A systematic review and meta-analysis was performed to evaluate the effectiveness of antidepressants in reducing the poor evolution of COVID-19 disease (a composite variable including death, hospitalization and need for mechanical ventilation), and mortality, according the guidelines for Systematic Reviews of Interventions published by the Cochrane library.
Source of dataMEDLINE, EMBASE and COCHRANE LIBRARY were consulted up to February 25, 2022. Unpublished studies were searched on clinicaltrials.gov platform.
Selection of studiesSeven masked and unmasked, observational and experimental studies evaluating death, hospitalization and need for mechanical ventilation were selected. A second subgroup analysis with mortality variable was performed.
Data extractionA full risk of bias assessment was performed addressing issues such as information and confounding bias. ROB2 and Robins-I tools for randomized and no randomized studies were employed respectively. In the quantitative analysis, the risk of publication bias, heterogeneity, estimation of pooled measure and a sensitivity analysis was performed. The pooled final measure was calculated as odds ratio with its correspondent 95% confidence interval. A random effects model was used for this purpose due to the heterogeneity between included studies.
Finally, a sensitivity analysis was performed to assess the robustness of final pooled measure.
ResultsSeven studies were finally considered to calculate the final pooled measure. The effect of intervention was OR 0.73; 95% CI 0.56–0.94.
ConclusionsThe use of antidepressants, and specially SSRI could be effective for reducing the risk of poor progression of COVID-19 disease.
Se realizó una revisión sistemática y un metaanálisis para evaluar la eficacia de los antidepresivos en la reducción de la mala evolución de la enfermedad COVID-19 (variable compuesta que incluye muerte, hospitalización y necesidad de ventilación mecánica), y mortalidad, según las directrices para Revisiones Sistemáticas de Intervenciones publicadas por la biblioteca Cochrane.
Fuente de datosSe consultaron MEDLINE, EMBASE y la biblioteca Cochrane hasta el 25 de febrero de 2022. Se realizaron búsquedas de estudios no publicados en la plataforma clinicaltrials.gov.
Selección de estudiosSe seleccionaron estudios observacionales y experimentales, enmascarados y no enmascarados, que evaluaron la muerte, la hospitalización y la necesidad de ventilación mecánica. Se realizó un segundo análisis de subgrupos con la variable mortalidad.
Extracción de datosSe realizó una evaluación completa del riesgo de sesgo que abordó temas como el sesgo de información y los factores de confusión. Se emplearon las herramientas Rob2 y Robins-I para los estudios aleatorios y no aleatorios, respectivamente. En el análisis cuantitativo, se analizó el sesgo de publicación, heterogeneidad, y se estimó la medida combinada global. La medida final se calculó como odds ratio con su correspondiente intervalo de confianza del 95%. Para ello se utilizó un modelo de efectos aleatorios debido a la heterogeneidad entre los estudios incluidos.
Finalmente, se realizó un análisis de sensibilidad para evaluar la robustez de la medida final estimada.
ResultadosSe consideraron 7 estudios para calcular la medida agrupada final. El efecto de la intervención (tratamiento con antidepresivos) fue OR 0,73; IC 95% 0,56-0,94.
ConclusionesEl uso de antidepresivos, y especialmente de ISRS, podría ser eficaz para reducir el riesgo de mala evolución y mortalidad de la enfermedad COVID-19.
COVID19 disease, usually presents with mild symptoms, however, some people may develop more severe symptoms1,2 mediated by proinflammatory cytokines, such as IL-6.3,4 Therefore, IL-6 blocking drugs have been supposed to be effective in the treatment of cytokine storm.5 Selective serotonin reuptake inhibitors (SSRIs) used as antidepressants have been explored as anti-inflammatory agents, and research suggests that SSRIs may be useful in the treatment of SARS-CoV2 as they inhibit proinflammatory transcription factors,6 reduce inflammatory cytokine production through serotonergic mechanisms,7 and inhibit inflammatory responses.8
In addition to its role in inflammation, an antiviral effect on the replication of certain viruses, and in vitro inhibitory effects on SARS-CoV2 viral replication at a concentration of 0.8μg/ml9 have been identified for fluoxetine. Different research groups have also found a lower risk of poor evolution associated with COVID-19 in patients taking SSRIs.10–12
In addition to SSRIs, there is some evidence about the anti-inflammatory properties of tricyclic antidepressants.13 Clomipramine was one of the few antidepressants (with amitriptyline) showing anti-inflammatory properties in all studies partly mediated by effects on signaling cascades activated during viral infection.14,15
However, antidepressants can cause significant side effects in about 15% of patients.16 The most common side effects are constipation, daytime sleepiness, diarrhea, dizziness, dry mouth, headache, nausea, sexual problems, tremors, sleep problems, and weight gain.17 Although some of these side effects may be common to several families of antidepressants, others are more likely to occur depending on the type of antidepressant being taken.18
They generally appear during the first two weeks of treatment and usually disappear after the body gets used to the medication.17
COVID-19 pandemic has put society as a whole, on edge because of the need to seek effective treatments to deal with the possible complications of SARS-CoV2 infection. SSRIs have been explored as anti-inflammatory agents, and research suggests that SSRIs may be useful in the treatment of SARS-CoV2.10–12 Given the important inflammatory response in SARS-COV-2 infection, there is a clear need to further investigate the beneficial effect of these drugs in order to have evidence to justify their use in the treatment of patients with severe symptomatology, even in a prophylactic manner in patients with risk factor.
In order to explore this hypothesis, we performed a meta-analysis of all the studies published to date where the main variable was poor evolution of COVID-19 disease.
Material and methodsA systematic review and meta-analysis of the effectiveness of antidepressants in reducing the poor evolution of COVID-19 disease was performed. For this purpose, a literature search was performed in February 2022 in the MEDLINE, EMBASE and COCHRANE LIBRARY databases. Unpublished studies were also searched on the clinicaltrials.gov platform. A search by mesh term and free-text search was performed. The search strategy can be seen as supplementary material. There was no restriction on language, type of study or year of publication. Systematic review and meta-analysis has been carried according the guidelines for Systematic Reviews of Interventions published by the Cochrane library.
A full risk of bias assessment and a quantitative analysis of the selected studies were carried out. In the first one, topics as information, selection and reporting bias were assessed. In the quantitative analysis, it was assessed the risk of publication bias, the heterogeneity between studies, and calculated the pooled measure. A sensitivity analysis was carried out to assess the influence of each study on the pooled estimate and the stability of the final measure. Finally, certainty of the evidence has been judged with GRADE methodology.
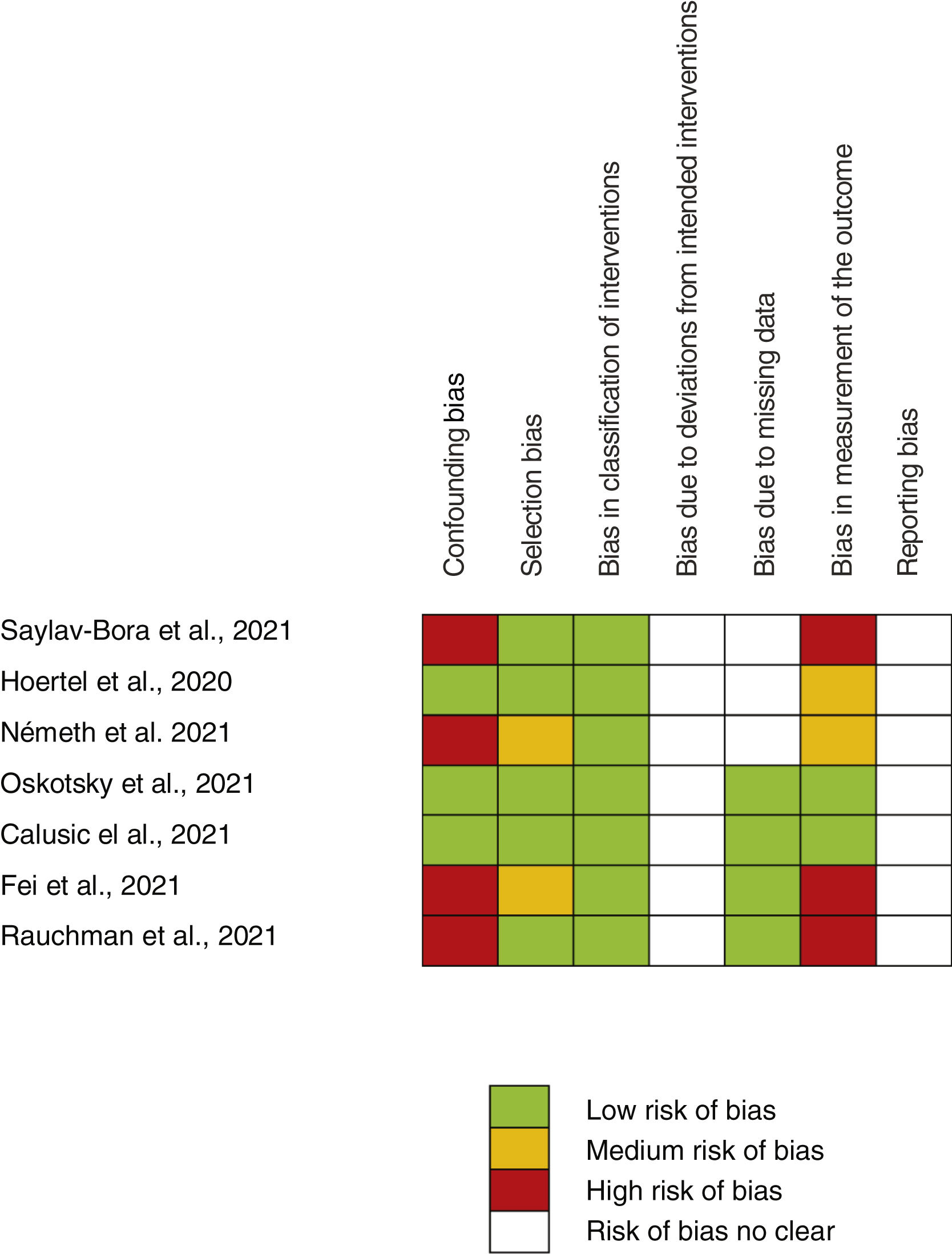
Risk of bias assessmentA full risk of bias assessment was performed through the tool developed by the Cochrane group, which is a domain-based assessment evaluation. Robins-I tool for non-random studies (confounding bias, selection bias, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of the outcome) and ROB2 tool for random studies (generation of randomized allocation sequence, blinding of participants and staff, blinding of outcome assessors, selective reporting of results, incomplete reporting) were employed. Each domain was classified as high, medium, low risk of bias, or risk of bias not clear. Risk for each study and the global risk for all studies were evaluated.
Estimation of pooled measuresMain endpoint evaluated was poor evolution disease (a composite variable including death, hospitalization and need for mechanical ventilation variables). The odds ratio (OR) and 95% confidence interval (95% CI) was calculated. A second meta-analysis with mortality was also carried out. A random effects model was used due to the heterogeneity between included studies.
Heterogeneity analysisThe heterogeneity between studies was assessed with DerSimonian and Laird Q-statistic test.18,19 Galbraith20 and L’Abbé21 graphics were also built. The I2 index was also calculated from the equation I2=(Q−df)/Q×100%, where Q is the Cochran homogeneity test statistic and df is the degrees of freedom (number of trials minus 1). Heterogeneity was considered important if I2>50%.22
Sensitivity analysisThe influence of each study on the overall estimate and, therefore the stability of the final measure was assessed. To address this, the meta-analysis was repeated as many times as the number of the selected studies, skipping one each time while combining the remaining others.
Publication biasPublication bias was assessed using Egger statistic,19 and the Funnel plot graph.
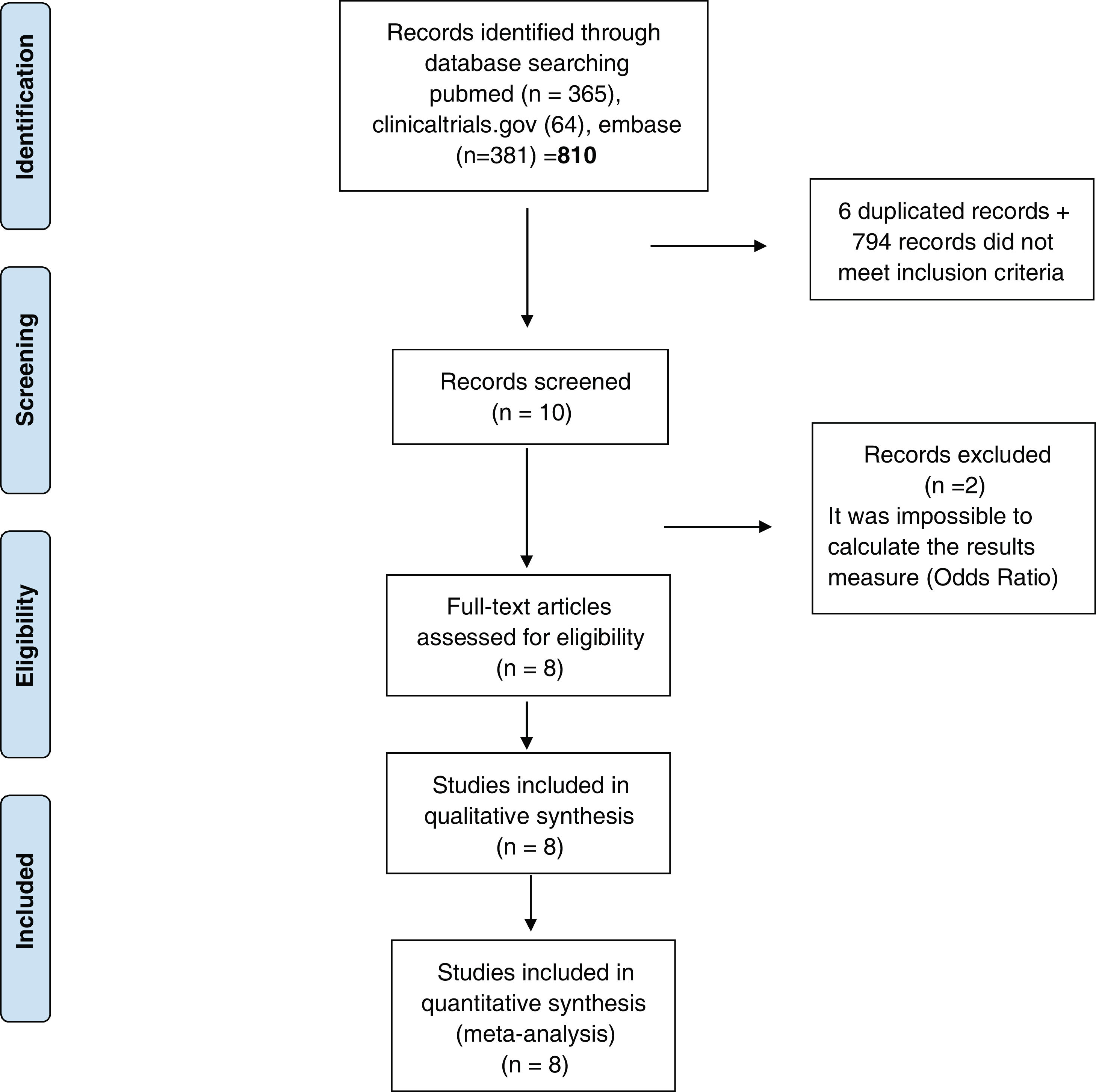
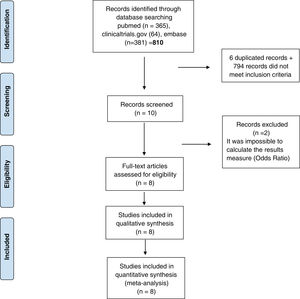
ResultsStudies included810 studies were identified for this meta-analysis (Supplementary Tables 1 and 2). After exclusion papers did not meet inclusion criteria (studies evaluating death, hospitalization and need for mechanical ventilation) and duplicated papers, 10 manuscripts were included for screening. After a more exhaustive review of the included studies, 2 of them were discarded it was impossible to calculate the final outcome measure23,24 (OR) (Fig. 1). Finally, 8 studies were included in the systematic review and meta-analysis10–12,25–29 and include data from 38,080 participants. Additional details of the included studies are presented in Supplementary Tables 1 and 2.
PRISMA 2009 flow diagram. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(6):e1000097. doi:10.1371/journal.pmed.1000097. Prisma statement check list included in Supplementary file.
In 3 studies, authors didn’t found any association between the use of antidepressant drugs and the reduction of mortality.25,28,29 In Bora25 and Fei's28 studies no adjustment for confounding variables was made. In Rauchman's29 study, no statistical differences were observed in mortality when adjusting for age, sex and race.
In 4 studies, authors found association between the use of antidepressants and a lower risk of poor evolution.10,12,26,27 In Németh26 and Calusic's studies,27 results were adjusted by confounding variables (Supplementary Table 2). In Ostosky's study,12 no adjustment for confounding variables was made but propensity score matching by age, sex, and race and ethnicity, comorbidities and SSRI prescription indications was performed (Supplementary Table 2).
The results of Hoertel's11 study are somewhat peculiar, as the raw data show a statistically significant increase in the risk of intubation or death, but when adjusting by sex, age, obesity, smoking status, and any medical condition associated with increased risk of severe COVID-19, results change drastically showing statistically significant reduction in this risk.
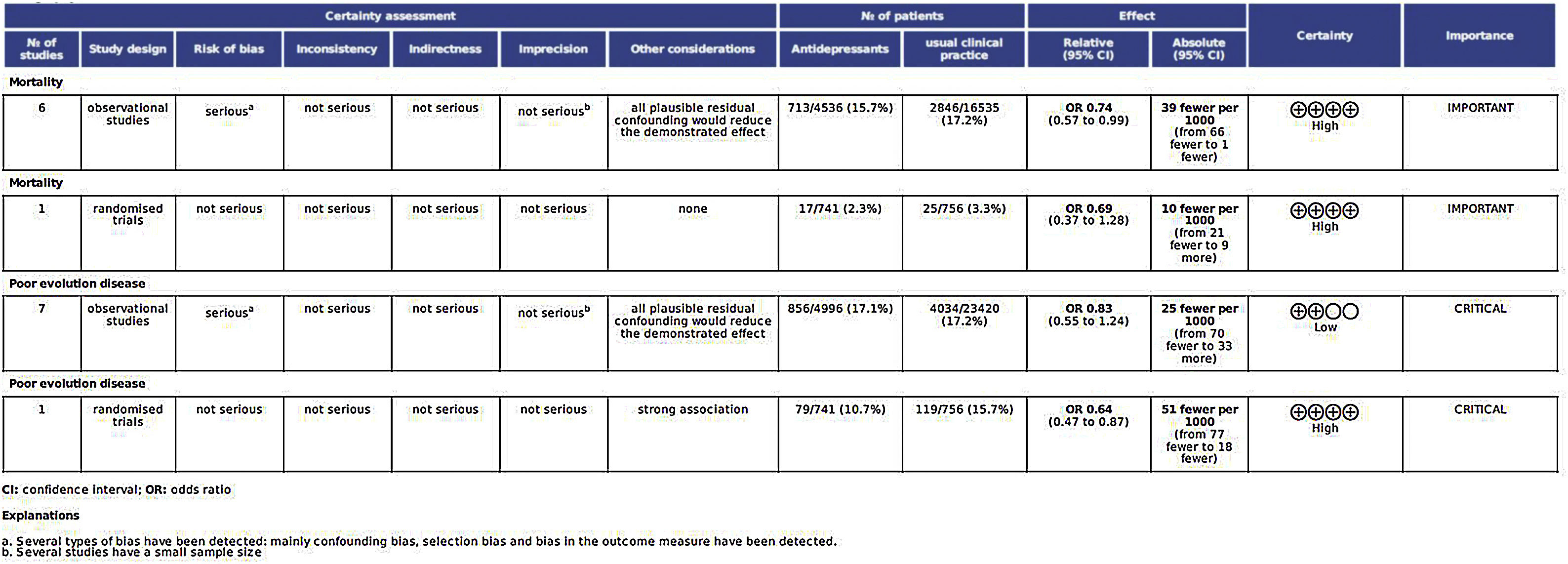
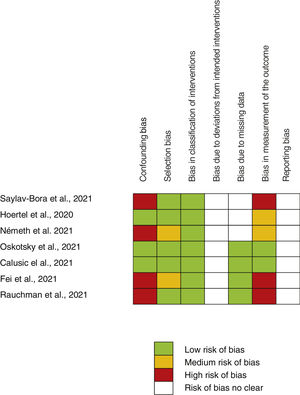
Assessment of main outcome “poor evolution of COVID-19 disease”Risk assessment of biasThe risk of bias of no randomized studies is shown in Fig. 2. Main bias observed were confounding bias25,26,28,29 and bias in the measurement of results.25,28,29Supplementary Fig. 1 shows the global risk of bias of non-random studies. Risk of bias of Reis et al. study10 (the only RCT-17) is low in all domains evaluated.
Heterogeneity analysisAccording to the results of the Dersimonian and Laird test,18 there is statistical heterogeneity among the studies (p<0.0001). Similar results can be drawn from Galbraith20 (Supplementary Fig. 2) and L’Abbé21 graphs (Supplementary Fig. 3). I2 index was 92%.
Publication biasThe p value of the statistical tests performed was greater than 0.05, suggesting a lack of publication bias. When analyzing the Funnel Plot (Supplementary Fig. 4), we realized that results do not come along with the statistical tests. Thus, the lack of bias cannot be assumed.
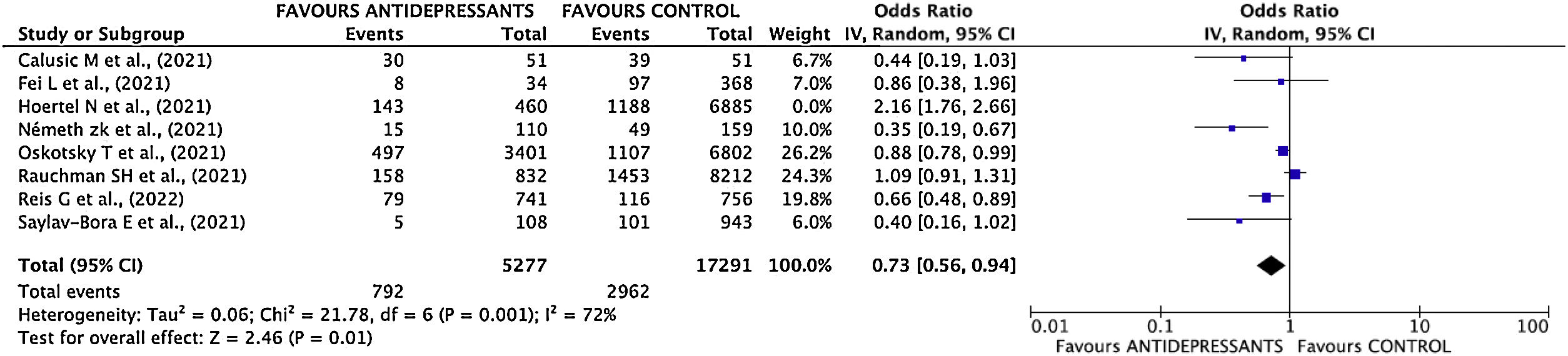
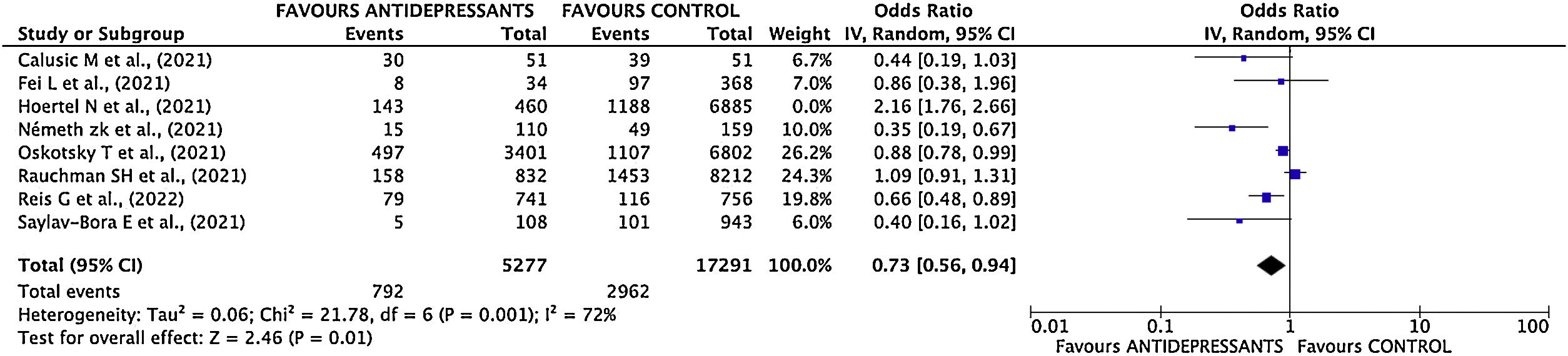
Estimation of pooled measurementThe results of the studies included in the meta-analysis have been combined using a random effects mode given the existence of heterogeneity. After combining the results, a total OR of 0.80; 95% CI 0.55–1.15 was estimated (Fig. 3).
Sensitivity analysisThe study with the greatest influence on the meta-analysis is Hoertel's one.11 When this study was deleted, and the meta-analysis was repeated, the greatest variation in the estimates of the overall effect was obtained. The estimated OR decreased (OR 0.73; 95% CI 0.57–0.94) (Supplementary Fig. 5 and Fig. 4). I2 index decreased to 73%.
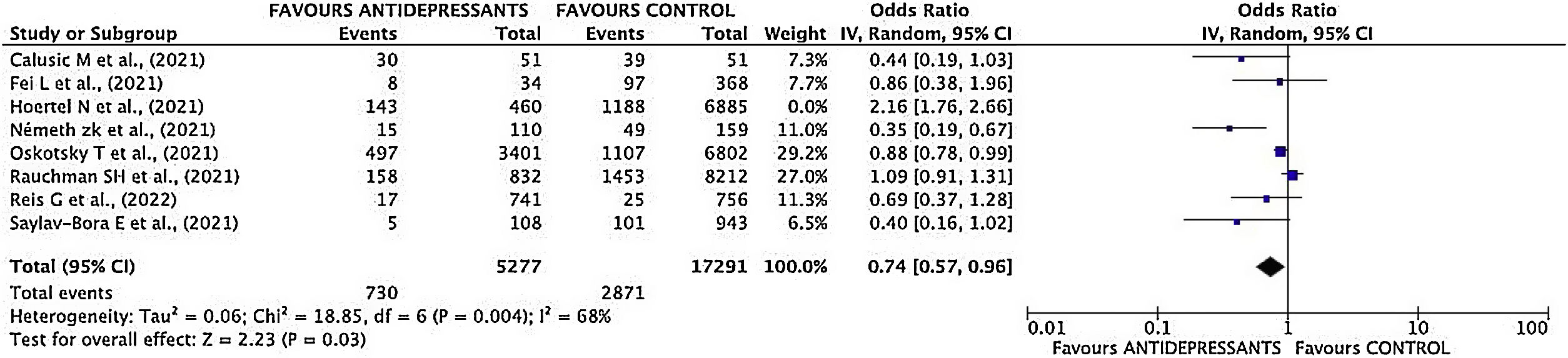
Mortality sub-analysisEstimation of pooled measurementOnly 7 studies were considered for the pooled mortality measure. Hoertel's study11 was excluded as the end point was a composite variable, making it impossible to break down each event included in it.
Results of mortality sub-analysis showed a decrease of mortality risk in patients treated with antidepressant drugs (OR 0.74; 95% CI 0.57–0.96) (Fig. 5).
Certainty of the evidence judgementsThe analysis of all factors affecting the quality of the studies is shown in Fig. 6.
DiscussionSSRIs have been explored as anti-inflammatory agents, and research suggests that SSRIs may be useful in the treatment of SARS-CoV2.10–12 In order to explore this hypothesis, we performed a meta-analysis of all the studies published to date to assess the effectiveness of these drugs to reduce the poor evolution and mortality. Results showed a favorable effect on both endpoint.
In the initial meta-analysis, no statistically significant differences are observed between the effectiveness of antidepressant use versus NO use (clinical practice) in the poor disease evolution outcome. However, sensitivity analysis showed a high influence of Hoertel's study11 on results, therefore, meta-analysis was repeated by deleting this study.
Once deleted Hoertel's study,11 the magnitude of the measure decreased considerably, from 0.80 to 0.73, showing also a statistically significant reduction in the risk of poor disease evolution in patients taking antidepressant drugs. The suppression of this study in the meta-analysis could be justified taking into to account that Hazard Ratio adjusted by confounding variables is favorable to the use of antidepressants, but the inclusion of raw data distorts totally the results of meta-analysis.
The estimated measure by deleting Hoertel's study11 suggests a statistically significant reduction in the risk of poor disease evolution in patients taking antidepressants; results which agree with obtained by Lee el al.30 and Nyirenda et al.,31 who assessed the effectiveness of fluvoxamine preventing hospitalization and mortality respectively.32
The exclusion of this study in the metaanalysis could be justified taking into to account that Hazard Ratio adjusted by confounding variables is favorable to the use of antidepressants, but the inclusion of raw data distorts totally the results of metaanalysis. Furthermore, results of Hoertel's11 study, when adjusting by sex, age, obesity, smoking status, and any medical condition associated with increased risk of severe COVID-19, results showing statistically significant reduction in the risk of intubation or death, in agreement with the results of metaanalysis.
On the other hand, as a result of the second meta-analysis, the sub-analysis of mortality showed a significant reduction in the risk of death in patients treated with antidepressants. Due to the moderate–low quality of included studies, the strength of the recommendation is weak, or not too strong, on both end points.
This meta-analysis presents several limitations: First, the quality of studies includes is low to moderate because the most of them are observational studies (Fig. 5). Second, several studies included hospitalized patients and others outpatients but, last ones with risk factors for severe COVID-19. Third, drugs are not the same (Supplementary Table 3), and in the most of papers authors didn’t collect the duration of treatment. To date, it is unknown whether the duration of treatment can influence the evolution of COVID-19 disease. Furthermore, drug evaluated are not the same, being fluvoxamine the most employed.
In relation to publication bias, studies with low sample size or with results not favorable to the evaluated intervention could be unpublished. In this meta-analysis, the authors did not find a favorable effect of antidepressants in three studies25,28,29 among 10 initially selected (even not included23, 24). This could lead us to think that there is possibly a publication bias, but that the lack of studies on the right side of the graph could also be due to the fact that the effect of antidepressants is really striking and powerful. Fifth, the authors do not indicate whether mortality is due to COVID-19 disease, so we assume that the variable analyzed is all-cause mortality.
This meta-analysis focuses mainly on the beneficial effect of SSRI antidepressant in the risk of poor evolution of disease. This effect could be due to the blocking of IL-6 release, as it was demonstrated by Fei et al.,28 with significant lower levels of interleukin-6 of recovered patients of the treated subgroup compared to recovered patients of not-treated subgroup (12.1 versus 25.4, p<0.001). In addition to the decrease on IL-6 levels, other possible mechanisms of action have been considered; such as the role of sigma-1 receptor in the early steps of viral RNA replication of SARS-CoV-2,33,34 and the inhibition of the acid sphingomyelinase/ceramide system which plays key roles in bacterial and viral infections.33,34 From the limited clinical data, fluvoxamine may be the most attractive candidate for early-stage COVID-19 patients. Fluvoxamine like all other SSRIs has favorable safety profiles, widespread availability, and is very low cost administered orally and used for children and adolescents,33 and it could prescribed in persons with COVID-19 as quickly as possible after SARS-CoV-2 infection confirmation33 in order to reduce clinical impairment.
In this sense, Lenze et al.23 demonstrated that the antidepressant fluvoxamine had affinity at the sigma-1 receptor, and could prevent clinical deterioration in adult outpatients infected with SARS-CoV-2. In relation to the last, Hashimoto34 also proposed that sigma-1 receptor plays a key role in the replication of SARS-CoV-2 in cells. Furthermore the author points out that the order of potency for SSRIs at the sigma-1 receptor is as follows: fluvoxamine>sertraline>fluoxetine>escitalopram>citalopram>paroxetine,34 suggesting that fluvoxamine could likely be a prophylactic drug for early-stage SARS-CoV-2-infected patients.35
In case of considering treatment with antidepressant drugs to reduce the risk of poor evolution and/or mortality in COVID-19 positive patients, SSRIs could be considered as primer choice because they are the most prescribed drugs for the treatment of the first episode of depression or a new episode of recurrent depression.36,37 Moreover, they are generally well tolerated and have advantages over tricyclic antidepressants including improved safety in overdose, reduced side-effect burden, and uncomplicated dosing regimens.38
On the other hand, primary care providers are important in recognizing and managing depression,39 and 79% of antidepressant prescriptions are written by providers who are not mental health care providers. Furthermore, the critical role of primary care in managing ambulatory COVID-19 and identifying those at higher risk for severe manifestations plays a key role in disease management.40 As a preventive strategy at times of higher incidence of the disease, could be considered to identify individuals with risk factors for developing more severe symptoms of COVID-19 in primary care consultations, and to offer them as a preventive manner, temporary treatment with this type of drug.
- •
IL-6 blocking drugs have been supposed to be effective in the treatment of cytokine storm.
- •
SSRIs (fluoxetine) have an antiviral effect on the replication of certain viruses.
- •
Fluoxetine has an inhibitory effect on SARS-CoV2 viral replication at doses of 0.8μg/ml.
- •
SSRIs have been explored as anti-inflammatory agents in the context of autoimmune and inflammatory diseases, and research suggests that SSRIs may be useful in the treatment of SARS-CoV2.
- •
SSRIs could be effective for reducing the risk of poor progression and mortality of COVID-19 disease. Prescribing antidepressants to patients with worse prognosis could be considered.
This review does not include patients’ data.
FundingWithout funding.
Conflict of interestAuthors declare no conflicts of interest.
Supplementary material associated with this article can be found in the online version available at doi:10.1016/j.aprim.2023.102771.