Posterior canal benign paroxysmal positional vertigo (pc-BPPV) causes physical, functional, and emotional impairment. The treatment is the Epley manoeuvre (EM).
ObjectiveThe purpose of the study was to compare the impact of the EM and a sham manoeuvre in primary care on self-perceived disability.
DesignRandomised, double-blind, sham-controlled clinical trial conducted in primary care with a follow-up of 1 year.
ParticipantsPatients aged ≥18 years old diagnosed with pc-BPPV according to the Dix–Hallpike test (DHT) were randomised to:
InterventionsIntervention (EM) group or a control (sham manoeuvre) group.
Main measurementsThe main study covariates were age, sex, history of depression and anxiety, presence of nystagmus in the DHT, patient-perceived disability assessed with the Dizziness Handicap Inventory – screening version (DHI-S). Data were analyzed using bivariate and multivariate mixed Tobit analyses.
ResultsOverall, 134 patients were studied: 66 in the intervention group and 68 in the control group. Median age was 52 years (interquartile range [IQR], 38.25–68.00 years. standard deviation, 16.98) and 76.12% of the patients were women. The DHT triggered nystagmus in 40.30% of patients. The median total DHI-S score for the overall sample at baseline was 16 (IQR, 8.00–22.00); 16 [IQR, 10.5–24.0] vs 10 [6.0–14.0] for women vs men (P<.001). Patients treated with the EM experienced a mean reduction of 2.03 points in DHI-S score over the follow-up period compared with patients in the sham group.
ConclusionsPc-BPPV affects the quality of life of primary care patients. A single EM can improve self-perceptions of disability by around 2 points on the DHI-S scale.
El vértigo posicional paroxístico benigno del canal posterior (pc-BPPV) causa deterioro físico, funcional y emocional. El tratamiento es la maniobra de Epley (ME).
ObjetivoEl propósito del estudio fue comparar el impacto de la ME y una maniobra simulada en Atención Primaria sobre la discapacidad autopercibida.
DiseñoEnsayo clínico aleatorizado, doble ciego y controlado realizado en Atención Primaria con un seguimiento de un año.
ParticipantesLos pacientes ≥18 años diagnosticados de pc-BPPV según la prueba de Dix-Hallpike (DHT) fueron aleatorizados para:
IntervencionesGrupo de intervención (EM) o un grupo de control (maniobra simulada).
Variables principalesLas principales variables del estudio fueron la edad, el sexo, los antecedentes de depresión y ansiedad, la presencia de nistagmo en la DHT, la discapacidad percibida por el paciente, evaluada con la versión de cribado del Inventario de discapacidad del vértigo (DHI-S). Los datos se analizaron mediante análisis Tobit mixtos bivariados y multivariados.
ResultadosSe estudió a 134 pacientes: 66 en el grupo de intervención y 68 en el grupo de control. La mediana de edad fue de 52 años (rango intercuartílico [IQR], 38,25-68,00 años; desviación estandar 16,98) y el 76,12% de los pacientes eran mujeres. La DHT desencadenó nistagmo en el 40,30% de los pacientes. La media del DHI-S para la muestra general al inicio del estudio fue de 16 (IQR 8,00-22,00); 16 (RIQ, 10,5-24,0) frente a 10 (6,0-14,0) para mujeres frente a hombres (p<0,001). Los pacientes tratados con ME experimentaron una reducción media de 2,03 puntos en la puntuación DHI-S durante el período de seguimiento en comparación con los pacientes del grupo simulado.
ConclusionesEl Pc-BPPV afecta a la calidad de vida de los pacientes de Atención Primaria. Una sola ME puede mejorar la autopercepción de la discapacidad en alrededor de 2 puntos en la escala DHI-S.
Benign paroxysmal positional vertigo (BPPV), the most frequent cause of vertigo, has an annual incidence ranging from 10.7 to 140 cases per 100000 inhabitants1 and a lifetime prevalence of 2.4%.2 According to one systematic review, between 4.3% and 39.5% of patients seen in primary care for dizziness had BPPV.3 Posterior canal BPPV (pc-BPPV) is the most common variant and accounts for 60–90% of all cases.4
Pc-BBPV can be diagnosed with relative ease in primary care, as a targeted history, a basic physical examination, and performance of the Dix–Hallpike test (DHT) are sufficient to establish a diagnosis.5 The DHT is considered to be positive when it causes both vertigo and nystagmus. This form of BPPV is known as objective BPPV (O-BPPV). According to some authors, however, the DHT may also be considered positive if the patient experiences symptoms without nystagmus.6 This form of BPPV is known as subjective BPPV (S-BPPV) and it accounts for around 11.5–48% of all cases.7
Many patients with BPPV experience impaired physical and functional performance and the condition can also have an effect on family and social life.8,9 Vertigo increases the risk of falls,10 particularly among elderly patients,11 and also causes psychological symptoms that can lead patients to avoid certain everyday situations.12
Perceived disability among patients with BPPV is usually assessed using standardised questionnaires, the most common being the Dizziness Handicap Inventory (DHI),13 for which several abbreviated versions have been created. One recent study comparing the original 25-item DHI with shorter versions reported that the Dizziness Handicap Inventory – screening version (DHI-S) was the best option as it shows strong correlation with the original DHI (r=0.86) and has acceptable internal consistency (test–retest r=0.95).14 The DHI-S was designed by Jacobson et al.15 and consists of 10 questions with the same “yes”, “sometimes” and “no” answer options as the original questionnaire. It is a self-administered questionnaire that can be completed in 4–5min, making it suitable for use in centres that have to deal with large numbers of patients such as primary care practices. It has been validated for use in Spain.16
The treatment of choice for BPPV is the Epley manoeuvre (EM), whose effectiveness has been demonstrated in numerous studies and systematic reviews.1,17–19 The EM is a canalith repositioning manoeuvre that has been associated with improved self-perceptions of disability among patients with BPPV.16,20 It has also proven effective in elderly patients21 and in the long term, even after a single treatment.22
Although performance of the EM is feasible in primary care,23 most of the studies that have evaluated its impact on perceived disability among patients with pc-BPPV have been performed in specialised clinics. Studies thus are needed to evaluate its impact in patients diagnosed and treated in primary care.1
The aim of the study was to compare the effect of the EM and a sham manoeuvre on self-perceived disability assessed using the DHI-S at 1 week, 1 month, and 1 year in primary care patients with pc-BPPV.
MethodsTrial designRandomised, double-blind, sham-controlled clinical trial with an allocation ratio of 1:1 conducted in two primary care practices.
ParticipantsPatients were recruited between November 2012 and January 2015, and three follow-up visits, held a week, a month, and a year after the intervention, were performed by six general practitioners (GPs) blinded to treatment allocation working at two primary care practices, which employ 26 GPs and offer care for 38305 people in L’Hospitalet de Llobregat (Barcelona, Spain).
Eligible participants included all adults (≥18 years) with symptoms of vertigo seen at the primary care practices, and patients clinically suspected to have pc-BPPV were systematically recruited by the GPs involved in the study. Patients who agreed to participate were referred for baseline evaluation by one of six GPs on the research team. Those who provided written informed consent and had a positive DHT, with or without nystagmus (O-BPPV or S-BPPV, respectively), were included. Patients with purely vertical nystagmus, nystagmus lasting >1min, or vertical or alternating nystagmus were excluded and referred to an ear, nose, and throat (ENT) specialist. The full list of exclusion criteria is available in the study protocol.24
Changes to trial designAlthough vestibular migraine was not an exclusion criterion for the trial,24 emerging evidence on the high prevalence of this condition25 and its overlapping symptoms with pc-BPPV suggested that patients with vestibular migraine might have been recruited for the trial. It was therefore decided to re-assess all patients after completion of the follow-up phase and to remove those who met the 2013 criteria for probable vestibular migraine.26 Results following the original trial design, including patients with probable vestibular migraine, are presented as supplementary material.
Interventions and comparisonsAt the baseline visit, the patients underwent a full physical examination and a complete medical history, including review of electronic medical records.
Patients in the intervention group were treated with a single EM. They were prescribed betahistine 8mg/8h and instructed to use the medication as required (maximum 3 times a day) until their symptoms improved.
Patients in the control group were prescribed betahistine as the same dosage. Instead of the EM, however, they received a sham manoeuvre consisting of laying the patient with his/her head turned towards the affected side for 5min.27
The GPs who administered the EM took part in a 2-h practical training session on diagnosing vertigo and applying the manoeuvre under the supervision of an ENT specialist to ensure consistent execution across patients. Two videos showing an investigator administering the DHT (https://www.youtube.com/watch?v=tJEFi5RFZEM) and the EM (https://www.youtube.com/watch?v=yAFx4-TFcGE) were also recorded.
VariablesThe 10 items on the DHI-S are scored with 0 for “no”, 2 for “sometimes”, and 4 for “yes”. The total possible score therefore ranges from 0 (no self-perceived disability) to 40 (worst possible self-perceived disability).
The independent variables were age, sex, DHT result (O-BPPV or S-BPPV), history of anxiety and/or depression (Yes/No) and treatment with benzodiazepines, antidepressants, and/or vertigo drugs (Yes/No) at the time of the baseline visit.
Sample sizeThe sample size was calculated for outcomes not analysed in this study. Based on a sample size of 65 and 61 patients in the study arms at week 1, the study had a power of 80% to detect a statistically significant difference (alpha=0.05) of 5.16 in DHI-S scores between groups (assuming a standard deviation of 1016 in a Wilcoxon test [power estimated by bootstrap]).
Randomisation and allocationPatients were assigned to the intervention and control groups using a unique list of randomisation sequences prepared in advance by the study statistician. The randomisation sequences were generated in ‘R: A language and environment for statistical computing’, version 2.14.2 (R Foundation for Statistical Computing, Vienna, Austria).
Statistical analysisData were analysed in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines,28 and comparisons between groups were based on the intention-to-treat principle.
Continuous variables (e.g., DHI-S scores) were expressed as median and interquartile range (IQR), while categorical variables (e.g., anxiety) were expressed as absolute and relative frequencies. For the between-group comparisons at each time point, the distribution of DHI-S answers was compared using the Chi-square test, while DHI-S scores were compared using the Wilcoxon test. These bivariate analyses were performed for the overall samples and stratified by sex and presence or absence of nystagmus at baseline.
A longitudinal multivariate regression model was built using DHI-S scores. Given the considerable proportion of 0 scores, a full mixed-effects multivariate Tobit regression model was adjusted to explain DHI-S scores by intervention, baseline DHI-S score, sex, baseline diagnosis (O-BPPV vs S-BPPV), and three-way interactions between all these variables and the treatment group, with adjustment for correlated intraindividual observations. Stepwise backward variable selection using the Akaike information criterion (AIC) was applied to obtain the final model. This reduced Tobit model contained all relevant variables and interactions; results are presented as marginal effects (medians of individual effects29,30) and statistical significance (P-value) of the associated coefficients.
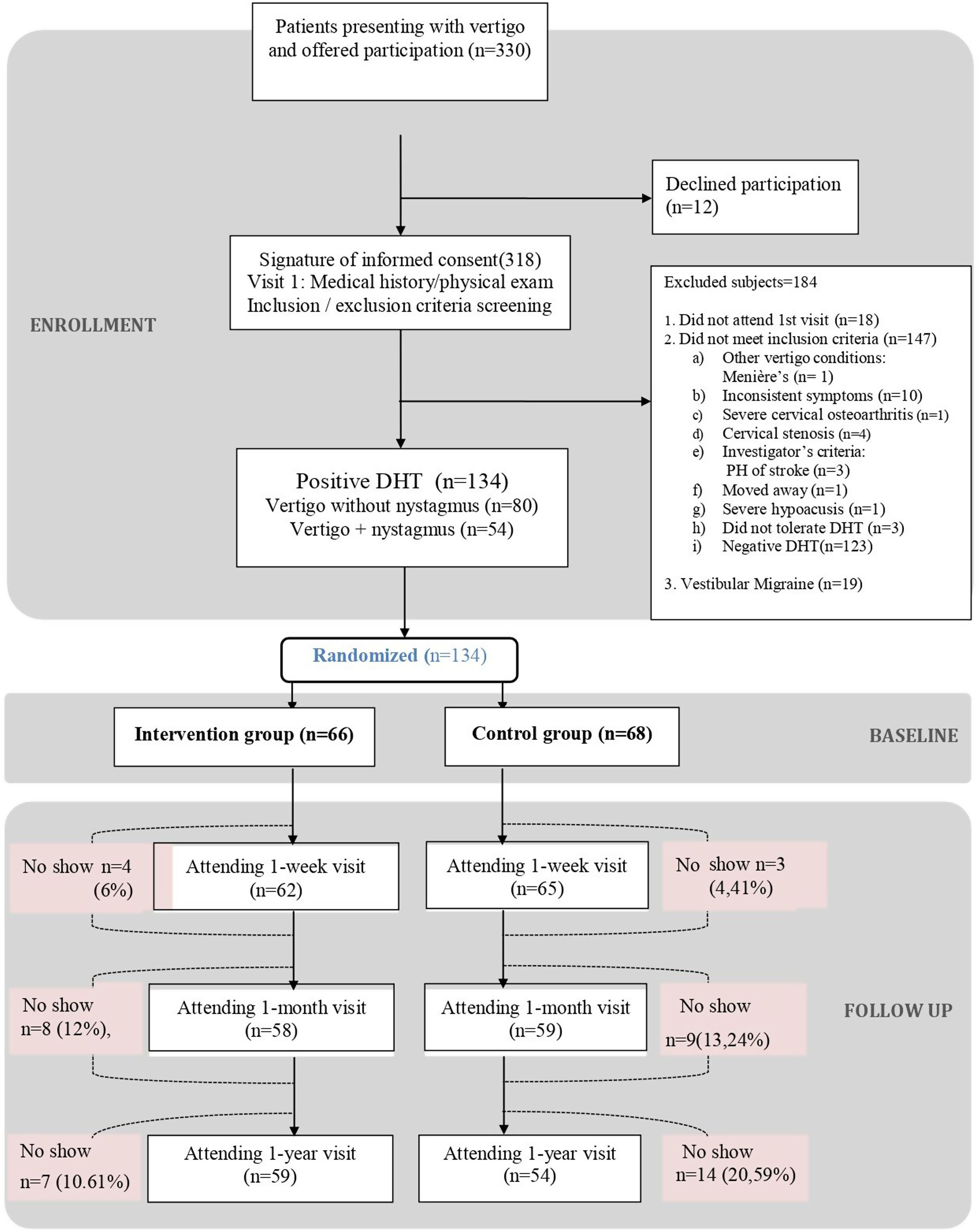
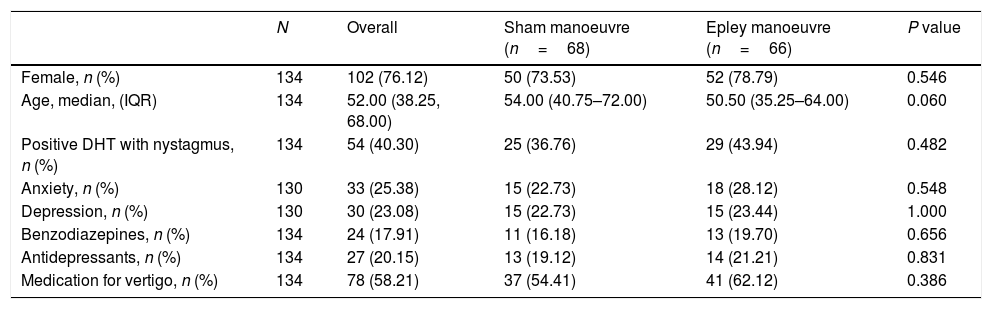
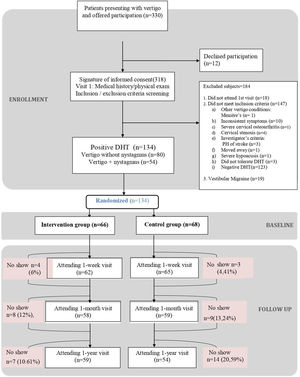
ResultsOf the 330 patients with suspected pc-BPPV analysed, 134 (40.6%) were included in the trial: 66 were randomised to the intervention group and 68 to the sham group. The reasons for exclusion are summarised in the study flowchart (Fig. 1). The main reason for exclusion was a negative DHT at baseline (n=123). Nineteen patients were removed retrospectively because they had criteria compatible with vestibular migraine. Their results, however, can be consulted in the supplementary material. The respective number of patients lost to follow-up at the three time points (week, month, and year) was 7, 17, and 21. The baseline characteristics for the overall sample and the intervention and control groups are shown in Table 1. The median age was 52 years (IQR, 38.25–68.00, standard deviation, 16.98) and 76.12% of the patients were women. The DHT triggered nystagmus in 40.30% of patients (O-BPPV). The proportion of patients with anxiety and depression was 25.38% and 23.08%, respectively. In total, 17.91% of patients were being treated with benzodiazepines, 20.15% with antidepressants, and 58.21% with vertigo drugs at the baseline visit. No significant differences were observed between the intervention and control group for any of the study variables.
Characteristics of the study participants overall and by intervention.
| N | Overall | Sham manoeuvre (n=68) | Epley manoeuvre (n=66) | P value | |
|---|---|---|---|---|---|
| Female, n (%) | 134 | 102 (76.12) | 50 (73.53) | 52 (78.79) | 0.546 |
| Age, median, (IQR) | 134 | 52.00 (38.25, 68.00) | 54.00 (40.75–72.00) | 50.50 (35.25–64.00) | 0.060 |
| Positive DHT with nystagmus, n (%) | 134 | 54 (40.30) | 25 (36.76) | 29 (43.94) | 0.482 |
| Anxiety, n (%) | 130 | 33 (25.38) | 15 (22.73) | 18 (28.12) | 0.548 |
| Depression, n (%) | 130 | 30 (23.08) | 15 (22.73) | 15 (23.44) | 1.000 |
| Benzodiazepines, n (%) | 134 | 24 (17.91) | 11 (16.18) | 13 (19.70) | 0.656 |
| Antidepressants, n (%) | 134 | 27 (20.15) | 13 (19.12) | 14 (21.21) | 0.831 |
| Medication for vertigo, n (%) | 134 | 78 (58.21) | 37 (54.41) | 41 (62.12) | 0.386 |
DHT: Dix–Hallpike test; IQR: interquartile range.
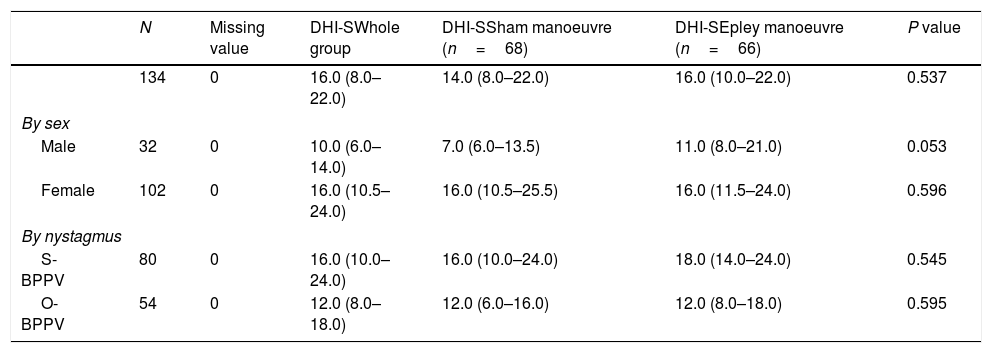
The median DHI-S score at baseline was 16 (IQR, 8.00–22.00). A higher median score was observed in women compared with men (16 [IQR, 10.5–24.0]) vs 10 [IQR, 6.0–14.0], P<0.001 [not shown in table]) and in patients without nystagmus at baseline (S-BPPV) than in those with nystagmus (16 [IQR, 10.0–24.0] vs 12 [8.0–18.0 for patients with O-BPPV]; P=0.033 [not shown in table]) (Tables 2a, 2b, 2c and 2d).
Baseline evaluation.
| N | Missing value | DHI-SWhole group | DHI-SSham manoeuvre (n=68) | DHI-SEpley manoeuvre (n=66) | P value | |
|---|---|---|---|---|---|---|
| 134 | 0 | 16.0 (8.0–22.0) | 14.0 (8.0–22.0) | 16.0 (10.0–22.0) | 0.537 | |
| By sex | ||||||
| Male | 32 | 0 | 10.0 (6.0–14.0) | 7.0 (6.0–13.5) | 11.0 (8.0–21.0) | 0.053 |
| Female | 102 | 0 | 16.0 (10.5–24.0) | 16.0 (10.5–25.5) | 16.0 (11.5–24.0) | 0.596 |
| By nystagmus | ||||||
| S-BPPV | 80 | 0 | 16.0 (10.0–24.0) | 16.0 (10.0–24.0) | 18.0 (14.0–24.0) | 0.545 |
| O-BPPV | 54 | 0 | 12.0 (8.0–18.0) | 12.0 (6.0–16.0) | 12.0 (8.0–18.0) | 0.595 |
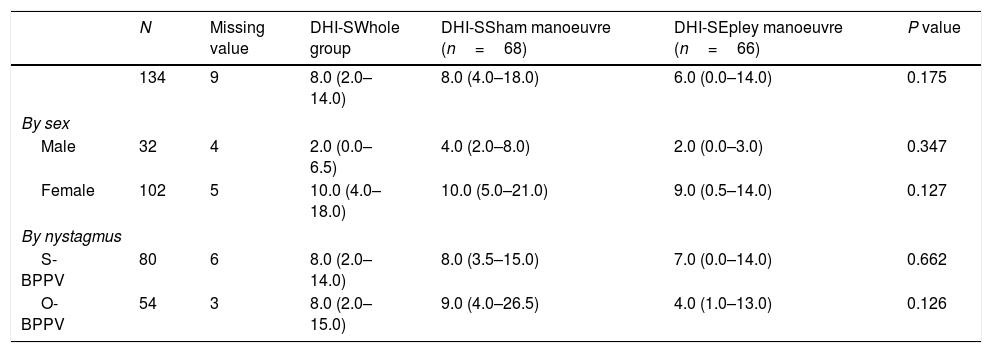
Evaluation at 1 week.
| N | Missing value | DHI-SWhole group | DHI-SSham manoeuvre (n=68) | DHI-SEpley manoeuvre (n=66) | P value | |
|---|---|---|---|---|---|---|
| 134 | 9 | 8.0 (2.0–14.0) | 8.0 (4.0–18.0) | 6.0 (0.0–14.0) | 0.175 | |
| By sex | ||||||
| Male | 32 | 4 | 2.0 (0.0–6.5) | 4.0 (2.0–8.0) | 2.0 (0.0–3.0) | 0.347 |
| Female | 102 | 5 | 10.0 (4.0–18.0) | 10.0 (5.0–21.0) | 9.0 (0.5–14.0) | 0.127 |
| By nystagmus | ||||||
| S-BPPV | 80 | 6 | 8.0 (2.0–14.0) | 8.0 (3.5–15.0) | 7.0 (0.0–14.0) | 0.662 |
| O-BPPV | 54 | 3 | 8.0 (2.0–15.0) | 9.0 (4.0–26.5) | 4.0 (1.0–13.0) | 0.126 |
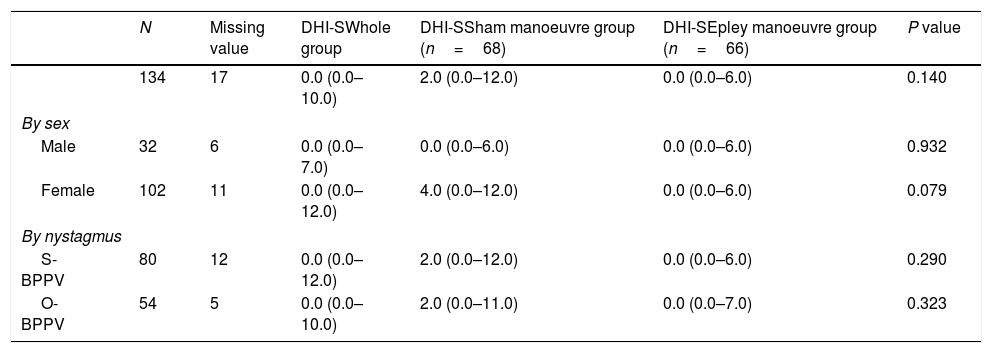
Evaluation at 1 month.
| N | Missing value | DHI-SWhole group | DHI-SSham manoeuvre group (n=68) | DHI-SEpley manoeuvre group (n=66) | P value | |
|---|---|---|---|---|---|---|
| 134 | 17 | 0.0 (0.0–10.0) | 2.0 (0.0–12.0) | 0.0 (0.0–6.0) | 0.140 | |
| By sex | ||||||
| Male | 32 | 6 | 0.0 (0.0–7.0) | 0.0 (0.0–6.0) | 0.0 (0.0–6.0) | 0.932 |
| Female | 102 | 11 | 0.0 (0.0–12.0) | 4.0 (0.0–12.0) | 0.0 (0.0–6.0) | 0.079 |
| By nystagmus | ||||||
| S-BPPV | 80 | 12 | 0.0 (0.0–12.0) | 2.0 (0.0–12.0) | 0.0 (0.0–6.0) | 0.290 |
| O-BPPV | 54 | 5 | 0.0 (0.0–10.0) | 2.0 (0.0–11.0) | 0.0 (0.0–7.0) | 0.323 |
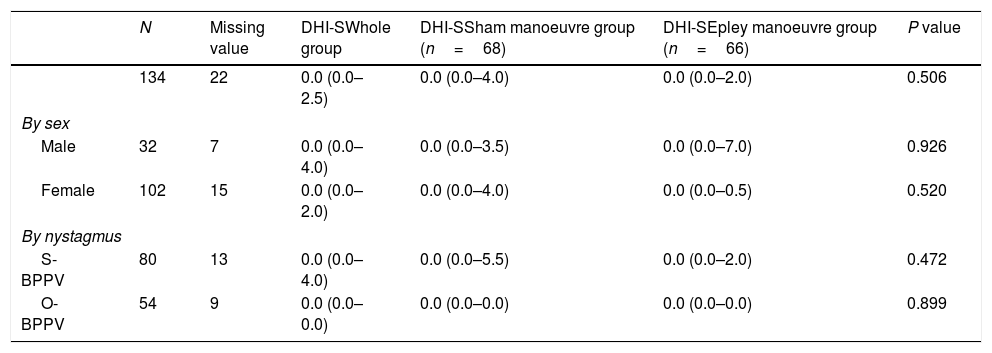
Evaluation at 1 year.
| N | Missing value | DHI-SWhole group | DHI-SSham manoeuvre group (n=68) | DHI-SEpley manoeuvre group (n=66) | P value | |
|---|---|---|---|---|---|---|
| 134 | 22 | 0.0 (0.0–2.5) | 0.0 (0.0–4.0) | 0.0 (0.0–2.0) | 0.506 | |
| By sex | ||||||
| Male | 32 | 7 | 0.0 (0.0–4.0) | 0.0 (0.0–3.5) | 0.0 (0.0–7.0) | 0.926 |
| Female | 102 | 15 | 0.0 (0.0–2.0) | 0.0 (0.0–4.0) | 0.0 (0.0–0.5) | 0.520 |
| By nystagmus | ||||||
| S-BPPV | 80 | 13 | 0.0 (0.0–4.0) | 0.0 (0.0–5.5) | 0.0 (0.0–2.0) | 0.472 |
| O-BPPV | 54 | 9 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.899 |
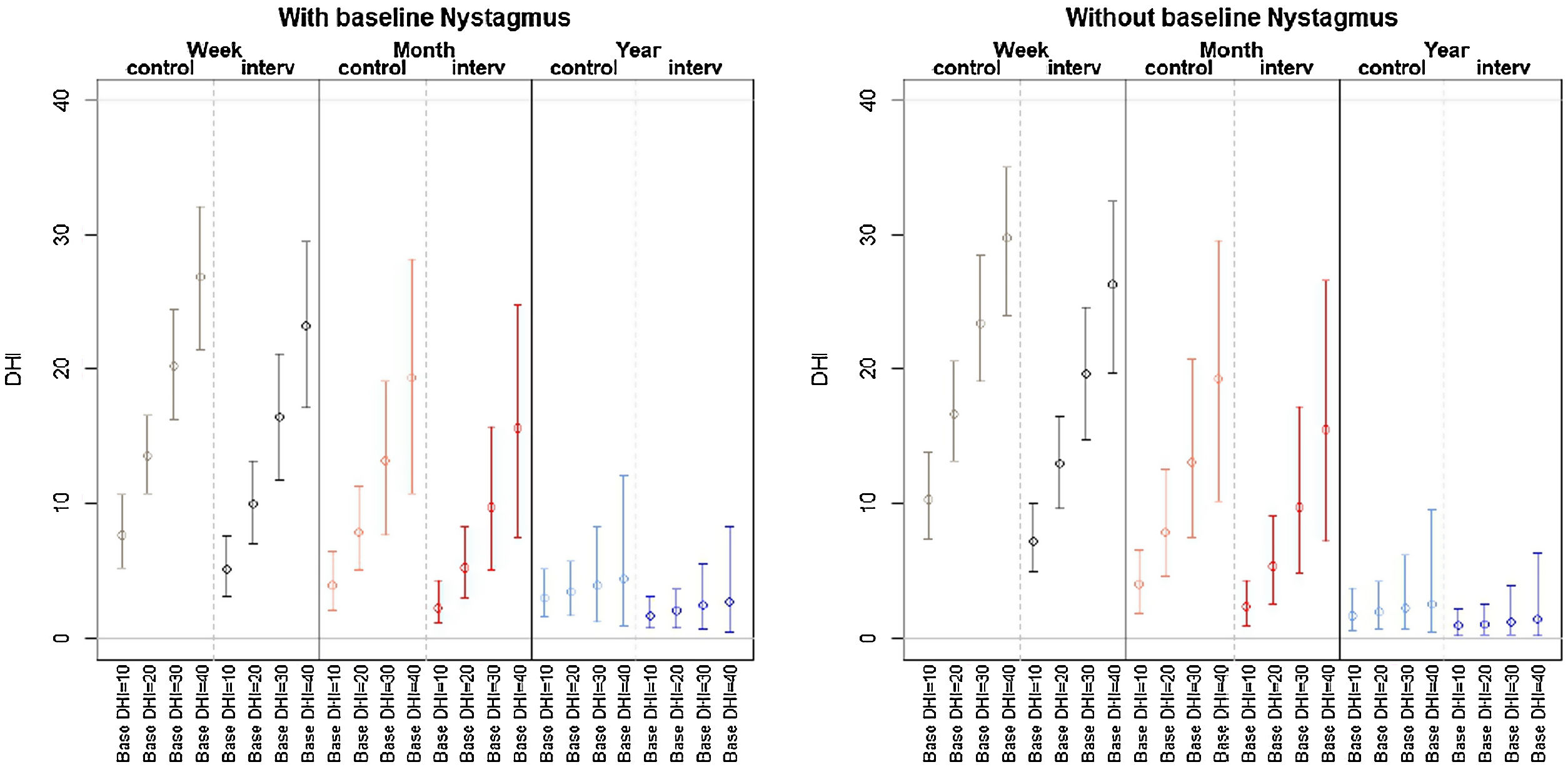
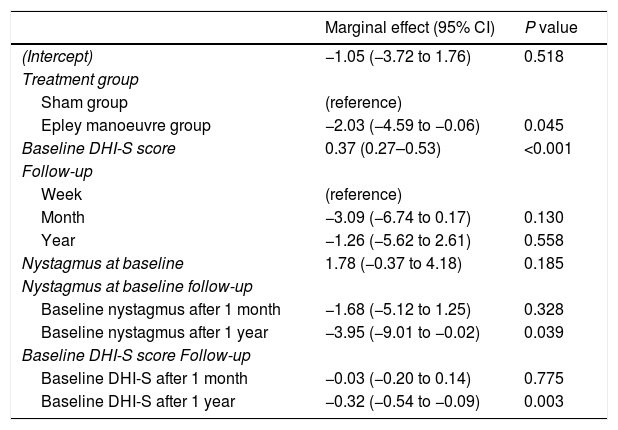
Patient-perceived disability during the follow-up period (week, month, year) was not influenced by a history of anxiety or depression or treatment with anxiolytics. The final multivariate model (the model with the best performance according to the AIC) showed that variations in DHI-S scores were explained by treatment group, baseline DHI-S score, follow-up (time point), and interaction between DHI-S score and time (all other interactions and effects did not provide enough likelihood to the model). Baseline DHI-S score was positively associated with DHI-S score during follow-up, with an initial marginal effect of 0.37 at week 1. The respective effects at 1 month and 1 year were 0.3 and 0.32 (significant decrease). According to the selection process, the interaction treatment×time did not provide the model with sufficient predictive accuracy for these coefficients to be retained. In the final model thus, the treatment effect did not vary significantly over time, showing a stable marginal effect of −2.03 (−4.59, −0.06) points (Table 3). The expected DHI values estimated by the multivariate model in Table 3 are shown in Fig. 2 according to the different exposure characteristics.
Multivariate mixed-effects regression.
| Marginal effect (95% CI) | P value | |
|---|---|---|
| (Intercept) | −1.05 (−3.72 to 1.76) | 0.518 |
| Treatment group | ||
| Sham group | (reference) | |
| Epley manoeuvre group | −2.03 (−4.59 to −0.06) | 0.045 |
| Baseline DHI-S score | 0.37 (0.27–0.53) | <0.001 |
| Follow-up | ||
| Week | (reference) | |
| Month | −3.09 (−6.74 to 0.17) | 0.130 |
| Year | −1.26 (−5.62 to 2.61) | 0.558 |
| Nystagmus at baseline | 1.78 (−0.37 to 4.18) | 0.185 |
| Nystagmus at baseline follow-up | ||
| Baseline nystagmus after 1 month | −1.68 (−5.12 to 1.25) | 0.328 |
| Baseline nystagmus after 1 year | −3.95 (−9.01 to −0.02) | 0.039 |
| Baseline DHI-S score Follow-up | ||
| Baseline DHI-S after 1 month | −0.03 (−0.20 to 0.14) | 0.775 |
| Baseline DHI-S after 1 year | −0.32 (−0.54 to −0.09) | 0.003 |
Final model built following stepwise backward selection of variables using the Akaike Information Criterion from an initial model featuring treatment adjusted for follow-up, baseline DHI-S score, sex, nystagmus at baseline, and three-way interactions between these factors and treatment and follow-up. DHI-S: Dizziness Handicap Inventory – screening version.
Patients’ perceptions of pc-BPPV-associated disability assessed by the DHI-S improved after treatment with a single EM performed by GPs, with a significant difference in DHI-S scores of −2.03 points compared with the sham group over the follow-up period; no significant variations were observed during this period.
Although the difference of −2.03 points was significant, it may not have been clinically relevant, as the minimal detectable difference for the long version of the DHI is 17.68 points in the 0–100 theoretical range.31 Our results should, therefore, be interpreted with caution and it is important to consider the theoretical score ranges of the different versions of the DHI. We might also have observed greater differences if we had treated certain patients with more than one EM (the recommended number is up to four).32
The median age of the patients in our series is similar to that observed in other studies at specialised clinics16,31 and primary care practices.23
Also supporting previous findings,2,20,33,34 women outnumbered men in our study, which is consistent with the higher prevalence of pc-BPPV in women.35–38 No significant differences between men and women were found in terms of response to the EM during follow-up, even though baseline DHI-S scores were higher among women. Sex was not a significant factor in the multivariate model. Pereira et al.20 also reported similar responses to the EM in men and women.
Baseline patient perceptions of disability in our series (median DHI-S score, 16) were similar to those reported by Ardıç et al.14 (mean, 16.4±0.71), but better than those reported by patients treated at a specialised clinic (mean±SD, 19.79±10.14)16 and patients aged over 60 years21 (mean, 17.19±SD 9.06). One possible explanation for the better scores in our series is that the patients were diagnosed and treated in primary care, avoiding the delays associated with referral to a specialist. Their younger age may also have played a part.
A high proportion of patients in our series had anxiety or depression. It is not uncommon for patients with BPPV to experience psychiatric-psychosomatic disorders such as depression or anxiety.39 Anxiety disorders, in fact, have been found to be 2.17 times (95% CI, 1.63–2.90, P<0.001) more common in patients with BPPV than in controls following adjustment for age, sex, and comorbidities.38
The main strength of our study is that it was conducted under typical conditions encountered in primary care practices, which is where most patients with BPPV are seen.
We acknowledge some limitations of this study. The higher proportion of patients with S-BPPV detected in our series compared with earlier series14,40 indicates that we may have underestimated the prevalence of O-BPPV. Patients seen in primary care often have early-stage nystagmus, which is more difficult to diagnosis, particularly if special tools such as Frenzel goggles or videonystagmography are not used.41 The gold standard diagnostic work-up for pc-BPPV does not include these tools,1 but they can increase the number of O-BPPV cases diagnosed. Testing primary care patients with S-BPPV in addition to those with O-BBPV (greater diagnostic sensitivity at the expense of specificity) in primary care may improve early diagnosis and treatment rates, and such an approach has been suggested by other authors.40 The decision not to use Frenzel goggles or videonystagmography in our study was deliberate as we wished to reproduce the conditions faced by GPs in routine practice. Response to the EM in terms of perceived disability was not influenced by the presence or absence of nystagmus at baseline, supporting previous findings by Huebner et al.40 and Marques et al.42 Nonetheless, we cannot completely rule out the possibility that some of the patients in the S-BPPV group may have had vestibular neuritis or vestibular migraine.
Our findings for perceived disability contrast with previous findings for the same series of patients showing that the EM reversed positive DHTs and resulted in less severe vertigo in patients with O-BPPV but not S-BPPV.43
Another possible limitation of the present paper is that initial sample size was calculated for other analyses, and this sample size was later reduced due to non-planned exclusion criteria. Although this could have led to lack of statistical power, our results found statistical significance for the main effect of the manoeuvre; further research is needed to discard any other possible effects, such as a time variation of the effect of the manoeuvre.
Implications for research and/or practiceThe DHI-S is a simple tool that can be administered in a matter of minutes. It would therefore be interesting to determine cut-off points for different levels of perceived disability (none, mild, moderate, and severe).
It would also be interesting to calculate the minimal detectable change and minimal clinically important difference for the DHI-S in order to determine the relevance of changes over time.
BPPV can cause considerable disability. Early treatment of pc-BPPV using repositioning manoeuvres can result in significant improvements, highlighting the importance of prompt treatment in all patients, including those seen in primary care. It would be very interesting to determine whether performance of more than one EM would improve the outcomes observed in this study.
ConclusionsPc-BPPV affects the quality of life of primary care patients. A single EM can improve self-perceptions of disability by around 2 points on the DHI-S scale. Nonetheless, although this difference is statistically significant, it may have little clinical relevance.
Benign paroxysmal positional vertigo, primary health care, Epley manoeuvre, health-related quality of life, randomised controlled trial.
- •
BPPV affects self-perceived disability.
- •
Performance of the EM is feasible in primary care.
- •
Patients’ perceptions of pc-BPPV-associated disability assessed by the DHI-S improved after treatment with a single EM performed by GPs.
This project received a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded on the 2013 call under the Health Strategy Action 2013–2016, within the National Research Programme oriented to Societal Challenges, within the Technical, Scientific and Innovation Research National Plan 2013–2016, with reference PI13/01396, co-funded with European Union ERDF funds. It was also funded by the cycle XIV (2013) research grant from the Jordi Gol Institute for Research in Primary care (IDIAP Jordi Gol) (2014/005E).
Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), provided the main amount of funding for the development and main management of the project. REAP funded the organisation of coordinating meetings and training.
IDIAP gave the grant to free up time for dedicate to the project as a predoctoral training of the author Jose Luis Ballve.
IDIAP Jordi Gol also funded the translation of this article into English.
Authors’ contributionsRCM, JLBM, YRM and IVB: conception, design and drafting, data collection and analysis, writing and final approval of manuscript. OCP: statistical analysis, design and drafting, analysis and interpretation of data, writing and final approval of manuscript. JAO: conception, design and drafting, analysis and interpretation of data, writing and final approval of manuscript. JPP, OLAA: data collection and analysis, critical review and final approval of manuscript.
Conflict of interestsThe authors declare that they have no competing interests.
The authors gratefully acknowledge the technical and scientific assistance provided by the Primary Healthcare Research Unit of Costa de Ponent Primary Healthcare University Research Institute IDIAP-Jordi Gol. We also thank Neus Profitós and Celsa Fernández who were responsible for safeguarding the randomisation sequence list. Finally, we thank all the participants of the Grupo de Estudio del Vértigo en Atención Primaria Florida: José Luis Ballvé Moreno, Yolanda Rando Matos, Estrella Rodero Pérez, Xavier Monteverde Curtó, Carles Rubio Ripollès, Noemí Moreno Farrés, Jean Carlos Gómez Nova, Johan Josué Villarreal Miñano, Diana Lizzeth Pacheco Erazo, Raquel Adroer Martori, Anna Aguilar Margalejo, Olga Lucia Arias Agudelo, Silvia Cañadas Crespo, Laura Illamola Martín, Marta Sarró Maluquer, Lluís Solsona Díaz, Rosa Sorando Alastruey (Equip d’Atenció Primària Florida Nord, Institut Català de la Salut, Hospitalet de Llobregat, Barcelona, Spain).
Ricard Carrillo Muñoz, Iván Villar Balboa, Austria Matos Méndez, Marta Bardina Santos (Equip d’Atenció Primària Florida Sud, Institut Català de la Salut, Hospitalet de Llobregat, Barcelona, Spain).
Oriol Cunillera Puértolas and Jesús Almeda Ortega (Unitat de Suport a la Recerca Costa de Ponent, Institut Universitari d’Investigació en Atenció Primària Jordi Gol Cornellà, Spain) Universitat Autònoma de Barcelona, Bellaterra (Cerdanyola del Vallès), Spain
Lead author: José Luis Ballvé Moreno.
Trial registration: ClinicalTrials.gov Identifier: NCT01969513. Retrospectively registered. First Posted: October 25, 2013. https://clinicaltrials.gov/ct2/show/NCT01969513.







![Expected values from Table 3 estimated by the Tobit model with the best predictive accuracy according to treatment received (Epley manoeuvre [interv] vs sham manoeuvre [control]). Base DHI: baseline Dizziness Handicap Inventory – screening version score. Expected values from Table 3 estimated by the Tobit model with the best predictive accuracy according to treatment received (Epley manoeuvre [interv] vs sham manoeuvre [control]). Base DHI: baseline Dizziness Handicap Inventory – screening version score.](https://static.elsevier.es/multimedia/02126567/0000005300000008/v2_202110050614/S0212656721001116/v2_202110050614/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
