-sectional study was conducted on a total of 10,000 subjects aged ≥40 years, from 36 municipalities. An ankle–brachial index (ABI) ≤ 0.9 in either leg was used as diagnostic criterion of PAD.
ResultsOverall PAD prevalence was 4.4% (4.7% females vs. 4.0% males), with diabetes being the most prevalent risk factor (23%). Among individuals self-reporting a history of acute myocardial infarction or stroke, PAD prevalence was 31.0% and 8.1%, respectively. After adjusting for potential confounders, PAD was significantly associated with hypertension (OR 4.6; 95% CI; 3.42–6.20), diabetes (4.3; 3.17–5.75), dyslipidaemia (3.1; 2.50–3.88), obesity (1.8; 1.37–2.30), and cigarette smoking (1.6; 1.26–1.94). Analysis for the interaction of risk factors showed that diabetes, dyslipidaemia, and obesity accounted for 13.2 times the risk for PAD (6.9–25.4), and when adding hypertension to the model, the risk effect was the highest (17.2; 8.4–35.1).
ConclusionsHypertension, diabetes, dyslipidaemia, and obesity, but not smoking were strong predictors of PAD. ABI measurement should be routinely performed as a screening test in intermediate and high-risk patients for CVD prevention. This could lead to an early intervention and follow-up on populations at risk, thus, contributing to improve strategies for reducing CVD burden.
Las enfermedades cardiovasculares (ECV) son la causa más importante de mortalidad en América Latina, mientras que la enfermedad arterial periférica (EAP) es la tercera causa de morbilidad cardiovascular aterosclerótica.
ObjetivosEstablecer la prevalencia de la EAP y la distribución de factores de riesgo tradicionales para ECV en una población del departamento del Cauca, Colombia.
MétodosSe realizó un estudio de corte transversal en un total de 10,000 sujetos ≥ 40 años de 36 municipios. Un índice tobillo-brazo ≤ 0.9 en cualquiera de las piernas fue utilizado como criterio de diagnóstico para EAP.
ResultadosLa prevalencia de EAP fue del 4.4% (4.7% en mujeres vs. 4% en hombres), siendo la diabetes el factor de riesgo más prevalente (23%). Entre los individuos con autorreporte de infarto agudo de miocardio y accidente cerebrovascular, la prevalencia de EAP fue del 31% y 8,1%, respectivamente. Después del ajuste por potenciales factores de confusión, la EAP estuvo asociada significativamente con hipertensión (OR: 4.6; IC 95%: 3.42-6.20), diabetes (4.3; 3.17-5.75), dislipidemia (3.1; 2.50-3.88), obesidad (1.8; 1.37-2.30) y consumo de cigarrillo (1.6; 1.26-1.94). El análisis de interacción entre los factores de riesgo mostró que diabetes, dislipidemia y obesidad presentaron 13.2 veces más riesgo para EAP (6.9-25.4), y cuando se agregó hipertensión al modelo, el riesgo fue el más alto (17.2; 8.4-35.1).
ConclusionesLa medición del índice tobillo-brazo debe realizarse de forma rutinaria en pacientes con riesgo intermedio/alto como prueba de cribado para la prevención de ECV, permitiendo la intervención temprana y el seguimiento de las poblaciones en situación de riesgo.
Peripheral arterial disease (PAD), after acute myocardial infarction and stroke, is the third leading cause of atherosclerotic cardiovascular morbidity and mortality worldwide. It is estimated that 202 million people in the world are affected with PAD, from whom 45 million are expected to die from coronary or cerebrovascular disease during a 10-year period.1 Although the number of individuals with PAD has increased over the last decade by 28.7% in low or middle income countries,1 few epidemiological studies have been conducted to establish reliable estimates of prevalence and distribution of risk factors; specially in Latin America, where cardiovascular diseases (CVD) have become the leading cause of death and disability.2 Therefore, studies are still needed for a better understanding of the etiology and disease distribution, and for developing more effective policies and programs for preventing and managing PAD.
The ankle–brachial index (ABI), the ratio of the ankle and brachial systolic blood pressures, is often used as a surrogate marker for PAD. An ABI of 0.9 or less is generally considered abnormal and suggests PAD.3 The ABI is currently considered the most effective tool to screen PAD, being particularly important in detecting PAD in asymptomatic individuals.4 In fact, it has been suggested that ABI measurement may reduce morbidity and mortality through the early detection and treatment of PAD and other CVD.2–4 Furthermore, population-based cohort studies have established the ABI as an independent risk indicator of atherothrombotic events and as a risk predictor of CVD.4–6
In Colombia, CVD has a mortality rate of 107.3/100,000 men and 50.6/100,000 women, and thus, PAD also represents a public health concern.7,8 The aim of this study was to establish the prevalence of PAD using the ABI to screen subjects over the age of 40 years from the department of Cauca, Colombia. In addition, the association between sociodemographic and traditional CVD risk factors and PAD risk was estimated.
MethodsStudy design and populationThis cross-sectional, population-based study was conducted between June of 2011 and May of 2013 in 36 municipalities from the department of Cauca, located at the southwest of Colombia. Corresponding media through television, radio and newspapers was used to recruit population participants. All attending subjects who agreed to participate in the study were screened and surveyed. Inclusion criteria were men or women over the age of 40 years, regardless of provenance. All the questionnaires, procedures, and protocols were reviewed and approved by the Ethics Committee for Scientific Research at the University of Cauca; the guidelines used in the review were based on the bioethical principles established in the Helsinki in 1975 declaration and the parameters outlined in Resolution 8430 of the Colombian Ministry of Health in 1993.
Data collectionAfter signing a consent form, each volunteer was interviewed by a trained health professional to fill out a structured questionnaire to establish socio-demographical characteristics (age, gender, provenance, educational level, and occupational status), personal clinical history (hypertension, diabetes, dyslipidemia, obesity, acute myocardial infarction and brain ischemia), and smoking habits (never, former, current). During examination, height, weight, and resting blood pressure were measured to establish the presence of cardiovascular risk factors. Thus, arterial hypertension was considered when having systolic arterial pressure ≥140mm Hg and/or a diastolic arterial pressure ≥90mm Hg. Body mass index (BMI) was calculated as weight divided by squared height (kg/m2). Subjects were divided in three weight categories: normal weight (BMI less than 25), overweight (25–29.99) and obesity (≥30). In order to corroborate the presence of personal risk factors, blood samples were drawn for biochemical analyses and medical records were reviewed for clinical diagnosis. Thus, subjects were considered to have dyslipidemia if they had a fasting cholesterol level ≥200mg/dL, HDL level <40mg/dL for men and <50mg/dL for women, or triglycerides ≥150mg/dL (hypertriglyceridemia), or with a previous diagnosis of hypercholesterolemia or were under medication use. For the biochemical analyses, LDL-cholesterol >100mg/dL was considered high and a low HDL-cholesterol (<40mg/dL, for both gender) was considered hypoalphalipoproteinemia. The lipid triad was defined as triglycerides ≥150mg/dL, HDL-cholesterol <40mg/dL (man) or <50mg/dL (women), and LDL-cholesterol >100mg/dL. A triglycerides to HDL-cholesterol ratio (TG/HDL-c) >4 was considered as elevated. Diabetes was defined as having a fasting glucose level ≥126mg/dL, clinical history of diabetes or diabetes treatment.
ABI measurementPatients were asked to rest in a supine position for 10min. Afterwards, the systolic blood pressure (SBP) was measured in the brachial artery for each arm, using a sphygmomanometer (WelchAllyn) and an 8-mHz Doppler device (Huntleigh 500 D, Huntleigh Technology). The cuff was then placed in the distal calf and the Doppler was used to determine the SBP of both posterior tibial and dorsalis pedis arteries of each lower limb. The ABI for each leg was calculated by dividing the higher of the posterior tibial or dorsalis pedis pressure by the higher of the right or left arm SBP. According to the recommendations of the American Heart Association PAD was defined as having an ABI≤0.9 in either leg, between 0.91 and 1.40 was considered normal, and when >1.4 was classified as suggestive of calcified non-compressible arteries.3 The lower of the two ABI values obtained was used for the diagnosis of PAD. All sphygmomanometers were calibrated for the study and the ABI test was performed by trained health professionals.
Statistical analysisData analyses were performed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Prevalence of PAD was estimated as the number of subjects with an ABI≤0.9 over the total number of subjects collected in the study. Continuous variables were expressed using the mean and the standard deviation and the Student's t-test was used to assess mean differences between study groups. Discrete variables were expressed as frequencies and proportions and the Chi-squared test was used to assess distribution differences. To determine the association between each variable and disease status, subjects with ABI>1.4 were excluded and conditional logistic regression analysis was carried out to calculate both crude odds ratios (ORs) and 95% confidence intervals (CIs). To assess the effect of potential confounders, ORs were initially adjusted in the model by adding as covariates age (in years as a continuous variable), gender (males vs. females), and provenance (urban vs. rural). For further analysis, ORs were fully adjusted in a multivariate model adding as categorical variables occupation status, education level, cigarette smoking, obesity, hypertension, diabetes, and dyslipidemia. Interactions between risk factors were evaluated for those showing a significant increase on PAD risk. The interaction analysis was carried out using the macros of the SPSS statistical package.9 A probability level of <0.05 was used as the criterion of significance. Significance levels (p values) correspond to two-sided tests.
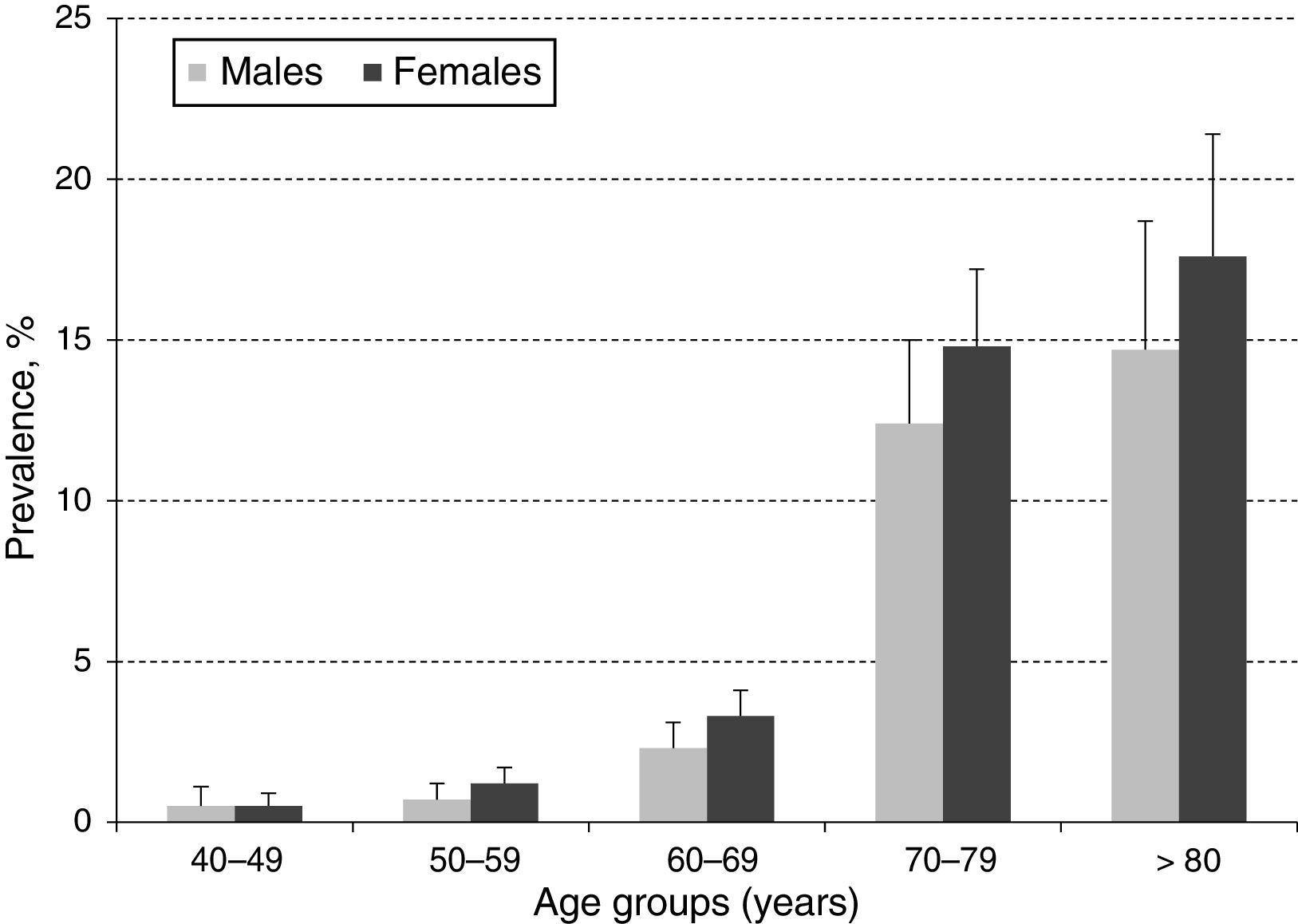
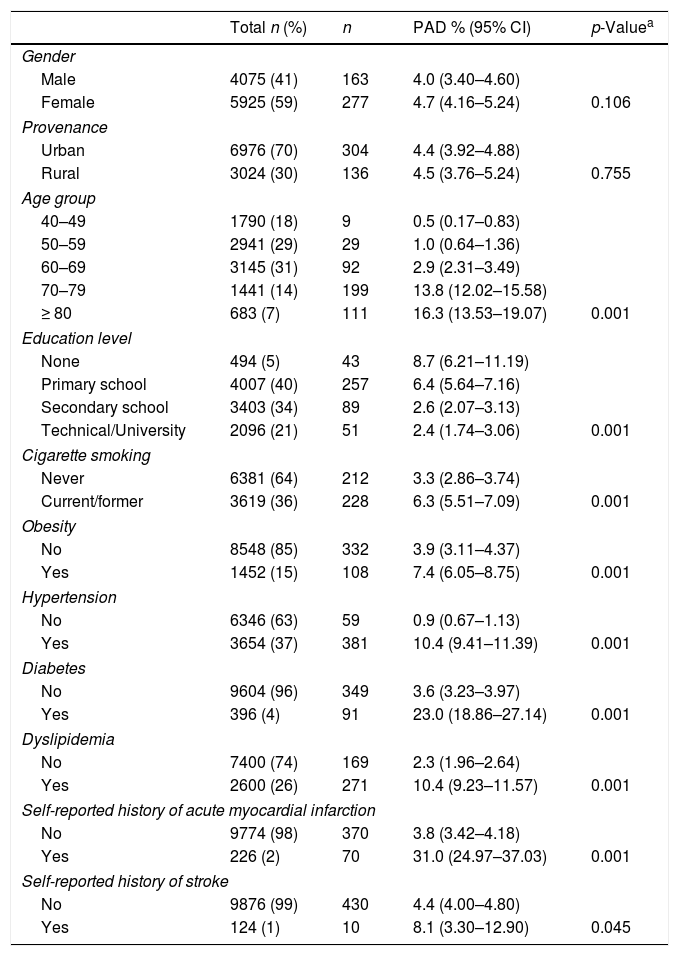
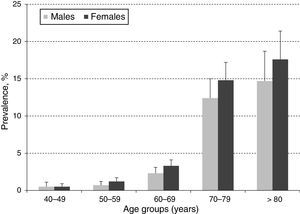
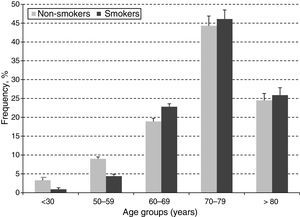
ResultsA total of 10,000 subjects were screened for PAD. As shown in Fig. 1, the prevalence of PAD increased with aging and was consistently higher in females compared to males in all groups, except for those 40–49 years of age. Table 1 shows the prevalence of PAD in the total population stratified by demographic variables and presence of CVD risk factors. The overall prevalence of PAD was 4.4%; 4.0% in male and 4.7% in females. PAD was equally prevalent in urban and rural communities. As expected, aging sharply increased PAD prevalence from a low rate of 0.5% in subjects 40–49 years up to 13.8% and 16.3% in subjects 70–79 and ≥80 years, respectively. Furthermore, PAD subjects were more often diabetic (23%), hypertensive (10.4%), dyslipidemic (10.4%), unschooled (8.7%), obese (7.4%), and current/former cigarette smokers (6.3%). Finally, the prevalence of PAD among individuals self-reporting a history of acute myocardial infarction or stroke was 31.0% and 8.1%, respectively.
Prevalence of PAD by selected population characteristics.
| Total n (%) | n | PAD % (95% CI) | p-Valuea | |
|---|---|---|---|---|
| Gender | ||||
| Male | 4075 (41) | 163 | 4.0 (3.40–4.60) | |
| Female | 5925 (59) | 277 | 4.7 (4.16–5.24) | 0.106 |
| Provenance | ||||
| Urban | 6976 (70) | 304 | 4.4 (3.92–4.88) | |
| Rural | 3024 (30) | 136 | 4.5 (3.76–5.24) | 0.755 |
| Age group | ||||
| 40–49 | 1790 (18) | 9 | 0.5 (0.17–0.83) | |
| 50–59 | 2941 (29) | 29 | 1.0 (0.64–1.36) | |
| 60–69 | 3145 (31) | 92 | 2.9 (2.31–3.49) | |
| 70–79 | 1441 (14) | 199 | 13.8 (12.02–15.58) | |
| ≥ 80 | 683 (7) | 111 | 16.3 (13.53–19.07) | 0.001 |
| Education level | ||||
| None | 494 (5) | 43 | 8.7 (6.21–11.19) | |
| Primary school | 4007 (40) | 257 | 6.4 (5.64–7.16) | |
| Secondary school | 3403 (34) | 89 | 2.6 (2.07–3.13) | |
| Technical/University | 2096 (21) | 51 | 2.4 (1.74–3.06) | 0.001 |
| Cigarette smoking | ||||
| Never | 6381 (64) | 212 | 3.3 (2.86–3.74) | |
| Current/former | 3619 (36) | 228 | 6.3 (5.51–7.09) | 0.001 |
| Obesity | ||||
| No | 8548 (85) | 332 | 3.9 (3.11–4.37) | |
| Yes | 1452 (15) | 108 | 7.4 (6.05–8.75) | 0.001 |
| Hypertension | ||||
| No | 6346 (63) | 59 | 0.9 (0.67–1.13) | |
| Yes | 3654 (37) | 381 | 10.4 (9.41–11.39) | 0.001 |
| Diabetes | ||||
| No | 9604 (96) | 349 | 3.6 (3.23–3.97) | |
| Yes | 396 (4) | 91 | 23.0 (18.86–27.14) | 0.001 |
| Dyslipidemia | ||||
| No | 7400 (74) | 169 | 2.3 (1.96–2.64) | |
| Yes | 2600 (26) | 271 | 10.4 (9.23–11.57) | 0.001 |
| Self-reported history of acute myocardial infarction | ||||
| No | 9774 (98) | 370 | 3.8 (3.42–4.18) | |
| Yes | 226 (2) | 70 | 31.0 (24.97–37.03) | 0.001 |
| Self-reported history of stroke | ||||
| No | 9876 (99) | 430 | 4.4 (4.00–4.80) | |
| Yes | 124 (1) | 10 | 8.1 (3.30–12.90) | 0.045 |
CI: confidence interval; PAD: peripheral arterial disease.
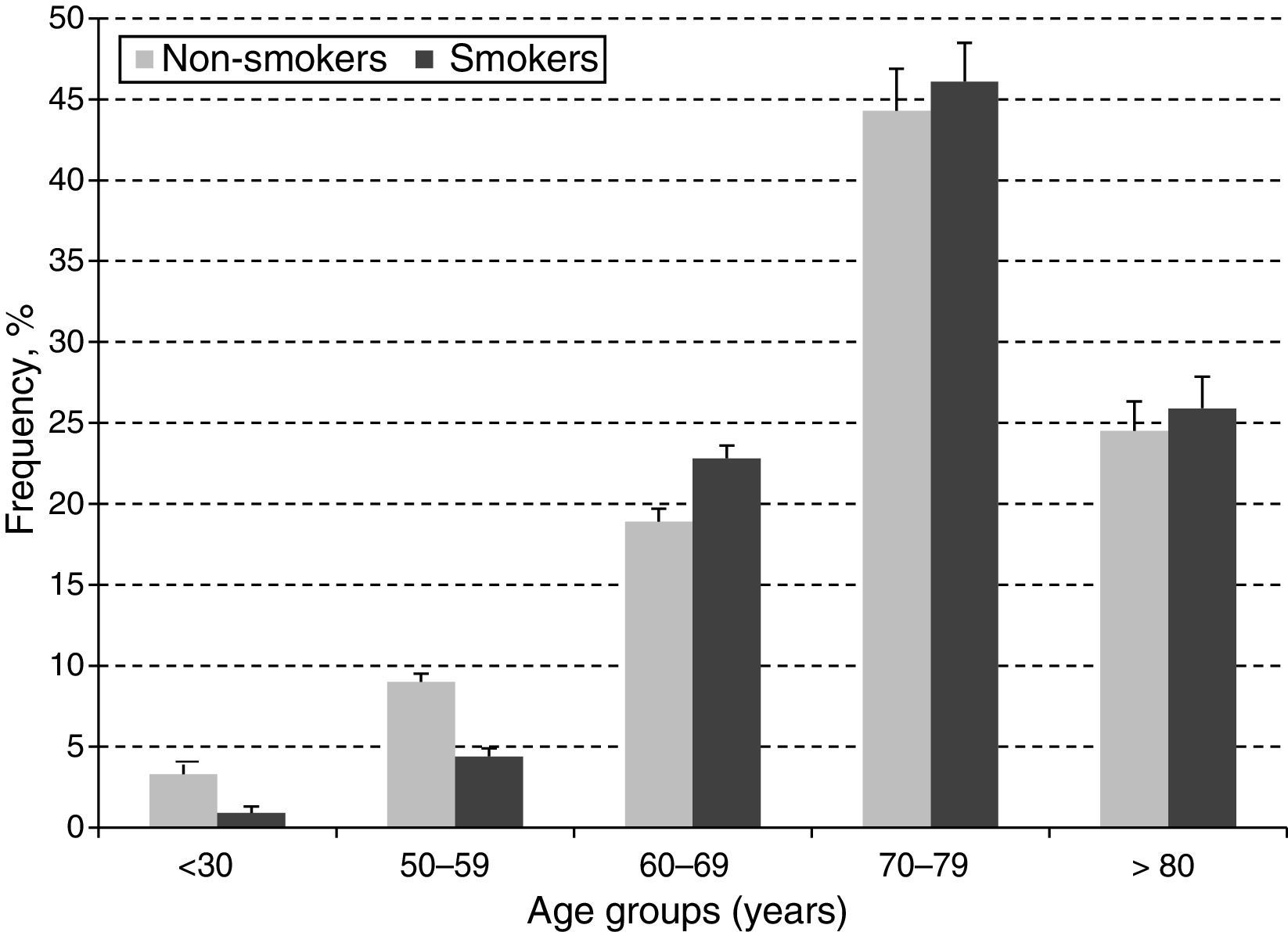
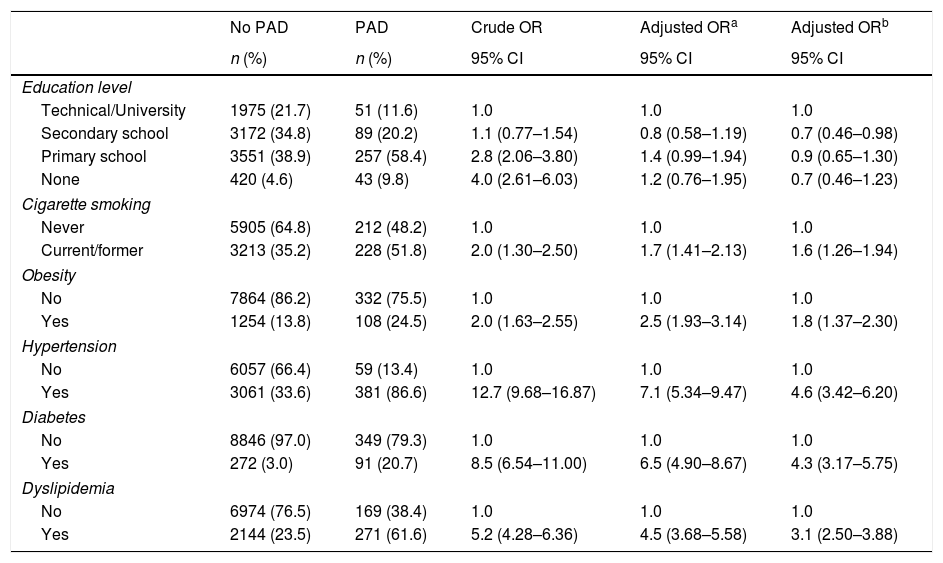
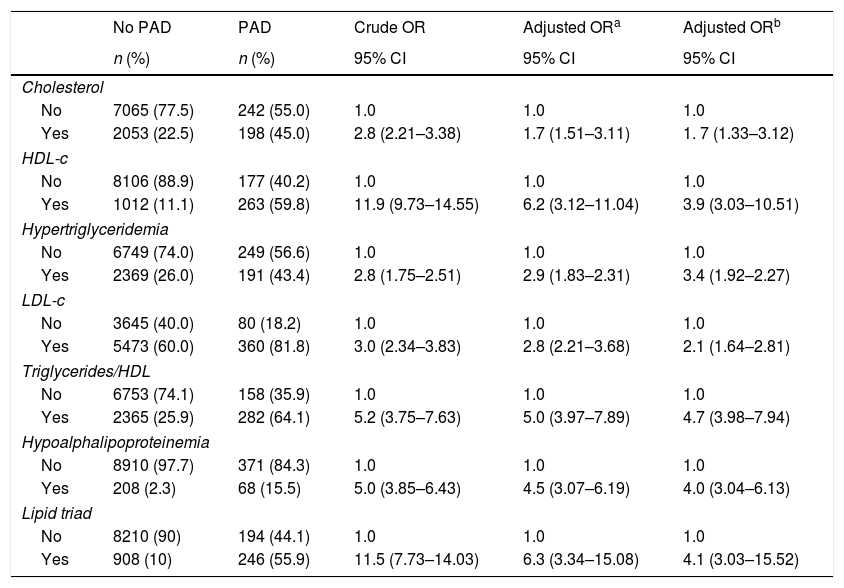
In order to estimate the odds ratio (OR) for each of the above-mentioned CVD risk factors, 442 subjects with ABI >1.4 were excluded. Thus, a total of 3853 (40.3%) males with a mean age of 61.48 years (SD: 11.26 years) and 5705 (59.7%) females with a mean age of 60.37 years (SD: 11.46 years) were included in this analysis. As shown in Table 2, unschooling, cigarette smoking, obesity, hypertension, diabetes and dyslipidemia were significantly associated to an increase in risk for PAD in the crude (unadjusted) OR model. However, after adjusting in the multivariate regression model, hypertension (OR 4.6; 95% CI 3.42–6.20), diabetes (OR 4.3; 95% CI 3.17–5.75), dyslipidemia (OR 3.1; 95% CI 2.50–3.88), obesity (OR 1.8; 95% CI 1.37–2.30) and cigarette smoking (OR 1.6; 95% CI 1.26–1.94) were significantly associated to an increase on PAD risk. In contrast, attending to secondary school was a protective factor for PAD, reducing the risk by 30% (OR 0.7; 95% CI 0.46–0.98). When looking at cigarette smoking frequency among PAD cases by age groups (Fig. 3), cigarette consumption was more often observed among subjects older than 60 years of age, but the difference between the groups was not statistically significant (p=0.107). For the lipid profile analysis (Table 3), the TG/HDL ratio was strongly associated to PAD (OR 4.7; 95% CI 3.9–7.9), followed by the lipid triad (OR 4.1; 95% CI 3.0–15.5), hypoalphalipoproteinemia (OR 4.0; 95% CI 3.0–6.1), HDL-c (OR 3.9; 95% CI 3.0–10.5), hypertriglyceridemia (OR 3.4; 95% CI 1.9–2.2), LDL-c (OR 2.1; 95% CI 1.6–2.8), and cholesterol (OR 1.7; 95% CI 1.3–3.1), respectively.
Selected CVD risk factors and odds ratio (95% CIs) for PAD.
| No PAD | PAD | Crude OR | Adjusted ORa | Adjusted ORb | |
|---|---|---|---|---|---|
| n (%) | n (%) | 95% CI | 95% CI | 95% CI | |
| Education level | |||||
| Technical/University | 1975 (21.7) | 51 (11.6) | 1.0 | 1.0 | 1.0 |
| Secondary school | 3172 (34.8) | 89 (20.2) | 1.1 (0.77–1.54) | 0.8 (0.58–1.19) | 0.7 (0.46–0.98) |
| Primary school | 3551 (38.9) | 257 (58.4) | 2.8 (2.06–3.80) | 1.4 (0.99–1.94) | 0.9 (0.65–1.30) |
| None | 420 (4.6) | 43 (9.8) | 4.0 (2.61–6.03) | 1.2 (0.76–1.95) | 0.7 (0.46–1.23) |
| Cigarette smoking | |||||
| Never | 5905 (64.8) | 212 (48.2) | 1.0 | 1.0 | 1.0 |
| Current/former | 3213 (35.2) | 228 (51.8) | 2.0 (1.30–2.50) | 1.7 (1.41–2.13) | 1.6 (1.26–1.94) |
| Obesity | |||||
| No | 7864 (86.2) | 332 (75.5) | 1.0 | 1.0 | 1.0 |
| Yes | 1254 (13.8) | 108 (24.5) | 2.0 (1.63–2.55) | 2.5 (1.93–3.14) | 1.8 (1.37–2.30) |
| Hypertension | |||||
| No | 6057 (66.4) | 59 (13.4) | 1.0 | 1.0 | 1.0 |
| Yes | 3061 (33.6) | 381 (86.6) | 12.7 (9.68–16.87) | 7.1 (5.34–9.47) | 4.6 (3.42–6.20) |
| Diabetes | |||||
| No | 8846 (97.0) | 349 (79.3) | 1.0 | 1.0 | 1.0 |
| Yes | 272 (3.0) | 91 (20.7) | 8.5 (6.54–11.00) | 6.5 (4.90–8.67) | 4.3 (3.17–5.75) |
| Dyslipidemia | |||||
| No | 6974 (76.5) | 169 (38.4) | 1.0 | 1.0 | 1.0 |
| Yes | 2144 (23.5) | 271 (61.6) | 5.2 (4.28–6.36) | 4.5 (3.68–5.58) | 3.1 (2.50–3.88) |
Lipid profiles and odds ratio (95% CIs) for PAD.
| No PAD | PAD | Crude OR | Adjusted ORa | Adjusted ORb | |
|---|---|---|---|---|---|
| n (%) | n (%) | 95% CI | 95% CI | 95% CI | |
| Cholesterol | |||||
| No | 7065 (77.5) | 242 (55.0) | 1.0 | 1.0 | 1.0 |
| Yes | 2053 (22.5) | 198 (45.0) | 2.8 (2.21–3.38) | 1.7 (1.51–3.11) | 1. 7 (1.33–3.12) |
| HDL-c | |||||
| No | 8106 (88.9) | 177 (40.2) | 1.0 | 1.0 | 1.0 |
| Yes | 1012 (11.1) | 263 (59.8) | 11.9 (9.73–14.55) | 6.2 (3.12–11.04) | 3.9 (3.03–10.51) |
| Hypertriglyceridemia | |||||
| No | 6749 (74.0) | 249 (56.6) | 1.0 | 1.0 | 1.0 |
| Yes | 2369 (26.0) | 191 (43.4) | 2.8 (1.75–2.51) | 2.9 (1.83–2.31) | 3.4 (1.92–2.27) |
| LDL-c | |||||
| No | 3645 (40.0) | 80 (18.2) | 1.0 | 1.0 | 1.0 |
| Yes | 5473 (60.0) | 360 (81.8) | 3.0 (2.34–3.83) | 2.8 (2.21–3.68) | 2.1 (1.64–2.81) |
| Triglycerides/HDL | |||||
| No | 6753 (74.1) | 158 (35.9) | 1.0 | 1.0 | 1.0 |
| Yes | 2365 (25.9) | 282 (64.1) | 5.2 (3.75–7.63) | 5.0 (3.97–7.89) | 4.7 (3.98–7.94) |
| Hypoalphalipoproteinemia | |||||
| No | 8910 (97.7) | 371 (84.3) | 1.0 | 1.0 | 1.0 |
| Yes | 208 (2.3) | 68 (15.5) | 5.0 (3.85–6.43) | 4.5 (3.07–6.19) | 4.0 (3.04–6.13) |
| Lipid triad | |||||
| No | 8210 (90) | 194 (44.1) | 1.0 | 1.0 | 1.0 |
| Yes | 908 (10) | 246 (55.9) | 11.5 (7.73–14.03) | 6.3 (3.34–15.08) | 4.1 (3.03–15.52) |
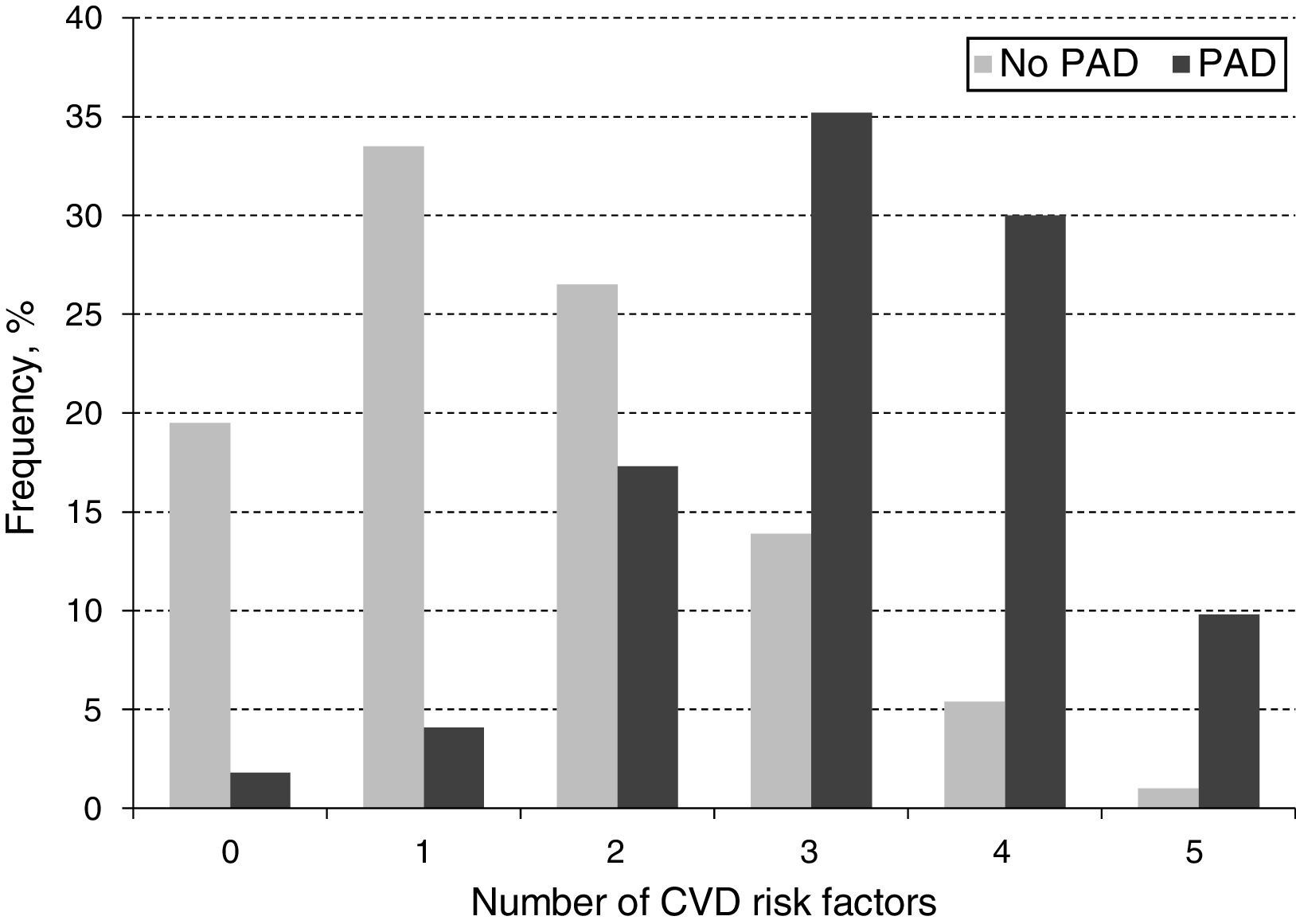
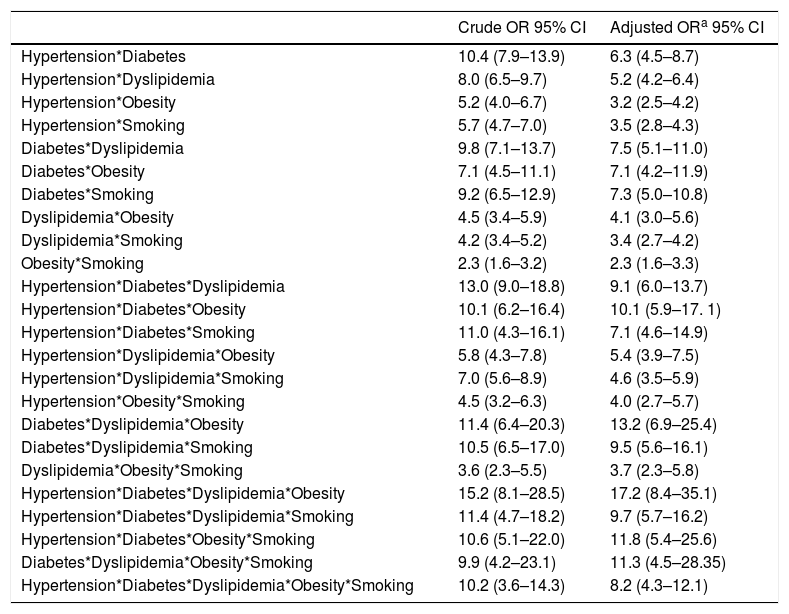
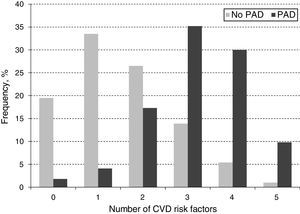
When looking at the frequency of harbored risk factors in the study population (Fig. 2), 94.1% of the PAD prevalence was explained by having a combination of two or more risk factors. In order to establish which combination of risk factors exerted the highest increase on PAD risk, an interaction analysis was conducted (Table 4). As shown for the pairwise interactions in the adjusted OR model, subjects who were both diabetic and dyslipidemic showed the highest PAD risk (OR 7.5; 95% CI 5.1–11.0), followed by diabetic and smokers (OR 7.3; 95% CI 5.0–10.8), and diabetic and obese (OR 7.1; 95% CI 4.2–11.9). Risk evaluation for having the interaction of three or more risks factors showed that diabetes, dyslipidemia and obesity accounted for 13.2 times the risk for PAD (95% CI 6.9–25.4), and when adding hypertension to the model, the risk effect was the highest (OR 17.2; 95% CI 8.4–35.1). Finally, when adding current/former cigarette smoking as the fifth risk factor to the model, the associated risk was decreased but it still remained significant (OR 8.2; 95% CI 4.3–12.1).
Interaction between selected CVD risk factors and odds ratio (95% CIs) for PAD.
| Crude OR 95% CI | Adjusted ORa 95% CI | |
|---|---|---|
| Hypertension*Diabetes | 10.4 (7.9–13.9) | 6.3 (4.5–8.7) |
| Hypertension*Dyslipidemia | 8.0 (6.5–9.7) | 5.2 (4.2–6.4) |
| Hypertension*Obesity | 5.2 (4.0–6.7) | 3.2 (2.5–4.2) |
| Hypertension*Smoking | 5.7 (4.7–7.0) | 3.5 (2.8–4.3) |
| Diabetes*Dyslipidemia | 9.8 (7.1–13.7) | 7.5 (5.1–11.0) |
| Diabetes*Obesity | 7.1 (4.5–11.1) | 7.1 (4.2–11.9) |
| Diabetes*Smoking | 9.2 (6.5–12.9) | 7.3 (5.0–10.8) |
| Dyslipidemia*Obesity | 4.5 (3.4–5.9) | 4.1 (3.0–5.6) |
| Dyslipidemia*Smoking | 4.2 (3.4–5.2) | 3.4 (2.7–4.2) |
| Obesity*Smoking | 2.3 (1.6–3.2) | 2.3 (1.6–3.3) |
| Hypertension*Diabetes*Dyslipidemia | 13.0 (9.0–18.8) | 9.1 (6.0–13.7) |
| Hypertension*Diabetes*Obesity | 10.1 (6.2–16.4) | 10.1 (5.9–17. 1) |
| Hypertension*Diabetes*Smoking | 11.0 (4.3–16.1) | 7.1 (4.6–14.9) |
| Hypertension*Dyslipidemia*Obesity | 5.8 (4.3–7.8) | 5.4 (3.9–7.5) |
| Hypertension*Dyslipidemia*Smoking | 7.0 (5.6–8.9) | 4.6 (3.5–5.9) |
| Hypertension*Obesity*Smoking | 4.5 (3.2–6.3) | 4.0 (2.7–5.7) |
| Diabetes*Dyslipidemia*Obesity | 11.4 (6.4–20.3) | 13.2 (6.9–25.4) |
| Diabetes*Dyslipidemia*Smoking | 10.5 (6.5–17.0) | 9.5 (5.6–16.1) |
| Dyslipidemia*Obesity*Smoking | 3.6 (2.3–5.5) | 3.7 (2.3–5.8) |
| Hypertension*Diabetes*Dyslipidemia*Obesity | 15.2 (8.1–28.5) | 17.2 (8.4–35.1) |
| Hypertension*Diabetes*Dyslipidemia*Smoking | 11.4 (4.7–18.2) | 9.7 (5.7–16.2) |
| Hypertension*Diabetes*Obesity*Smoking | 10.6 (5.1–22.0) | 11.8 (5.4–25.6) |
| Diabetes*Dyslipidemia*Obesity*Smoking | 9.9 (4.2–23.1) | 11.3 (4.5–28.35) |
| Hypertension*Diabetes*Dyslipidemia*Obesity*Smoking | 10.2 (3.6–14.3) | 8.2 (4.3–12.1) |
CI: confidence interval; PAD: peripheral arterial disease; OR: odds ratio.
CVD are the leading cause of death in Latin America, with ischemic heart disease as the principal cause in most countries.1 Global attention has been devoted to understanding CVD; however, little observance has been dedicated to PAD as few epidemiological studies have been conducted, especially in low or middle-income countries. Colombia is experiencing a rapid population growth, being today the third-most densely inhabited country in Latin America after Mexico and Brazil. In addition, years of armed conflict have obligated thousands of people to migrate from rural to urban areas, a phenomenon that has affected their access to education, basic needs and health care.10 While the ongoing recovering of Colombia's economy has improved the living standards in urban areas, the population exposure to environmental and life style risk factors such as poor diet, cigarette consumption, and physical inactivity, among others has also increased.11 Therefore, disease pattern and level of exposure to risk factors vary depending on the particular conditions of each country, and thus, the strategies to prevent and control disease burden cannot be transversally applied.
In the present study, the overall prevalence of PAD was 4.4%, being higher among women (4.7%) compared to men (4.0%) but consistently increasing with aging in both genders (Fig. 1). This observed overall PAD prevalence is lower than the previously reported for other Latin American studies conducted in Ecuador (7%), Brazil (10.5%), and Mexico (10%).12–14 The observed differences might be due, in part, to selection criteria, population characteristics, study setting (rural vs. urban), and sample size. However, these differences may, in fact, reflect precisely across-countries variation on population exposure to know risk factors such as smoking, hypertension, dyslipidemia, diabetes, obesity and history of CVD.15 As stated before, the migration from rural to urban settings is increasingly exposing the Colombian population to CVD risk factors, and thus, the observed PAD prevalence although low, raises important public health challenges to control and manage CVD burden.
With regards to gender, our results are consistent with a recent meta-analysis, including 34 community-based studies with a total of 112,027 individuals, showing that in low or middle-income countries, PAD prevalence was consistently higher in women compared to men up to 85–89 years of age, although the difference narrowed with aging.1 These gender-based prevalence differences could be related to “unidentified risk factors” or might represent a survival advantage for women, with men being more likely to experience death from concomitant coronary heart disease.15 In addition, our study shows, as well as in many others, that the ABI increased with aging.2–4 This is probably due to an increased prevalence of other atherosclerosis risk factors with aging, which also triggers the progression of PAD.16,17 Furthermore, our study confirms the role of traditional CVD risk factors on PAD prevalence (Table 1), which have been consistently reported as major predictors of morbidity and mortality,18 and support the argument for PAD prevalence variation depending on the population distribution of CVD risk factors.1,15 Finally, our results corroborate previously reported observations of a cross-sectional study conducted in Bucaramanga, Colombia. This established the prevalence of CVD risk factors in a random sample of the general population (2989 subjects, 15–64 years old), showing that smoking, hypertension, obesity, high cholesterol, and diabetes were significantly prevalent, calling for actions to control the ongoing CVD epidemic.19
Based upon our data (Table 2), an alarming increase in CVD could be expected in the coming decades in the study population, as smoking, hypertension, dyslipidemia, and obesity were quite prevalent among non-PAD subjects with proportions of 35.2, 33.6, 23.5, and 13.8%, respectively. Among PAD cases, hypertension was the strongest predictor for disease risk with an adjusted OR of 4.9, followed by diabetes>dyslipidemia>obesity. In particular, our study shows that the TG/HDL ratio was the most important contributor to dyslipidemia, increasing the risk for PAD in 4.7-times after adjustment (Table 3), which is also consistent with previous studies indicating that the TG/HDL ratio is a powerful independent indicator of extensive coronary artery disease, heart failure, and atherosclerosis.20–22 On the other hand, our study shows that cigarette smoking was weakly associated to PAD, increasing the risk to 1.6 times (95% CI 1.35–2.02) in the adjusted OR model. Worldwide, cigarette smoking is the most important risk factor associated to PAD, increasing the risk for the disease in up to 7 times compared to non-smokers.23 However, our results are consistent with a recent meta-analysis study showing that cigarette smoking is strongly associated to PAD in high income countries (meta-OR for current smoking of 2.72; 95% CI 2.39–3.09) while in low or middle income countries, as is the case for Colombia, cigarette smoking plays a lesser role (meta-OR 1.42; 95% CI 1.2–1.62).1 Finally, PAD subjects reported more often having a history of acute myocardial infarction (31%) and stroke (8.1%). In a follow-up study, it was previously established that PAD patients present a 3.1-times increase in risk for death from all causes, 5.9 from CVD, and 6.6 from coronary heart disease.15–19,24 Altogether, our results suggest the use of ABI as a sensible, low cost method to indirectly suspect the presence of atherosclerotic events in other arterial beds, which can help to implement intervention and follow-up strategies among individuals with medium to high risk for CVD. Such recommendation is also supported by other studies that proclaim the use of the ABI in primary health care for early identification of PAD in populations with prevailing CVD risk factors.25–27
In addition to establishing the distribution of traditional CVD risk factors and their effect on PAD risk, we investigated the frequency distribution of harboring a combination of CVD risk factors on PAD subjects (Fig. 2). Accordingly, the majority of PAD subjects harbored the presence of three (35.2%) or four (30%) CVD risk factors and a lesser percentage presented five (11.6%) simultaneously. This observation may be partly explained by the fact that the sample for subjects harboring three or four CVD risk factors had a higher number of individuals, thus, allowing for a greater identification of PAD cases within these categories. It could also be related to a reduction of life expectancy among subjects harboring five CVD risk factors compared to the other those in the three or four CVD risk factors’ categories (Mean age 71.94±8.37 vs. 74.51±8.98, respectively, p=0.058), which could lead to underestimating PAD prevalence given the less number of subjects on this group.
It should be noted that in our study population the four most important risk factors for PAD were hypertension, diabetes, dyslipidemia, and obesity, increasing the disease risk up to 17.2-times (Table 4). On the other hand, cigarette smoking did not contribute to a significant increase in PAD risk, as it could have been expected when adding it as the fifth interaction term in the model, but it rather reduced the risk. The higher effect of obesity compared to cigarette smoking on PAD risk when interacting with hypertension, diabetes and dyslipidemia highlights the importance of controlling poor diet and irregular physical exercise to manage PAD. The lower risk effect of smoking could be due to subject's self-awareness to consume fewer cigarettes when presenting multiple risk factors or subjects being less capable of economically sustaining the habit while having to invest on medication to treat other health conditions. A review on the relationship between obesity and smoking28 indicates that an inverse relationship between these two factors has been established by epidemiological studies,29 as in the general population, smokers usually weigh less than non-smokers.30 However, obese smokers tend to consume more cigarettes due to the reinforcement effect of nicotine and are at higher risk given the simultaneous presence of other lifestyle risk factors, including low fruit and vegetable intake, less physical activity and higher alcohol consumption.28 In the present study, a dose-response relationship between the number of cigarettes smoked per year and the increase in risk of PAD was not established. Therefore, we cannot conclude with certainty about the effect of cigarette smoking exposure level or its interaction with other CVD risk factors on PAD risk in the study population.
Our study presented some limitations, including: (a) study population was not recruited at random but rather by convenience, and thus, our observations might not entirely represent the general population; (b) although the ABI test has been validated and widely used for the screening of PAD and prediction of CVD, additional evaluation through Doppler ultrasound would have been recommended for a more accurate diagnose of PAD cases; (c) finally, data on the number of cigarettes and years of exposure were not collected, and thus, the risk posed by the intensity of cigarette consumption on PAD was not estimated in the study population, which could partly explain why a strong increase in risk for PAD was not observed among smokers.
This epidemiological study represents, to the best of our knowledge, the largest conducted so far in Latino America and the first community-based cross-sectional study in Colombia using the ABI to establish the prevalence of PAD in an adult population. We conclude that PAD prevalence was relatively low (4.4%), considering the overall mean age of the study population (61±11.4 yrs) and the prevalence distribution of traditional CVD risk factors. However, our study confirms the role of these factors on disease risk, favors the argument for PAD prevalence variation depending on CVD risk factor distribution, and supports the use of ABI measurement for PAD diagnosis among intermediate to high-risk patients. Therefore, these observations are of relevance for clinicians as, based upon our results, patients can be early identified at higher CVD disease risk to receive a more immediate intervention. Finally, given the strong, interaction effect between hypertension, diabetes, dyslipidemia, and obesity on the risk of PAD, our results provide scientific evidence for local health authorities to support the need for better policies and strategies aimed to prevent and control the observed risk factors for reducing CVD burden.
Ethical responsibilitiesProtection of people and animalsThe authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in agreement with the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors state that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is in the possession of the correspondence author.
Financial supportThis work was supported in part by a grant from the Department of Science, Technology and Innovation (COLCIENCIAS), Colombia (No. 110351929119).
Conflict of interestThe authors declare no conflicts of interest.
The authors express their gratitude to all staff members of Hospitals in the municipalities of El Tambo, Puracé, Silvia, Mercaderes, Rosas, Timbío, Bolivar, La Sierra, La Vega, Cajibio, Argelia, Tunia, Sotará, Piendamó, Miranda, El Bordo, Santander de Quilichao, Totoró, Almaguer, Inzá, Puerto Tejada, Balboa, Morales, San Sebastián, Belalcazar, Coconuco, Sucre and the Saint Joseph University Hospital, Nueva EPS and Salud Vida in Popayán. We are also indebted to all the volunteers who participated in the study. Finally, we acknowledge the collaboration of the administrative personnel of the Human Genetics Laboratory and the Vice-presidency for Research from the University of Cauca.