Catheter-based ablation of isthmus-dependent common atrial flutter results in very high success rates and almost no complications. However, bidirectional conduction block through the isthmus may be challenging in a small percentage of patients regarding the use of high power and high temperature settings during radiofrequency delivery. Anatomical and physiological circumstances may be the reason for such difficulties to achieve bidirectional block at the cavo-tricuspid isthmus. However, in the present case we show edema formation after multiple shots of radiofrequency delivery at the cavo-tricuspid isthmus, which complicates the achievement of bidirectional conduction block.
La ablación con catéter del flutter de tipo común dependiente del istmo es un procedimiento con elevada tasa de éxito y rara vez complicaciones. Sin embargo, en un pequeño porcentaje de casos puede resultar complicado conseguir el objetivo de bloquear bidireccionalmente el istmo durante el procedimiento de ablación a pesar de incrementar la potencia y temperatura del catéter de radiofrecuencia. Las características anatómicas y fisiológicas del istmo cavo-tricuspídeo pueden ocasionar dificultades en la consecución del bloqueo bidireccional. Sin embargo, en el presente caso mostramos cómo la propia aplicación de radiofrecuencia puede ocasionar edema importante tras múltiples aplicaciones con las consiguientes dificultades para conseguir el bloqueo bidireccional del istmo.
Common atrial flutter is one of the most common arrhythmias treated with catheter-based ablation. The procedure is safe and results in very high short- and long-term success rate among the current ablation procedures.1 The aim of the procedure is to block bidirectional conduction through the cavo-tricuspid isthmus rather than acute termination of the arrhythmia, which may give rise to recurrences in the follow-up. However, conduction block through the isthmus may be challenging in a small percentage of patients due to anatomical and physiological circumstances such as the presence of isthmus pouch, extensive Eustachian ridge and catheter instability.2 Radiofrequency delivery may also complicate the aim of achieving isthmus blockage because of protein denaturalization and edema formation.3 The latter may lead to persistence of conduction in specific gaps underneath edema, which also complicates identifying electrical signals due to electrogram attenuation. In addition, attempting to use either higher power and temperature by non irrigated-tip catheters or deeper lesions by irrigated-tip catheters is not exempt of complication risk. Thus, the trajectory of the right coronary artery is just underneath the isthmus, which makes it sensitive to vascular damage with deeper lesions. Although such a possibility has been barely reported and the vascular flow may protect any potential excessive increase in temperature, the distance between the endocardium and the coronary artery varies from 2 to 17mm depending on the septal, central or lateral location at the cavo-tricuspid isthmus area.4 The latter makes the artery sensitive to damage in those patients, where the artery is in intimate proximity to the endocardium.5
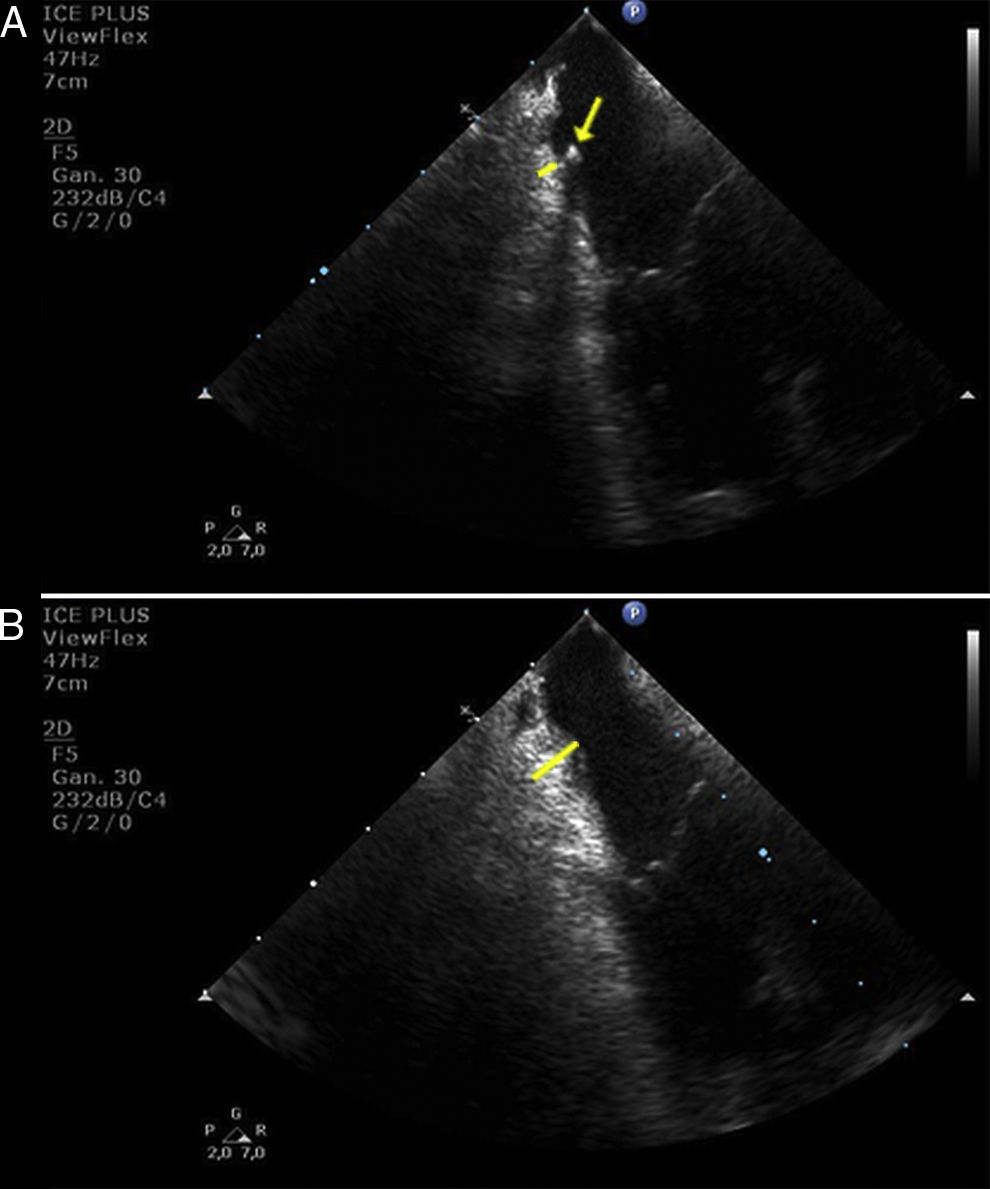
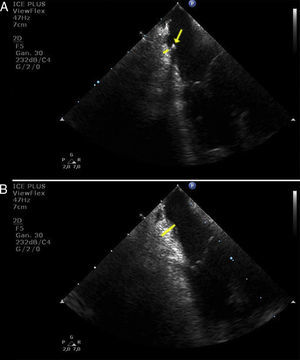
In the present report we show a striking image of edema formation during radiofrequency delivery at the cavo-tricuspid isthmus in a patient who underwent common atrial flutter ablation using real-time monitoring by intracardiac echocardiography. Fig. 1 shows the baseline thickness of the isthmus before radiofrequency delivery (panel A, yellow line). The yellow arrow points the transversal section of the multipolar catheter positioned around the tricuspid annulus. Complete conduction blockage through the isthmus was challenging despite the use of 8-mm non-irrigated-tip catheter and progressive increase in generator power up to 90W. Isthmus blockade was achieved after 832s of radiofrequency delivery. In Fig. 1, panel B, we show a significant increase in the thickness of the isthmus at the end of the procedure (Yellow line), and no complications were present. However, we postulate that the use of intracardiac echocardiography may help to decide when to stop the ablation procedure and postpone it for a second procedure in two weeks, upon resolution of the acute edema. Such an approach might avoid the above-mentioned severe potential complications due to deeper lesions and higher chances of right coronary artery damage. In addition, remaining conduction gaps might be easily identified and ablated after edema disappears. Therefore, it might be reasonable to use an intracardiac echocardiography probe in difficult ablation procedures after multiple ablation lesions and long radiofrequency delivery time.
Edema formation at the cavo-tricuspid isthmus. (A) baseline thickness of the cavo-tricuspid isthmus (yellow line) and cross-sectional view of a multipolar catheter positioned around the tricuspid annulus (yellow arrow). (B) significant increase in thickness at the cavo-tricuspid isthmus after radiofrequency delivery (yellow line).