Epiblepharon is a congenital eyelid malposition due to a horizontal skin fold and redundant orbicular muscle, resulting in the inturning of the eyelashes.
ObjectiveWe report our experience in the non surgical correction of symptomatic epiblepharon using a pretarsal injection of 5IU of Botulinum toxin type A (BoNT-A) in the orbicular muscle.
Materials and methodsPatients with epiblepharon younger than 2 year were included in the study and their charts were reviewed. Symptoms and signs of epiblepharon were evaluated before and after treatment with BoNT-A.
Results40 patients were included (28 girls [70%]). Average age at treatment was 11 months (range, 4–24 months). 76 eyelids were treated with BoNT-A. A statistically significant improvement in symptoms, lash-corneal touch and punctate corneal epitheliopathy were reported after the treatment with 5IU BoNT-A. Average final follow-up was 25.5 weeks (range, 4–92 months).
ConclusionsThe present study evidences that a pretarsal BoNT-A injection is an effective and safe treatment for the correction of symptomatic epiblepharon in patients younger than 2 years.
El epiblefaron es una malposición palpebral congénita ocasionada por un pliegue redundante de piel y músculo orbicular que invierte las pestañas hacia el globo ocular.
ObjetivoReportamos nuestra experiencia en la corrección no quirúrgica del epiblefaron sintomático usando una inyección en el músculo orbicular pretarsal de 5 Unidades de toxina botulínica tipo A (TbA).
Material y métodosRevisamos los expedientes de los pacientes menores de 2 años con epiblefaron tratados con TbA. Evaluamos los síntomas y signos del epiblefaron, previo y posterior al tratamiento.
ResultadosSe incluyeron un total de 40 pacientes (28 niñas [70%]). La edad media de presentación fue 11 meses (rango 4–24). Se trataron 76 párpados con TbA. Obtuvimos una mejoría estadísticamente significativa de los síntomas, del contacto cilio corneal y de la afectación corneal tras la aplicación de 5 unidades de TbA. El periodo medio de seguimiento fue de 25,55 semanas (rango 4–92).
ConclusionesCon este estudio demostramos que la aplicación de TbA es un tratamiento efectivo y seguro para la corrección del epiblefaron sintomático en niños menores de 2 años.
Epiblepharon is a congenital eyelid malposition characterized by a horizontal skin fold and the underlying orbicular muscle that pushes the eyelids towards the ocular globe.1 Clinic expressions depend on the amount of inverted eyelashes and the grade of ocular friction. The most common symptoms of epiblepharon comprise ocular discomfort, red eye, secretion and tearing.2 Its prevalence diminishes with age and is more frequent in the Asian population.3
Epiblepharon resolves spontaneously between 2 and 3 years of age with the growth of facial structures. However, in highly symptomatic children with corneal compromise, treatment is required to avoid visual capacity impairment. Several surgical treatments are available for severe cases. However, the purpose of the present study is to assess the efficacy of a nonsurgical treatment consisting in the application of TbA over the pre-tarsal portion of the orbicular muscle.4 The application of TbA for treating symptomatic epiblepharon was first described by Chen and Nava-Castañeda.
Material and methodsA retrospective, longitudinal study carried out in the Conde de Valenciana Ophthalmological Institute (I.A.P.), CDMX. The records of all patients with epiblepharon between January 2012 and July 2017 were analyzed. The present study fulfills the requirements of the Helsinki Declaration and was approved by the Research Committee of the Conde Valenciana 17CI 09015008 Ophthalmological Institute. The study included patients with epiblepharon diagnostic initially treated with TbA before 2 years of age. The study excluded patients with systemic diseases, previous eyelid surgery or concomitant eyelid malpositioning. In addition, the patients who did not return for follow-up after the application of TbA were also excluded from the study.
The following clinic record data were obtained: age, sex, pathological history and laterality of the compromised eye. The dependent variables comprised tearing, ciliocorneal contact, and the overall dosage of TbA units applied to each eyelid. Tearing was measured as absent or present. Ciliocorneal contact was defined as presence of keratitis assessed with fluorescein staining. These dependent variables were measured with a nominal dichotomic scale in the checkup prior to the application of TbA and in the subsequent checkup visits.
All the procedures took place in the consulting practice. The patients were adequately immobilized with the help of the parent who wrapped the patient in a surgical sheet, leaving the head uncovered. The infant was then placed on the hospital gurney with the head securely held. Ocular anesthetic consisting in 5% tetracaine hydrochloride drops (Ponti- Ofteno, Sophia, Mexico) were placed on the surface of the eye. The injection site was cleaned with a cotton swab with 70 % alcohol. The treatment consisted in the injection of 5 units of ona-BoNTA (Botox®, Allergan, Inc, Irvine, CA, USA) utilizing a 1 ml syringe with a 31 G insulin needle (Plastipak, Mexico State, Mexico). Said product was applied on the medial region of the pre-tarsal portion of the orbicular muscle at a distance of 3−4 mm from the free palpebral edge of the compromised side.4
Descriptive statistics were taken for all variables and inferential statistics with χ2 and Fishers exact test for analyzing dependent variables. The statistical SPSS application (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) was used, and a p < 0,05 was applied for significance.
ResultsOf all analyzed files, 40 patients fulfilled the inclusion criteria and had at least one post-TbA visit. TbA was applied on 76 eyelids because in 4 patients epiblepharon was symptomatic in one eye only. Overall, 70% (28 patients) were female. The average age at the first TbA dose was 11 months (range 4–24). Mean follow-up time of patients was 25.55 weeks (range 4–92). Each TbA application comprised 5 units. In 59 eyelids TbA was applied only once, in 16 eyelids its was applied twice and in one eyelid it was applied 3 times. Mean time between applications was 19 ± 8 weeks.
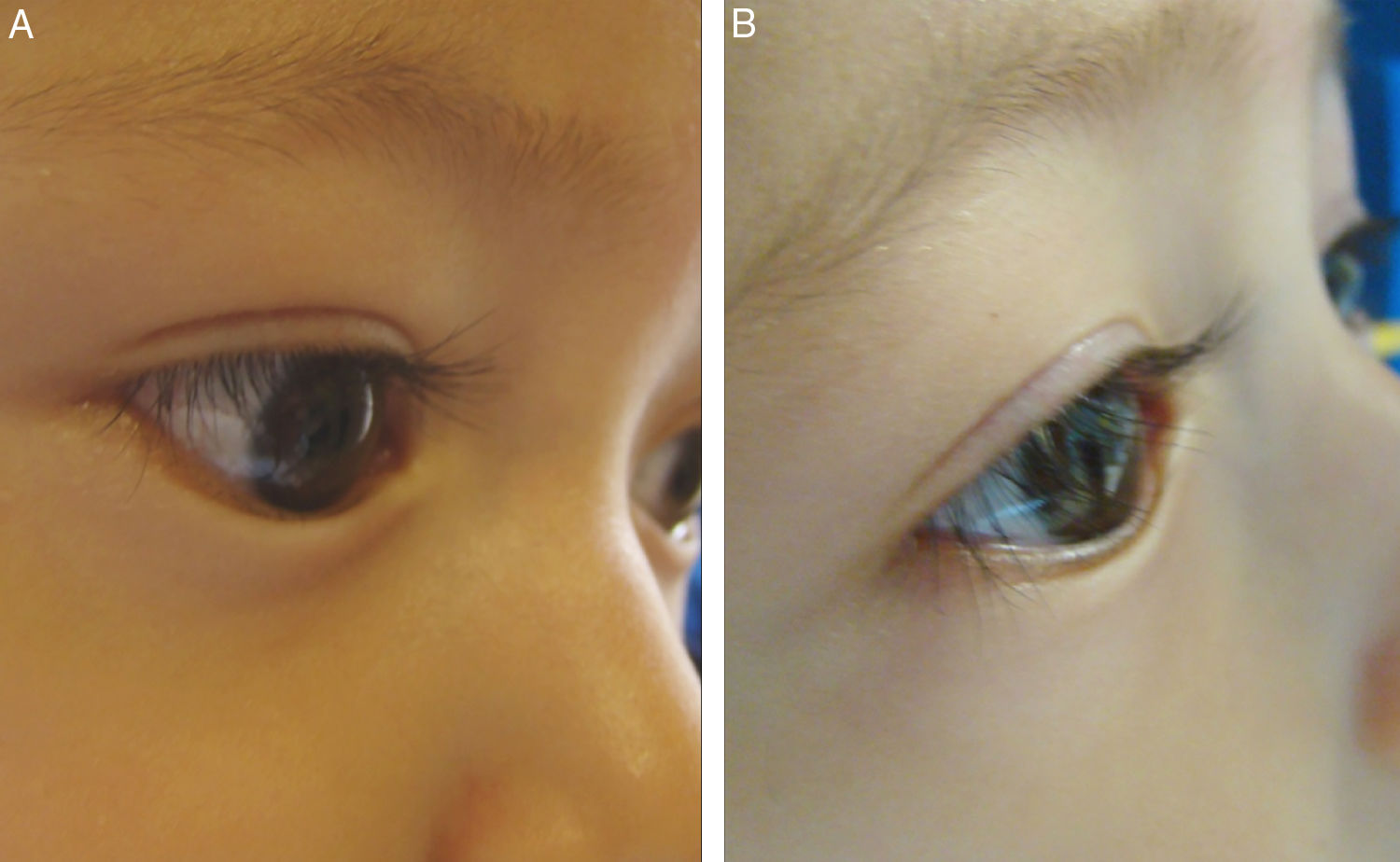
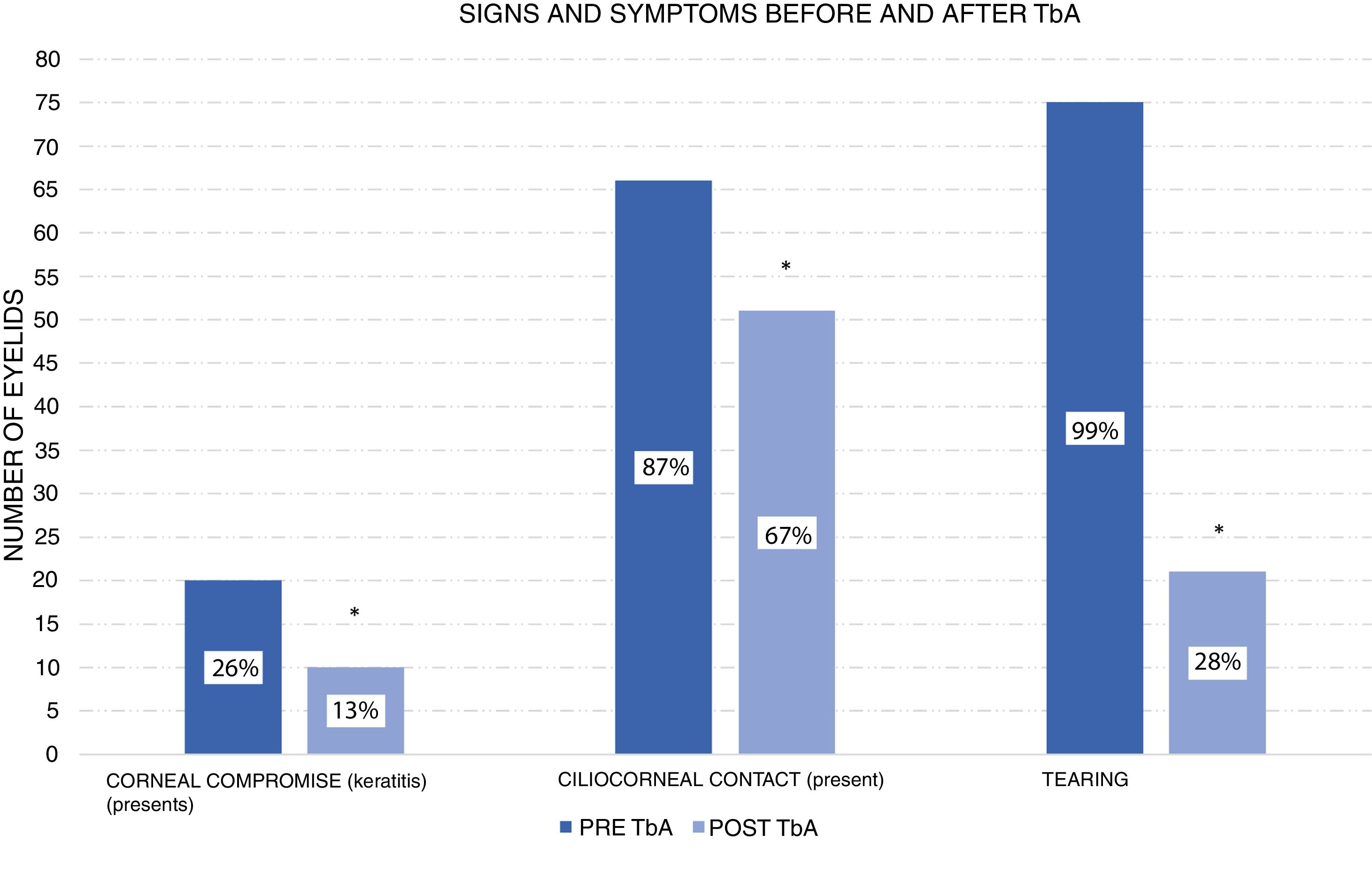
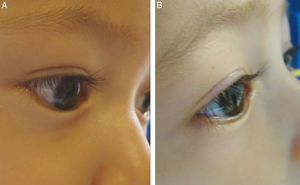
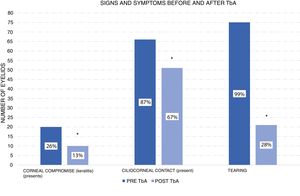
According to the corneal compromise classification developed by Khwarg and Lee,1 74% (56 eyes) were in stage I without exhibiting corneal compromise, with 26% (20 eyes) classified in stage II, involving under 2/3 corneal compromise. After the application of 5 UI TbA units in the first control visit only 13% of eyes exhibited corneal compromise, while the remaining 87% (66 eyes) exhibited ciliocorneal contact prior to the application of TbA. Said percentage diminished to 67% (51 eyes) after the application of TbA. Figs. 1 and 2 show clinic results obtained in 2 study patients. All eyes exhibited symptoms of tearing prior to the application of TbA, while in the subsequent visits tearing was absent in 72% (48 eyes). These changes in epiblepharon signs and symptoms were statistically significant (Fig. 3).
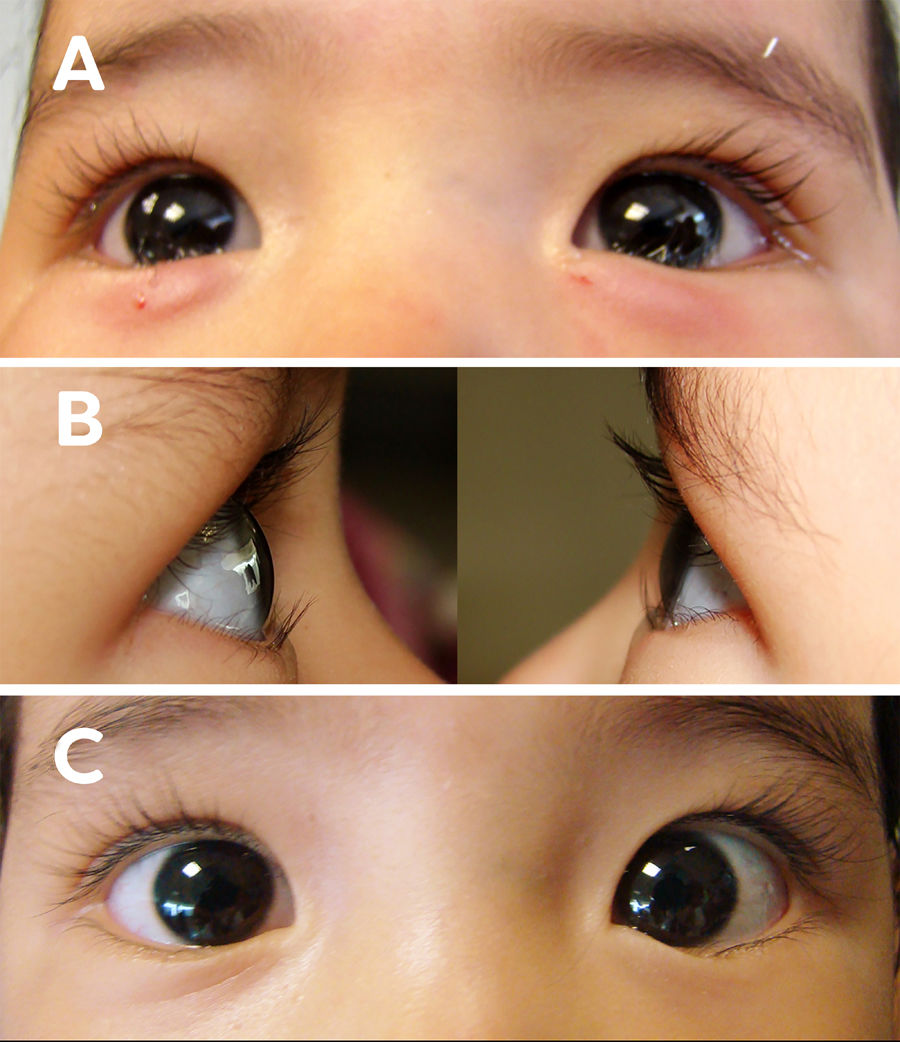
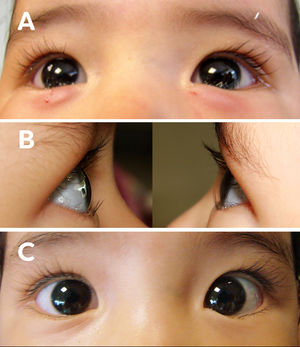
Female patient, age 1, with bilateral epiblepharon. (A) clinic photograph immediately after treatment with 5 UI of TbA in the pre-tarsal region of the orbicular muscle. (B) clinic photographs at one month of treatment with 5 UI of TbA, showing right and left eye respectively without ciliocorneal contact. (C) frontal clinic photograph of both eyes at one month of treatment with 5 UI of TbA.
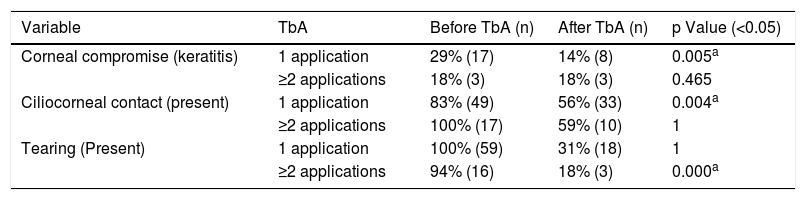
The eyelids that received more than one TbA application exhibited improvements in symptomatology and ciliocorneal contact. However, no changes were observed for corneal compromise (Table 1).
Comparison of signs and symptoms of epiblepharon before and after treatment, indicating in the number of TbA applications.
| Variable | TbA | Before TbA (n) | After TbA (n) | p Value (<0.05) |
|---|---|---|---|---|
| Corneal compromise (keratitis) | 1 application | 29% (17) | 14% (8) | 0.005a |
| ≥2 applications | 18% (3) | 18% (3) | 0.465 | |
| Ciliocorneal contact (present) | 1 application | 83% (49) | 56% (33) | 0.004a |
| ≥2 applications | 100% (17) | 59% (10) | 1 | |
| Tearing (Present) | 1 application | 100% (59) | 31% (18) | 1 |
| ≥2 applications | 94% (16) | 18% (3) | 0.000a |
No complications secondary to TbA application were observed.
At the end of the follow-up period, epiblepharon was resolved in 43% of cases (33 eyelids). In these patients, the mean follow-up time was 33 weeks (range 4–90). As for the remaining 57% (43 eyelids) that did not exhibit complete resolution of epiblepharon, tearing symptomatology disappeared in 56% after treatment with TbA.
DiscussionEpiblepharon is a frequent disease in ophthalmological clinic practice. In the institution of the authors, the prevalence is of, patients. It resolves naturally with the growth of facial structures. However, while epiblepharon is present, its symptoms such as, tearing, secretion produce discomfort in patients. In addition, it could compromise the integrity of the cornea, produce keratitis due to ciliocorneal contact. Severe cases exhibit epithelial defects, leukoma that could diminished visual acuity. It is important to treat epiblepharon with ocular lubricants, preferably in the form of creams, as, they assist in protecting the cornea.5 However, when symptoms persist despite treatment, the use of TbA injected in the pre-tarsal portion of the lower eyelid orbicular muscle is proposed. This technique was first described by Chen, Nava-Castañeda who treated eyelids with epiblepharon with a single dose of 12,5, IU of TbA (abo-BoNTA, Dysport®Ipsen LTD, Wrexham, United Kingdom), found a statistically significant improvement of symptoms, signs of epiblepharon4.
TbA is a neurotoxin produced by Clostridium botulinum. It bonds with cholinergic nerve endings, penetrating the cells and producing the inhibition of acetylcholine release.6 TbA has been utilized to weaken muscle fibers in various diseases. It has been used in eyelids to diminish the function of the orbicular muscle by injecting the toxin on the muscle belly. Steel et al.7 reported improvement in involutive entropion after the use of TbA. Christiansen et al.8 utilized 5 U of TbA in a child with congenital entropion with positive results and no adverse effects. Deka and Saikia9 assessed the efficacy of senile as well as congenital entropion treatment in 17 patients who were administered a botulinum toxin injection on the preseptal portion of the orbicular muscle, obtaining a temporary resolution of entropion between 8 and 26 weeks.
The present study demonstrates that the application of TbA in patients under 2 years of age with symptomatic epiblepharon diminishes symptoms, corneal compromise and ciliocorneal contact in a statistically significant proportion. TbA diminishes the strength of the orbicular muscle that exerts less pressure of the eyelids against the ocular globe, thus improving tearing.
Khwarg and Lee1 proposed a classification to assess the severity of epiblepharon according to the following morphologic characteristics: height of the skin fold, ciliocorneal contact and keratopathy. According to the corneal scale of the Khwarg and Lee classification, the patients of the present study exhibited a statistically significant improvement of corneal compromise after the application of TbA. Despite the fact that 67% of eyelids treated with TbA exhibited persistence of contact between the eyelashes and the cornea, epiblepharon improvement can be inferred because tearing disappeared in 72% of eyes. Another feasible explanation for the results of the study is that during the ophthalmological examination ciliocorneal contact was present due to increased voluntary contraction of the orbicular muscle while in other periods of the day it may have improved.
No adverse effects were reported during the follow-up period due to the application of TbA. None of the patients exhibited palpebral retraction or lagophthalmos due to diminished orbicular muscle contraction caused by TbA. In addition, TbA diffusion did not impair ocular motility.
The application of TbA at the pre-tarsal level of the orbicular muscle is a minimally invasive technique that does not require general anesthesia. The authors consider that as the injection is made with a thin needle and in a small amount it can be safely performed in the consulting practice. The fact remains that the distress of the patient cannot be evaluated although it could be compared to that of a vaccine. An alternative would be to carry out the procedure in a surgical theater applying breathing anesthesia with laryngeal mask.
The present procedure is reversible considering that the toxin activity is of about 6 months although this period can vary between patients. In the present study, the highest number of TbA applications was 3 in a single eyelid. In patients that respond positively to TbA, a further 5-unit dose can be applied when the effect of the toxin wears off and epiblepharon becomes again symptomatic. The time between doses is variable for each patient and depends on the onset and severity of symptoms. For this reason, frequent follow-up of patients is recommended. In the present study, the mean interval between doses was approximately 4 months. A larger sample of eyelids treated with more than one botulinum toxin application would be necessary to produce standardizable results.
Non-surgical procedures for treatment symptomatic epiblepharon include hyaluronic acid injections in the lower eyelid. Taban et al.10 described the use of 0.3 ml Restylane (Q-Med AB, Sweden) injected in the retractor muscle plane and reported epiblepharon correction in 2 clinic cases.
Naik et al.11,12 proposed the use of hyaluronic acid in the sub-orbicular plane between the skin fold and the palpebral margin. In their recent study12 said authors assessed epiblepharon correction in 10 eyelids after transcutaneous injection of Juvederm hyaluronic acid (Juvederm Ultra, Allergan, Irvine, CA, USA) or Restylane (Medicis Aesthetics Inc, Scottsdale, AZ, USA), with a mean dose of 0.19 ml (range 0,1-0,3 ml). All treated cases exhibited improvement of symptoms, corneal compromise and ciliocorneal contact. The application of hyaluronic acid was done under anesthesia with laryngeal mask. The authors consider that in addition to being a nonsurgical procedure the application of botulinum toxin is less expensive and safer than hyaluronic acid injection. A clinic trial should be carried out comparing both techniques in order to obtain conclusive results.
One of the limitations of the present study is its retrospective nature and the absence of a control group.
In the case that, at age 2, a patient does not exhibit epiblepharon resolution and it remains symptomatic or evidences corneal compromise, surgery should be considered for correcting the defect under general anesthesia. However, the implicit risks of anesthesia should always be taken into account. The surgery generally oerfired by the authors in their hospital for correcting epiblepharon involves the excision of skin and the redundant orbicular muscle through a subciliary incision. A more detailed study should be carried out to compare the percentage of spontaneous epiblepharon resolution compared to surgical resolution in the authors’ institution.
ConclusionsThe present study demonstrates that the application of 5 TbA units injected in the pre-tarsal region of the lower eyelid orbicular muscle is a safe technique that improves epiblepharon symptoms and signs in patients under 2 years of age.
It would be necessary to carry out a randomized multi-center clinic trial to determine the effectiveness of TbA application in comparison to the hyaluronic acid injection treatment as well as conservative treatment. Said trial should also study the number of botulinum toxin applications required and the interval between each dose in order to optimize the symptom-free period in patients with symptomatic epiblepharon. It is important to follow-up patients until epiblepharon resolution is achieved to avoid corneal damage and improve their quality of life.
FundingThe present research has not received specific aid from public, commercial or nonprofit sector agencies or organizations.
Conflict of interestNo conflict of interests was declared by the authors.
Please cite this article as: de la Fuente Díez Y, Olvera Morales O, Chen López CY, Tovilla Canales JL, Nava Castañeda A. Experiencia del uso de la toxina botulínica tipo A para el tratamiento sintomático del epiblefaron en pacientes menores de 2 años. Arch Soc Esp Oftalmol. 2020;95:9–14.