Case report
A 28-year-old woman was referred to our department, she had a history of 10- day progressive dyspnea, five hours previous to her admission at the hospital, and she presented suddenly oppressive anterior chest pain without irradiations, accompanied by palpitations at rest and orthopnea. Physical examination showed heart rate (HR) of 110 beats/min, blood pressure (BP) of 120/40 mmHg and the jugulars veins were distended. There was a grade 4/6 continuous murmur along the left parasternal line without thrill. There was also hepatomegaly 2-2-1. The electrocardiogram showed tachycardia and incomplete right bundle branch block. An increase of pulmonary vascularity and normal cardiac silhouette was seen in the chest x-ray.
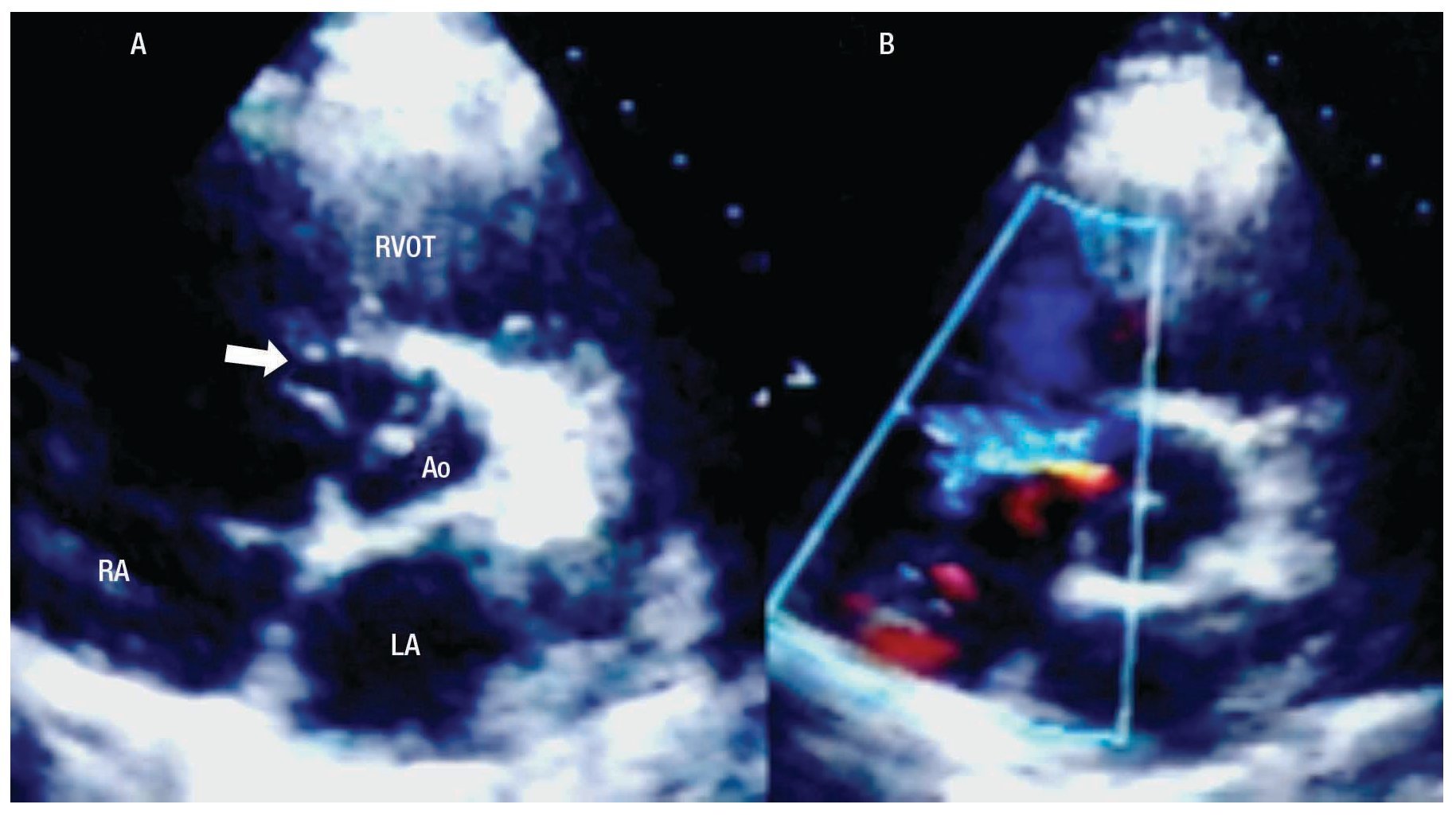
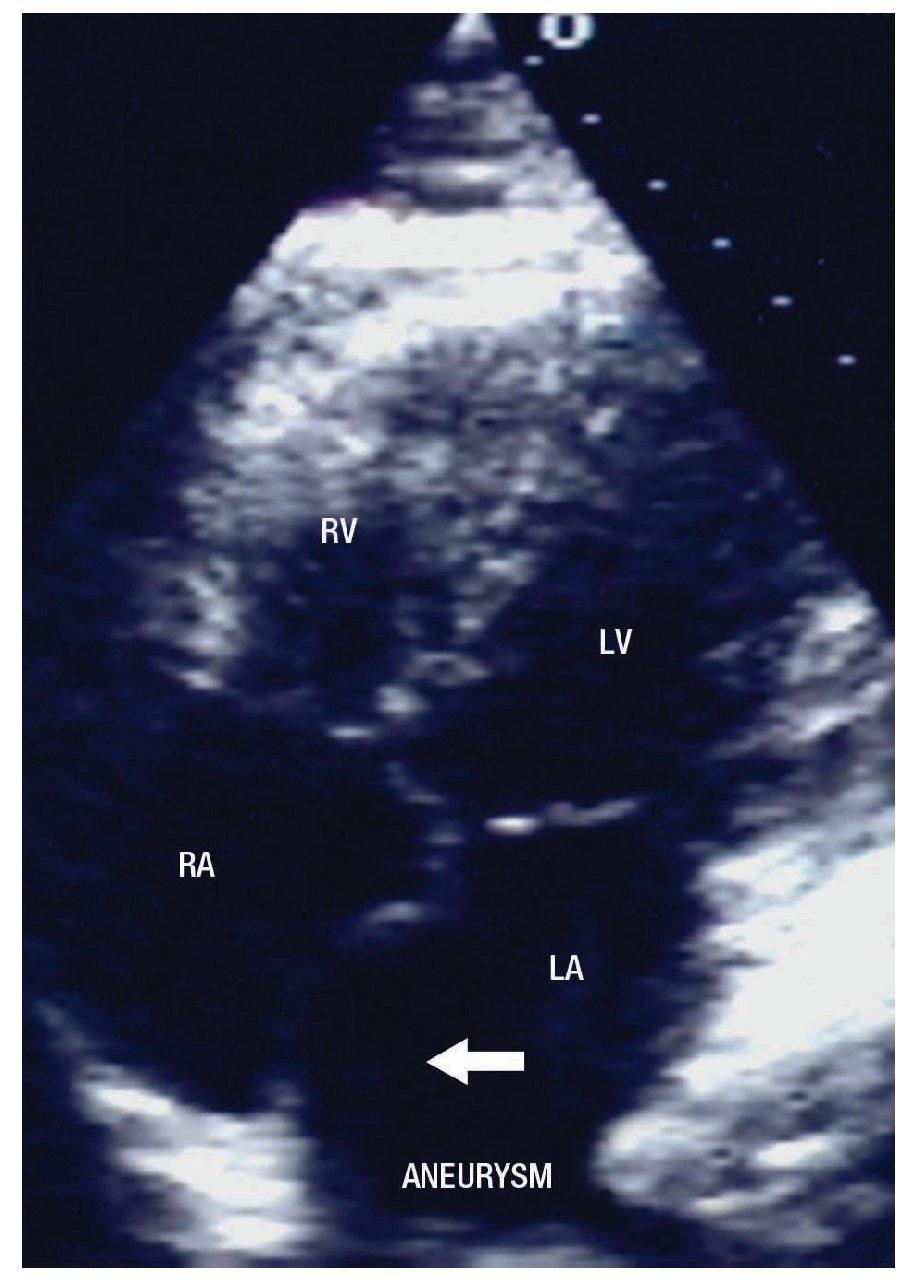
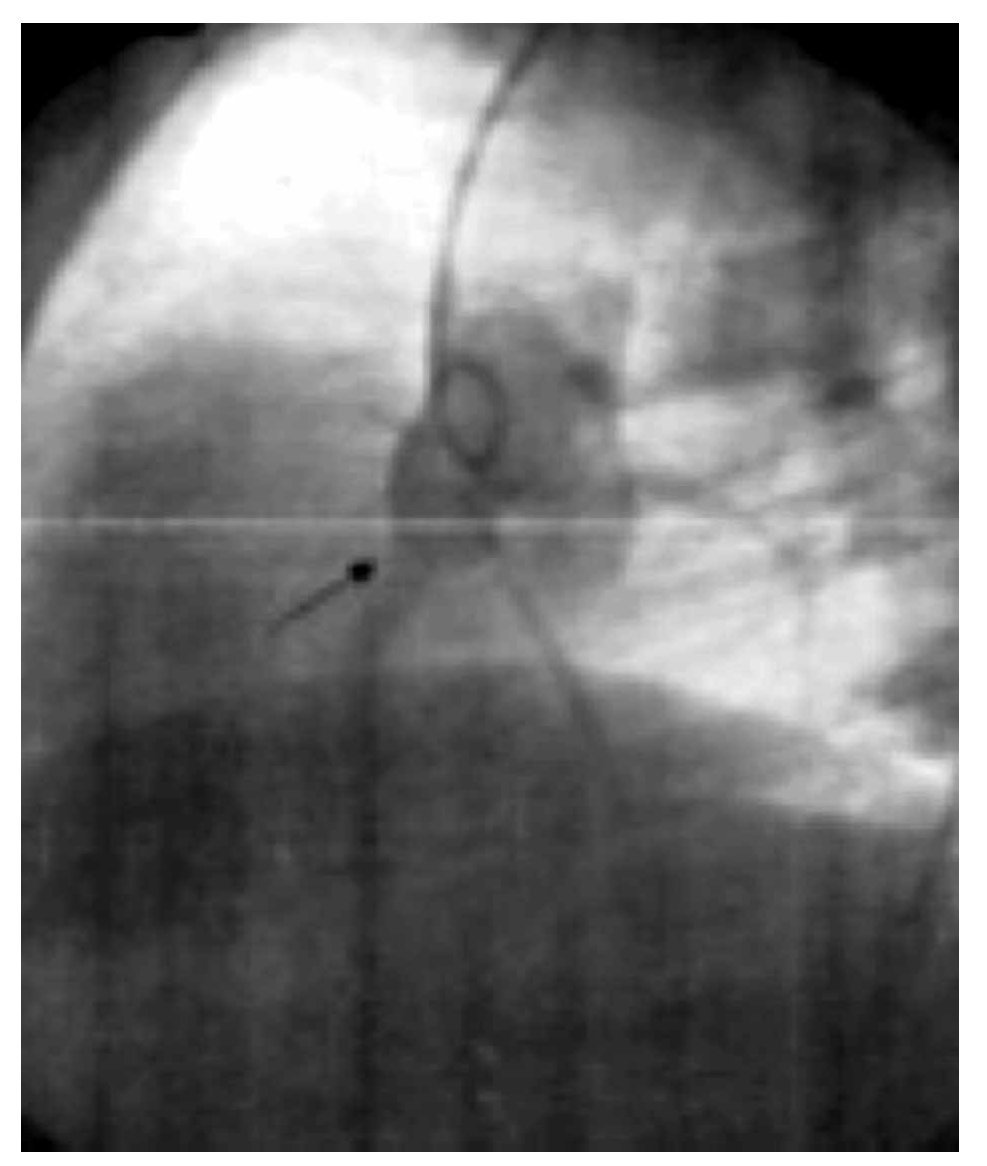
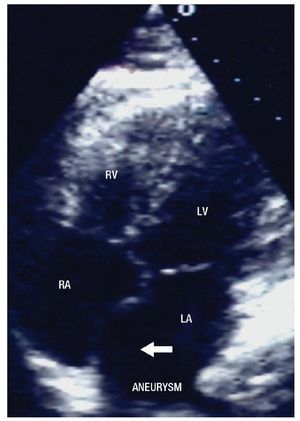
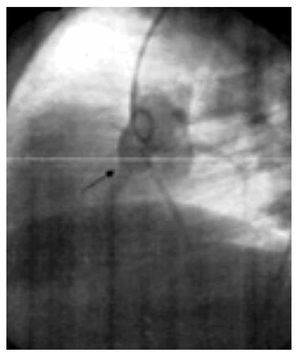
The two-dimensional Doppler echocardiogram documented a short aneurysmal dilatation of the right sinus of Valsalva, protruding into the right atrium. The place of rupture was shown with color flow imaging, revealing a unidirectional continuous mosaic jet from the aorta to the right heart chamber, (Figure1A, 1B). In a four chamber view with posterior angulation, an interatrial septal aneurysm (Figure 2) with a 19 mm base and an 11 mm right excursion beyond the plane of the atrial septum was found. No association with patent foramen ovale was observed with agitated saline solution (baseline and Valsalva maneuver). This defect had all the features to be deemed an aneurysm, the cutoff criteria reported in an autopsy study by Silver and Dorsey1 was used. Cardiac catheterization with contrast medium injected into the ascending aorta showed shunting from the right sinus of Valsalva to the right atrium (Figure 3). Oximetries and pressure in this cavity were increased. Surgical closure of the communication was performed under extracorporeal circulation. The aortotomy showed the sinus of Valsalva aneurysm's origin in the right coronary sinus. The right atrium was open and the aneurysm´s coat was seen, including the basal and medium portion of the interventricular septum. Resection of the aneurysm´s coat was made and replaced with a 1.5 cm diameter woven Dacron patch. Aortic valve replacement was not considered necessary. Laboratory tests searching for connective tissue disease were negative. In the early postoperative period, the patient developed variable degrees of atrioventricular block, and a definite VVI pacemaker was implanted. The patient was discharged on the 10th postoperative day being in a NYHA functional class I.
Figure 1. A. Two-dimensional short axis view at the level of the great arteries, showing the site of the rupture (arrow) of the right coronary sinus aneurysm into the right atrium. B. With color Doppler flow, the turbulent flow through the site of the rupture is evidenced. Abbreviations: RA=Right atrium, RV= Right ventricle, LA=Left atrium, RVOT=Right ventricular outflow tract, Ao=Aortic valve
Figure 2. Four chambers apical view with posterior angulation, showing the atrial septal aneurysm (arrow). Abbreviations: LA=Left atrium, LV= Left ventricle, RA= Right atrium, RV= Right ventricle.
Figure 3. Aortography at the root of the aorta, in left anterior oblique projection with abnormal flow in the ruptured aneurysm of the right sinus of Valsalva (arrow).
Discussion
Aneurysm of the sinus of Valsalva (ASV) is a rare cardiac abnormality, occurring in 0.15% to 1.5% of patients who undergo cardiopulmonary bypass. They may be congenital or acquired. A congenital aneurysm is caused by separation or failed fusion of the aortic media layer with the fibrous annulus of the aortic valve. Acquired aneurysm of sinus of Valsalva can develop as the result of traumatic injury,2 endocarditis,3 syphilis,4 Behcet´s disease5 or Marfan´s syndrome. The first reports of ASV appeared in the 19th century and Lillehei et al.6 reported the first successful surgical repair in 1957.
Aneurysms are generally silent for prolonged periods of time. Regarding complications, the most frequent occurrence is rupture. It occurs in most cases between the third and fourth decades of life.7 The clinical presentation of this entity varies considerably due to the fact that it can be asymptomatic. It can be found in a postmortem or angiographic study, or they present with cardiogenic shock and death. This wide variety in initial clinical presentations can be due to the size of the shunt; little shunts are asymptomatic, and big aortocardiac fistulas cause a clinical presentation similar to an acute aortic regurgitation.8
There are numerous complications that can originate from a Valsalva aneurysm, including obstruction of the right ventricular outflow tract, infectious endocarditis, and thrombus formation, with systemic or local embolic events.7 The compression of the origin of the coronaries or the obstruction of their ostia can cause ischemia or necrosis. Ischemia has been reported as a conditioning factor for ventricular fibrillation in some patients.9 Whereas, in others, ventricular fibrillation is secondary to the dissection of the interventricular septum due to a ruptured aneurysm at this site. In this case, complete atrioventricular block usually accompanies the clinical presentation.10
Aneurysms are most frequently localized at the right sinus of Valsalva (76.8%) and at the non-coronary sinus (20.2%). Left sinus of Valsalva localization is not very frequent (3%).11 The least frequent sites are the pericardium, pleural space, interventricular septum,9 or the left ventricle chambers.
Eventhough the first report of a Valsalva aneurysm diagnosed by echocardiography was in 1974,12 currently the gold standard for the diagnosis of these lesions continues to be cardiac catheterization with aortography. Development of new generation ultrasonography machines has made transthoracic and, especially, the transesophageal echocardiography a useful tool in the confirmation of the diagnosis.8,13 Additionally, it may help in the differential diagnosis of other abnormalities causing continuous murmurs like patent ductus arteriosus, aorto-pulmonary window, or coronary fistulas. Nuclear magnetic resonance imaging is equally useful,14 but more expensive and less available than echocardiography. The latter is a better and faster diagnostic tool, especially when dealing with critically ill patients.
Congenital sinus of Valsalva aneurysms can coexist with other malformations; the most common of which is the association with subaortic ventricular septal defect (25-55%) and aortic regurgitation. Other associated abnormalities less frequently found are pulmonary stenosis, patent ductus arteriosus, atrial septal defect, subaortic stenosis, and Fallot's Tetralogy.7
Regarding the atrial septal aneurysm,1,15 this is an infrequent finding in adult patients. Its formation may be secondary to raised interatrial pressure gradients, producing a bulging septal shift toward the low-pressure side.
However, it has been found also in patients with normal atrial pressure,15 suggesting a primary malformation.16
The coexistence of sinus of Valsalva aneurysms and atrial septal aneurysms is very rare. Atrial septal aneurysm is believed to be a disorder of connective tissue,17 which is manifested along time in adults. In fact, connective tissue abnormalities must be suspected when both defects coexist together. Replacement of the valve was not considered necessary in the absence of aortic valve dysfunction. Regarding the atrioventricular block, this was temporal, because in a follow-up revision of the patient, she had sinus rhythm, alternanting with pacemaker rhythm (data not shown). We consider that the presence of this block was secondary to the inflammatory process of the surgery.
The present case illustrates the rare coexistence of two congenital malformations, as are sinus of Valsalva aneurysm and aneurysm of the interatrial septum.
Corresponding author: Nilda Espinola Zavaleta.
Instituto Nacional de Cardiología Ignacio Chávez. Echocardiography in Out-patient's Clinic.
Telephone: 5573-2911 ext. 1196.
E-mail:niesza2001@hotmail.com
Received on November 26, 2008;
accepted on March 29, 2010.