Community-acquired pneumonia (CAP) is treated with penicillin in some northern European countries.
ObjectivesTo evaluate whether high-dose penicillin V is as effective as high-dose amoxicillin for the treatment of non-severe CAP.
DesignMulticentre, parallel, double-blind, controlled, randomized clinical trial.
Setting31 primary care centers in Spain.
ParticipantsPatients from 18 to 75 years of age with no significant associated comorbidity and with symptoms of lower respiratory tract infection and radiological confirmation of CAP were randomized to receive either penicillin V 1.6 million units, or amoxicillin 1000mg three times per day for 10 days.
Main measurementsThe main outcome was clinical cure at 14 days, and the primary hypothesis was that penicillin V would be non-inferior to amoxicillin with regard to this outcome, with a margin of 15% for the difference in proportions. EudraCT register 2012-003511-63.
ResultsA total of 43 subjects (amoxicillin: 28; penicillin: 15) were randomized. Clinical cure was observed in 10 (90.9%) patients assigned to penicillin and in 25 (100%) patients assigned to amoxicillin with a difference of −9.1% (95% CI, −41.3% to 6.4%; p=.951) for non-inferiority. In the intention-to-treat analysis, amoxicillin was found to be 28.6% superior to penicillin (95% CI, 7.3–58.1%; p=.009 for superiority). The number of adverse events was similar in both groups.
ConclusionsThere was a trend favoring high-dose amoxicillin versus high-dose penicillin in adults with uncomplicated CAP. The main limitation of this trial was the low statistical power due to the low number of patients included.
En algunos países la neumonía adquirida en la comunidad (NAC) se trata con penicilina.
ObjetivoEvaluar si penicilina V a dosis altas es igual de efectiva que amoxicilina a dosis altas en la NAC no complicada.
DiseñoEnsayo clínico paralelo, doble ciego, controlado y multicéntrico.
EmplazamientoTreinta y un centros de salud en España.
ParticipantesSe reclutaron pacientes de 18 a 75 años de edad sin comorbilidad asociada importante, con síntomas de infección respiratoria inferior y confirmación radiológica de neumonía, que fueron asignados aleatoriamente a 1,6M unidades de penicilina V o amoxicilina 1.000mg, 3 veces al día, durante 10 días.
Mediciones principalesLa variable de resultado principal fue curación clínica a los 14 días y se planteó la hipótesis de que penicilina no era inferior a amoxicilina con un margen de 15% para la diferencia de proporciones. Registro EudraCT 2012-003511-63.
ResultadosSe aleatorizaron 43 personas (amoxicilina: 28; penicilina: 15). Se observó curación clínica en 10 pacientes asignados a penicilina (90,9%) y en 25 asignados a amoxicilina (100%), observándose una diferencia de –9,1% (IC 95%: –41,3 a 6,4%; p=0,951) para no inferioridad. En el análisis por intención de tratar amoxicilina fue 28,6% superior a penicilina V (IC 95%: 7,3% a 58,1%; p=0,009 para superioridad). El número de eventos adversos fue similar en ambos grupos.
ConclusionesSe observó una tendencia de un mayor beneficio de amoxicilina frente a penicilina en adultos con NAC no complicada. La principal limitación fue la baja potencia estadística debido al bajo número de pacientes incluidos.
Community-acquired pneumonia (CAP) remains a burden in the modern world.1 Respiratory bacteria constitute the major group of causative organisms. In approximately 50% of the cases the pathogen cannot be identified; however, Streptococcus pneumoniae is the organism most frequently isolated throughout all the studies and settings, even among outpatients.2,3
In some European countries, mainly those in Northern Europe, pneumonia is treated with penicillin V since the resistance of pneumococci to this antibiotic is low.4 However, in most European countries amoxicillin is the first-choice antibiotic for uncomplicated cases.5–7
The reasons for this study are: the cut-off points which determine whether a pneumococcus is susceptible to penicillin changed in 2008,8 and according to recently studies pneumococcal resistance to penicillin (minimum inhibitory concentration [MIC] >2μg/ml) has fallen drastically.9 There is no correlation between pneumococcal infection by a strain resistant to penicillin and therapeutic failure with β-lactams prescribed at adequate doses.10 The use of narrow-spectrum antibiotics is needed because of the dearth of new antimicrobials and the link between the consumption of broad-spectrum antibiotics and the emergence and spread of antibacterial resistance11; no clinical study comparing amoxicillin and penicillin V in pneumonia in adults has been published to date.
The aim of this trial was to determine whether high-dose penicillin V was as effective as high-dose amoxicillin for the treatment of uncomplicated CAP in a Mediterranean adult population.
Material and methodsThis is a prospective, parallel-group, randomized, double-blind, trial in primary healthcare centers in Spain carried out from November 2013 to April 2016. All patients provided written informed consent. The study protocol has been published elsewhere.12
Patients aged 18-75 without significant associated comorbidity attending a primary care physician with signs and symptoms of lower respiratory tract infection and radiological confirmation of the diagnosis of pneumonia were invited to participate. We permitted the inclusion and randomisation of patients without radiological confirmation if there was a high suspicion of pneumonia as suggested in previous studies13: patients with lower respiratory tract infection, temperature higher than 38.5 °C and productive cough; if chest X-ray performed in the following 24h, did not confirmed pneumonia, patients were excluded. Due to difficulties in the recruitment of patients an amendment to the previous protocol was made, broadening the age range of subjects, considering some other comorbidities and also taking into account patients who had taken urinary antiseptics in the previous days.12
Exclusion criteria were subjects under 18 or over 75 years of age, severe impairment of signs (impairment of consciousness, respiratory rate >30 breaths/min, heart rate >125 beats/min, systolic blood pressure <90mm Hg, diastolic blood pressure <60mm Hg, temperature >40°C, oxygen saturation <92%), hypersensitivity to β-lactams, important alteration on chest X-ray, (alveolar infiltrate in more than one lobe or bilateral, pleural effusion and/or pulmonary cavitation), problems to comply with treatment at home, lack of tolerance to oral treatment, significant comorbidity (bronchial asthma, renal failure, hepatic cirrhosis, heart failure, chronic obstructive pulmonary disease, ischemic heart disease, stroke, type 1 diabetes mellitus, immunosuppression, terminal disease), pregnancy or lactation, hospitalization in the last month, consumption of antibiotics in the last two weeks, difficulty to attend the programmed visits or refusal to participate in the study.
Participants and investigators remained blinded to the treatment received. Patients were randomly assigned to receive either penicillin V 1,600,000IU (two 800,000IU pills) or amoxicillin 1000mg (two 500mg pills) thrice a day during 10 days. Subject numbers were assigned sequentially as each subject entered the study. The subjects were assigned through a schedule based on the randomization plan. Since this was a multicentre study a block procedure was planned for the allocation of the study medication. Each container included 66 pills (60 pills for the treatment plus 6 extra pills). The dose of penicillin used was not marketed in our country and drugs were entirely prepared and labeled by the Pharmacy Unit of the Hospital Universitari Son Espases (Palma de Mallorca). The batches were small and every batch required stability analysis every 6 months, thereby exceeding the initial budget. A data and safety monitoring board was created to ensure patient safety and treatment efficacy during recruitment.
The difficulties in drug preparation, the limited budget and the elevated number of centers opened, made it impossible to maintain the 4 block per center distribution, and finally the medication was allocated following a simple randomization according to center needs.
Use of antithermic drugs or analgesics (acetaminophen, acetylsalicylic acid or ibuprofen), bronchodilators or any other medication, except oral systemic antibiotics, that the patient was talking was allowed.
Variables. The main outcome was clinical cure at 14 days, defined as the absence of fever, resolution or improvement of cough, improvement of general well-being and resolution or reduction of crackles. Any clinical result other than the anterior was considered as treatment failure. Secondary outcomes were efficacy at day 30 after the initiation of antibiotic treatment, radiological resolution or improvement one month after the initiation of the treatment, complete clinical resolution at day 14 defined as the total resolution of acute symptoms and signs related to the infection, and the presence of adverse events. The radiologists responsible for diagnosing pneumonia and confirming the radiological cure were blind to the clinical data and the treatment administered.
The nature of the study and scheme to the visit program was explained to the patient and informed consent was obtained. All the eligible patients had a chest X-ray (postero-anterior and lateral views) demonstrating pneumonic infection. Patients, were randomized to one of the 2 treatment groups and the medication was given. At the first follow-up visit, at day 3 (by phone or at the center), worsening of the clinical situation was evaluated to determine whether a change in the antibiotic treatment was necessary, compliance and possible secondary effects of the treatment were evaluated. At the second follow-up visit, at day 14, the clinical evolution of the signs and symptoms was evaluated, and possible secondary effects of the treatment. On the last follow-up visit, at day 30, the clinical outcome of the signs and symptoms of the pneumonia was evaluated and a new chest X-ray was performed (postero-anterior and lateral views) to confirm radiological resolution. The medication was discontinued if significant adverse events. The variables were registered in an electronic case report form designed by the Fundació Institut Català de Farmacologia, Barcelona.
Sample size calculation and statistical analyses: The objective of the study was to demonstrate that penicillin V was not inferior to amoxicillin. Considering a success rate of 85% for the group treated with amoxicillin,14,15 a total of 105 patients were required in each treatment group (total of 210) to detect a noninferiority margin of 15% at maximum between the two treatments with a minimum power of 80% considering an alpha error of 2.5% for a unilateral hypothesis and maximum possible losses of 15%.
The intention-to-treat (ITT) population included all randomized patients with confirmation of pneumonic infection receiving at least one dose of the study drug and the per-protocol (PP) population included patients adherent to protocol with adequate treatment compliance and absence of major protocol violations.
Descriptive results of the outcomes were reported as number and percentages. To evaluate the differences on baseline characteristics, Fisher's exact tests for categorical variables and non-parametric tests for continuous measures were carried out. Comparisons between groups were performed using exact binomial distribution for the efficacy endpoints and adverse events,16 95% confidence intervals (CI) for the differences in success percentages were estimated and p-values provided. All analyses were conducted with R statistical package, version 3.2.5.
The study was approved by the Institutional Review Board (IDIAP Jordi Gol Clinical Investigation Ethic Committee) and the Spanish Agency of Medicines and Health Products. Written informed consent was obtained from all participants. Trial registration: EudraCT number 2012-003511-63.
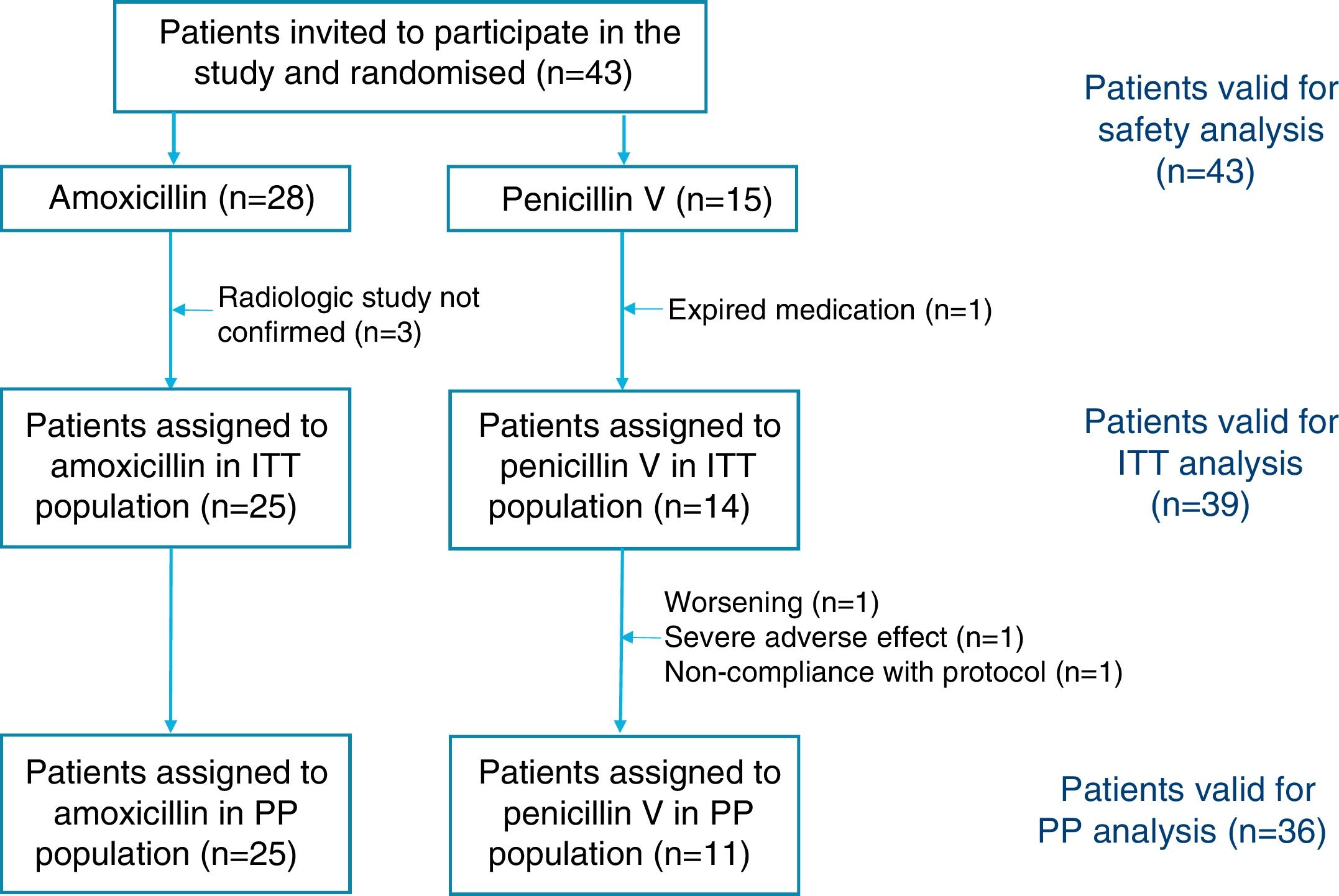
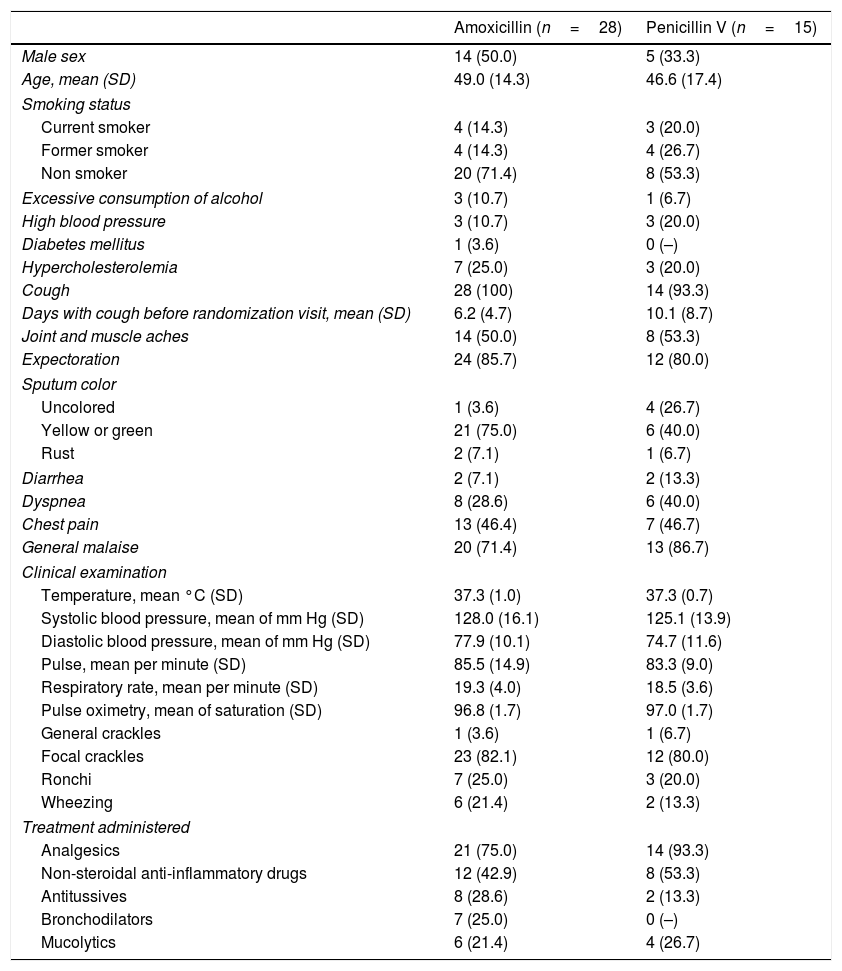
ResultsThe required sample size of 210 patients could not be achieved although 31 centers were opened (10 centers were initially planned). A total of 43 subjects were screened and randomized (28 patients in the amoxicillin arm and 15 patients in the penicillin arm) and constituted the safety population. The demographic and clinical characteristics of the randomized population were well matched between the groups (Table 1). A total of 4 patients were excluded from the ITT population: 3 had a normal chest X-ray and one was mistakenly given expired medication and was withdrawn the following day. A total of 3 patients were lost during follow-up and were all excluded from the PP population.
Baseline characteristics of the patients randomized into two groups to receive either amoxicillin or penicillin V. Values are numbers (percentages) unless stated otherwise.
| Amoxicillin (n=28) | Penicillin V (n=15) | |
|---|---|---|
| Male sex | 14 (50.0) | 5 (33.3) |
| Age, mean (SD) | 49.0 (14.3) | 46.6 (17.4) |
| Smoking status | ||
| Current smoker | 4 (14.3) | 3 (20.0) |
| Former smoker | 4 (14.3) | 4 (26.7) |
| Non smoker | 20 (71.4) | 8 (53.3) |
| Excessive consumption of alcohol | 3 (10.7) | 1 (6.7) |
| High blood pressure | 3 (10.7) | 3 (20.0) |
| Diabetes mellitus | 1 (3.6) | 0 (–) |
| Hypercholesterolemia | 7 (25.0) | 3 (20.0) |
| Cough | 28 (100) | 14 (93.3) |
| Days with cough before randomization visit, mean (SD) | 6.2 (4.7) | 10.1 (8.7) |
| Joint and muscle aches | 14 (50.0) | 8 (53.3) |
| Expectoration | 24 (85.7) | 12 (80.0) |
| Sputum color | ||
| Uncolored | 1 (3.6) | 4 (26.7) |
| Yellow or green | 21 (75.0) | 6 (40.0) |
| Rust | 2 (7.1) | 1 (6.7) |
| Diarrhea | 2 (7.1) | 2 (13.3) |
| Dyspnea | 8 (28.6) | 6 (40.0) |
| Chest pain | 13 (46.4) | 7 (46.7) |
| General malaise | 20 (71.4) | 13 (86.7) |
| Clinical examination | ||
| Temperature, mean °C (SD) | 37.3 (1.0) | 37.3 (0.7) |
| Systolic blood pressure, mean of mm Hg (SD) | 128.0 (16.1) | 125.1 (13.9) |
| Diastolic blood pressure, mean of mm Hg (SD) | 77.9 (10.1) | 74.7 (11.6) |
| Pulse, mean per minute (SD) | 85.5 (14.9) | 83.3 (9.0) |
| Respiratory rate, mean per minute (SD) | 19.3 (4.0) | 18.5 (3.6) |
| Pulse oximetry, mean of saturation (SD) | 96.8 (1.7) | 97.0 (1.7) |
| General crackles | 1 (3.6) | 1 (6.7) |
| Focal crackles | 23 (82.1) | 12 (80.0) |
| Ronchi | 7 (25.0) | 3 (20.0) |
| Wheezing | 6 (21.4) | 2 (13.3) |
| Treatment administered | ||
| Analgesics | 21 (75.0) | 14 (93.3) |
| Non-steroidal anti-inflammatory drugs | 12 (42.9) | 8 (53.3) |
| Antitussives | 8 (28.6) | 2 (13.3) |
| Bronchodilators | 7 (25.0) | 0 (–) |
| Mucolytics | 6 (21.4) | 4 (26.7) |
SD: standard deviation.
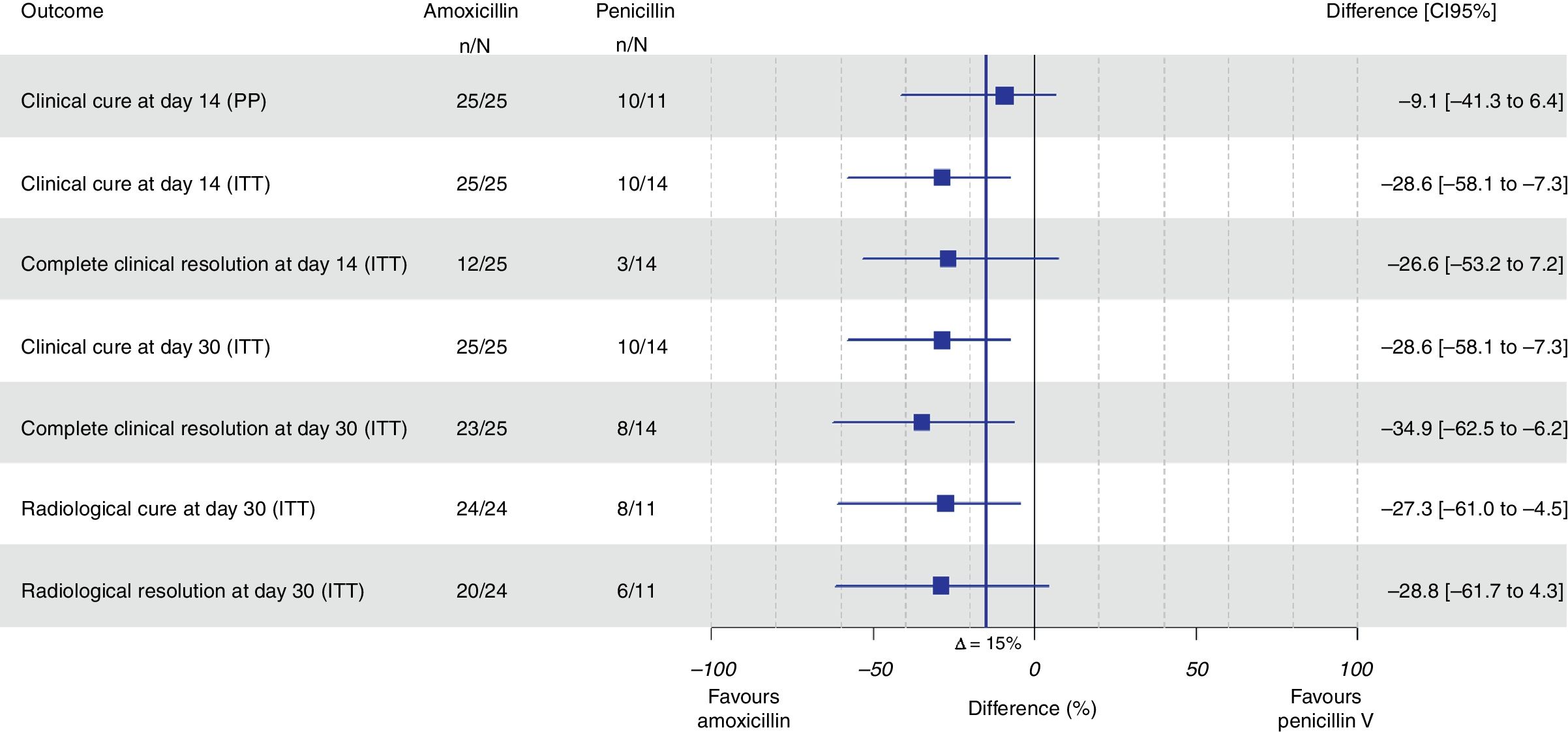
We cannot prove that penicillin V was not inferior to amoxicillin as clinical cure was observed in 10 patients assigned to penicillin (90.9%) and in 25 patients assigned to amoxicillin (100%) in the PP population with a difference of −9.1% (95% CI, −41.3% to 6.4%; p=0.951 for noninferiority). A total of 39 subjects (25 in the amoxicillin arm and 14 in the penicillin V arm) fulfilled all the criteria for the ITT population, and in this group amoxicillin was found to be superior to penicillin, with a difference of 28.6% (95% CI, 7.3–58.1%; p=0.009 for superiority) (Fig. 1).
All the results of this section refer to superiority comparisons in the ITT population (Fig. 1). Amoxicillin was found to be 28.6% superior to penicillin in terms of clinical resolution on day 30 (95% CI, 7.3–58.1%; p=0.009) and in terms of total resolution one month after the index visit (34.9% superior, 95% CI, 6.2–62.5%; p=0.002). Radiological resolution among patients assigned to amoxicillin was 27.3% superior to patients allocated to penicillin V (95% CI, 4.5–61%; p=0.027). A total of 4 adverse events were related to the study drugs: three cases among patients treated with amoxicillin and one case in the penicillin V group (10.7% vs. 6.7%, respectively) without any statistically significant differences. Three cases corresponded to epigastralgia and one corresponded to vaginal candidiasis.
DiscussionOn the basis on the results obtained we cannot prove that high doses of penicillin V in adults with non-severe CAP are not inferior to high doses of amoxicillin – the currently recommended treatment – in patients under the age of 75 years, but because of the limited number of patients, the confidence intervals were wide. However, when we tested for the superiority of amoxicillin, we did find significant statistically differences, with amoxicillin being 28.6% superior to penicillin V in clinical resolution on day 14 (95% CI, 7.3–58.1%).
The major limitation of this study was the limited number of patients included. This can lead to false negative results because of the width of the confidence intervals. The approval from the Spanish Agency of Medicines and Health Products was delayed and we were not able to start recruiting until November 2013. Since the funding was only available until April 2016 we were only able to recruit patients for two and a half years. In addition, the diagnosis of CAP in primary care is challenging, since a positive X-ray for pneumonic condensation is required for correct diagnosis. In an attempt to increase patient recruitment, GPs were allowed to recruit patients with a high suspicion of pneumonia, that were randomized before the chest X-ray was performed, and three patients were excluded because of normal radiological images. Many patients were also excluded because they had taken some doses of antibiotics, either because they had first bypassed the primary care professionals and gone to emergency departments, or they had taken leftover doses stored at home.17
Another limitation was the uneven number of patients per group. A proper block procedure randomization plan was prepared, but it was not possible to follow it due to budget problems. To increase the number of candidates for the trial, many additional centers were included, but it was impossible to make enough medication to send an entire block to every center, considering the small batches prepared by the pharmacy service and the stability analysis required per batch every six months. However, the probability of obtaining an imbalance greater than or equal to that observed (28 vs. 15 randomized patients) exceeds 6%, so that this disparity can be attributed to chance.
A possible limitation was the fact that microbiological studies were not taken into account. Bacterial eradication has been considered a secondary outcome in many studies undertaken up to now, but some experts recommend that this measure should not be considered in mild-moderate pneumonia.18 Moreover, GPs judge the response to treatment with eminently clinical rather than microbiological criteria.
Interventions and follow-ups were similar to clinical practice, a simple methodology, as it should be in primary care. The data collection was also simple enough to facilitate the inclusion of cases in primary care offices. Chest X-ray was absolutely necessary to confirm the diagnosis of pneumonia.
To our knowledge, no study comparing oral penicillin and oral amoxicillin in CAP in adults has been carried out to date. Some clinical trials have compared amoxicillin with penicillin, but these were performed in children and with parenteral doses.19–23 All these trials confirm that oral treatment with amoxicillin is equivalent to parenteral penicillin in children with CAP. Consequently, we cannot compare the results obtained in our randomized clinical trial with other studies. In a recent Cochrane review, including 11 randomized clinical trials and 3352 outpatients older than 12 years with a diagnosis of CAP, Pakhale et al. observed that there is inadequate evidence to recommend one antibiotic class over another.24 However, in our study amoxicillin is associated with better clinical outcomes than oral phenoxymethylpenicillin for the treatment of adults with uncomplicated CAP.
In Scandinavian countries, where penicillin V is considered the first choice for uncomplicated pneumonia, high doses ranging from 1 million to 1.5 million IU thrice-daily are recommended.4,25 According to data from the European Center for Disease Prevention and Control for the year 2014, resistant strains accounted for 20% of the pneumococci isolated in Spain.9 Most of this resistance rate corresponded to intermediate resistant organisms, against which higher doses of penicillin are recommended. From a microbiological standpoint, bacterial eradication is achieved when the concentration of antibiotic is above 40–50% of the time between β-lactams dose intervals. For partially resistant strains, it is recommended that this time be above 60%.26 We know that the bioavailability of penicillin V is very variable, ranging from 25% and 60%. However, one study found that the MICs achieved with penicillin V were higher than with penicillin G administered parenterally.27 Fredlund et al. in Sweden, showed that oral penicillin V was as effective as parenteral penicillin in patients with CAP when the former was administered during 10 days.28 Regarding the safety of the regimen of 1.6 million units (corresponding approximately to 1 gram of penicillin V) thrice-daily, this dose is not marketed in Spain, although a presentation of 1.5 million IU is available in Scandinavian countries. In addition, the British National Formulary recommends doses of up to 1 gram of penicillin V every 6h.29 We think that the dose of 1.6 million units taken thrice daily was fully justified in this clinical trial.
The statement that resistant organisms lead to failure is controversial. A large proportion of patients die because of the severity of their disease and the associated comorbidity but not due to failure of the antibiotic. Pallarés et al. provided evidence that the risk of death by pneumococcal pneumonia treated with benzylpenicillin or ampicillin was similar regardless of whether the patients were infected with germs resistant or not to penicillin.30 In other studies evaluating the effectiveness of penicillin in monotherapy for the treatment of non-meningeal pneumococcal infections within the first 48h in adults, an increase in mortality was not observed when the infection was caused by a strain with an MIC <2μg/ml.31 However, in our study amoxicillin is associated with better clinical outcomes than oral phenoxymethylpenicillin.
Some scientific societies, such as the Spanish Society of Family Medicine, recommend amoxicillin as the first-choice antibiotic for non-severe CAP in countries where pneumococcal resistance is observed,5–7 and according to the results of the present study, there is no reason to change this recommendation.
- •
The Spanish Society of Family Medicine recommends amoxicillin as the first-choice antibiotic for non-severe community-acquired pneumonia, since pneumococcal resistance is currently observed in Spain.
- •
The massive use of broad-spectrum antibiotics for respiratory tract infections (amoxicillin, amoxicillin and clavulanate and fluoroquinolones) is associated with a rapid increase of antimicrobial resistance.
- •
This trial planned to address hypothesis that the administration of high doses of a narrow-spectrum antibiotic – penicillin V – in adults with uncomplicated pneumonia was not less effective than high doses of amoxicillin.
- •
Amoxicillin was superior to penicillin V with statistically significant differences.
- •
These results might only be valid for countries with isolation of pneumococcal strains partially and highly resistant to penicillin, such as Spain.
The project received a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded in the 2011 call under the Health Strategy Action 2013–2016, within the National Research Program oriented to Societal Challenges, within the Technical, Scientific and Innovation Research National Plan 2008–2011, with reference PI11/02471, co-funded with European Union ERDF funds. It was also supported by the Spanish Clinical Research Network (SCReN), PT13/0002/0030. The funding bodies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interestDr Llor reports receiving research grants from the European Commission (Sixth and Seventh Programme Frameworks), Catalan Society of Family Medicine, Instituto de Salud Carlos III (Spanish Ministry of Health) and Alere and has received research fees from GlaxoSmithKline in the last 3 years. Dr Román-Rodríguez reports that he has received research grants or fees for speaking in the last 3 years from the Instituto de Salud Carlos III (Spanish Ministry of Health) and the following pharmaceutical companies: Astra Zeneca, Boehringer-Ingelheim, Chiesi, GSK, Menarini, Mundipharma, Novartis, Pfizer, Rovi, and Teva. MM has received speaker fees from Almirall, Boehringer Ingelheim, Pfizer, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols and Novartis, and consulting fees from Almirall, Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, Medimmune, Teva, Takeda, Novartis and Grifols. The other authors declare no competing interests.
We acknowledge the patients and the GPs who participated in this study as well as the contribution of the following professionals in the development of the project: Silvia Hernández, Olga Calviño (Primary Care Centre Jaume I), Marta Hernández (Primary Care Centre Salou), Josep M. Cots, Diego Sebastian, Cándida Espinosa, Jaume Puig (Primary Care Centre La Marina), Gràcia Garcia (Primary Care Centre El Temple), Antoni Viñas (Primary Care Centre Anglès), Juan Boj, Josep Balsells (Primary Care Centre Sant Pere), Javier Arranz, Antoni Jover (Primary Care Centre Arquitecte Benàssar), Antoni Ballester (Primary Care Centre Escola Graduada), Jesús Ortega, Ascensión Aicua (Primary Care Centre Rincón de Soto), Juan Carlos López Caro (Primary Care Centre Cotolino Sur), José Ramón López Lanza (Primary Care Centre Alisal), Mireia Guillén, Joan Llobera, Aina Soler (monitoring tasks in Balearic Islands), Olga Delgado, M. Mercedes Amengual, Pilar Rovira (fabricating the study drugs at Son Espases Hospital), Alicia Herranz (monitoring tasks in Madrid), Joan Vigo (guardian of the electronic platform), Guillem Frontera, Sara Bonet, Mònica Monteagudo (data and safety monitoring board), and Helena Pera.