Introduction
Health technology is defined as a group of drugs, devices, and medical or surgical procedures used in health care, and the organisational and support systems within which this care is provided.1,2 New health technology is that which has been recently, or will be shortly, introduced into clinical practice, and emerging is that which has not yet been put in place but is in a situation to be so, both having passed the clinical trial phase.3
To prevent the introduction of new technologies that could have undesirable effects on the population, the Office of Technology Assessment (OTA) was created in the USA in the 1970's.1 It was the first public technology assessment agency.
Since then, these agencies examine the short and long-term clinical, social, economic, and legal consequences, arising from the use of technologies (both desired and undesired effects).4
In 1993, International Network of Agencies for Health Technology Assessment (INAHTA) was created. Today it has 45 agencies from 22 countries, including Spain, among its members. In 1997, Euro-Scan, European Network for the Early Detection and Assessment of Emerging Technologies was established. Recently, the EUnetHTA (European Network for Health Technology Assessment) project has been started, to enable a more efficient exchange of information and health policies support.
Other important International Technology Assessment Societies and Networks are: HEN (World Health Organisation European Health Medical Evidence Network) and HTAi (Health Technologies Assessment International). Among the international ones in Spain, Iberamerican Cochrane Centre and the OPS/PAHO (Panamerican Health Organisation) are worthy of mention.
In 1999 the Health Technology Assessment Agency (AETS) of the Carlos III Health Institute began to develop an information system on new and emerging health technologies, known as SÍNTESIS. Its main objective is to identify new and emerging health technologies (except medicines) and compile relevant information on these technologies and their anticipated impact.2
The opinions of health personnel--in particular those of the doctors--are a good indicator of future technologies that are likely to appear in the health sector. On many occasions, the professionals acting as "fortune-tellers" of the technologies that will appear in the future.5
The objective is to analyse the correspondence between the technologies -which, in the opinion of medical specialists in Andalusia in 2003- that should be developed and the assessments that have been carried out and published by Spanish technology assessment agencies.
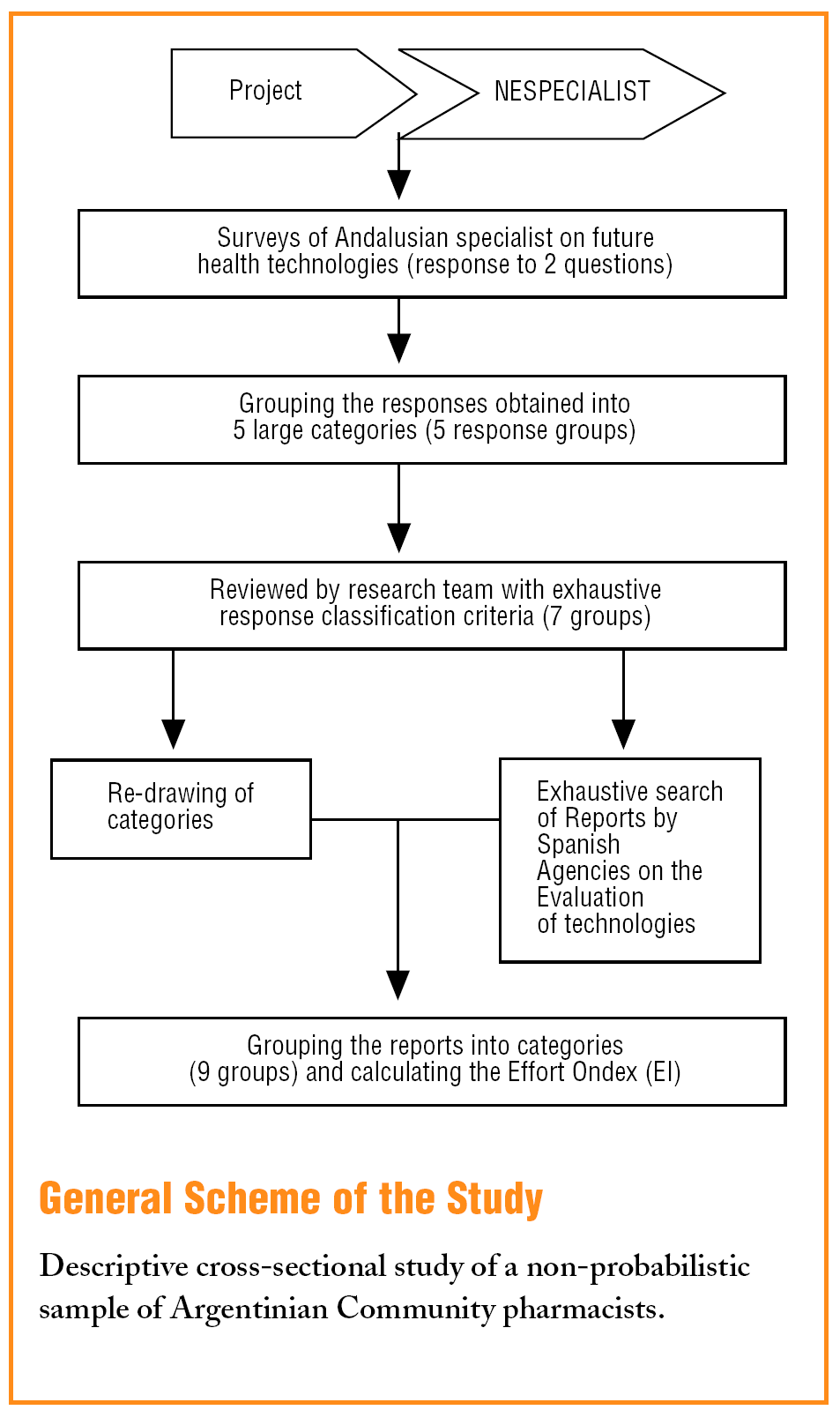
It is taken from the partial results of the NESPECIALIST study (specialised health training needs in the Andalusian Public Health System, PI SAS code: 0199/2005) and, PI FIS code: 06/90109), still not concluded.
Methods
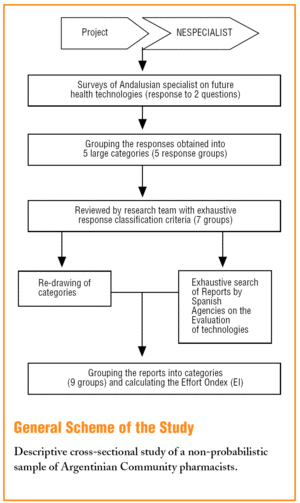
In the NESPECIALIST study--in 2002--147 Andalusian medical specialists were chosen. These professionals were identified by the research team as experts and professional leaders, who also had been advisors in health planning activities to the Andalusia Public Health System (SSPA).
They belong to 46 medical and surgical specialties, included in the National System of Health Specialist Training (the MIR System), with a minimum of 3, and a maximum of 5, professionals per specialty.
Using a questionnaire prepared ad hoc, they were asked about the future needs of specialists in our health system. For this article, the responses to 2 questions related to predicting the development or demand of future health technologies:
1. Which skills and/or appliance of new technologies in your specialty do you believe will be developed in the next 5-10 years in our health system?
2. Are there any others that, although you do not think they are going to be developed, you consider it important that should be developed to make our health system operate well? If yes, list them and say why you consider them important.
With the responses obtained, 5 large groups associated with "novel" health activities were established:
1. New diagnostic methods (including imaging and non-invasive diagnostics).
2. New treatments (medical, surgical, alternative, and psycho-social interventions).
3. Communication, information, network, and telematic systems.
4. Interventions technology assessments (impact, safety, efficacy and costs).
5. Planning and management (including information sources and clinical management).
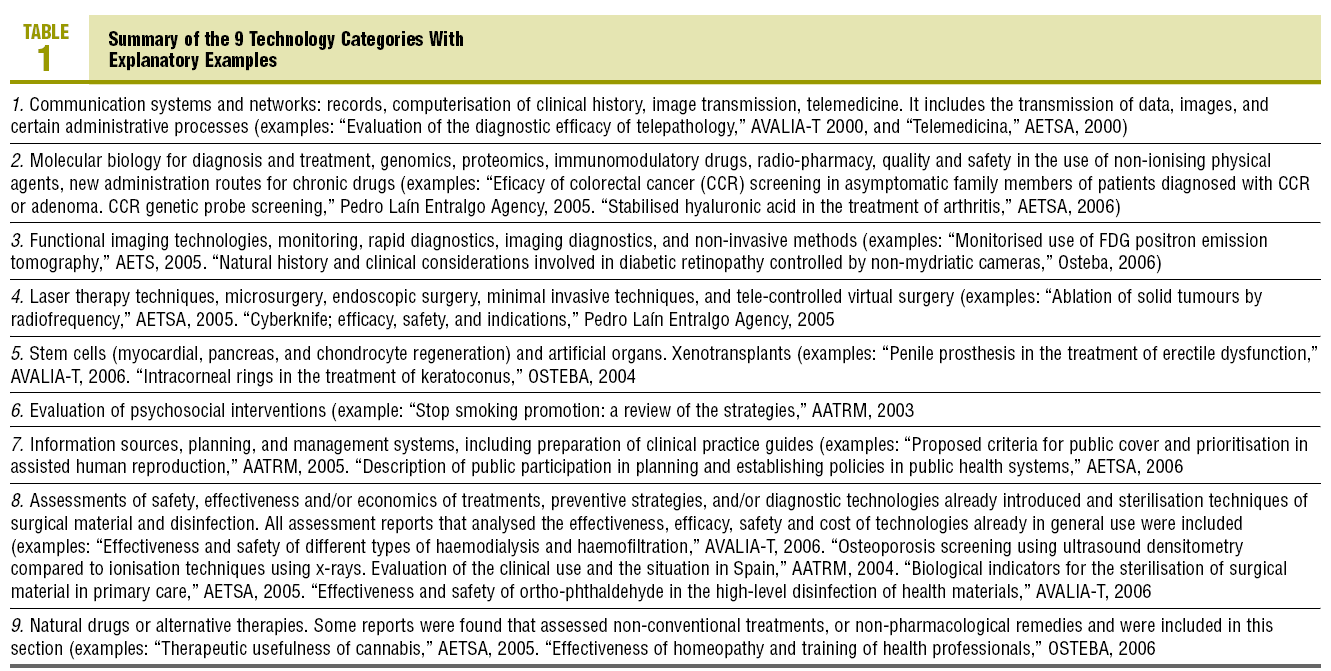
They were later increased to the 9 that are presented in the results, by consensus between the researchers, to be able to classify all the responses by the professionals and the assessments by the agencies.
An exhaustive search was then made on the Internet. Lists were made of assessment reports, short assessment reports and other publications that were written since their creation and reviewed in the Web pages of Spanish technology assessment agencies.
The information found was sub-divided into: a) those published between January 2005 and October 2006 ("current work" of the agencies), and b) assessment reports before January 2005.
Thus, it was checked whether the technologies soon to appear or be developed in the future (in the opinion of the specialists) had been assessed previously by the agencies and which had been included recently for assessment.
This period differentiation in the study seemed logical, in line with the proposals of other authors,2,6 when they define future technology (still has not been developed), emerging (before adopting it), new (that which is in the adoption phase), accepted (in general use), and obsolete (should be out of use).
All the technologies mentioned by the doctors and assessed by the agencies (established or not), were included in the analysis. This meant, the assessment of "novel technologies" using specific programmes or structures for this, and already established technologies, to evaluate their usefulness, cost and effectiveness at a determined time.
Results
Of the 147 specialists, 146 (99.32%) responded to the survey.
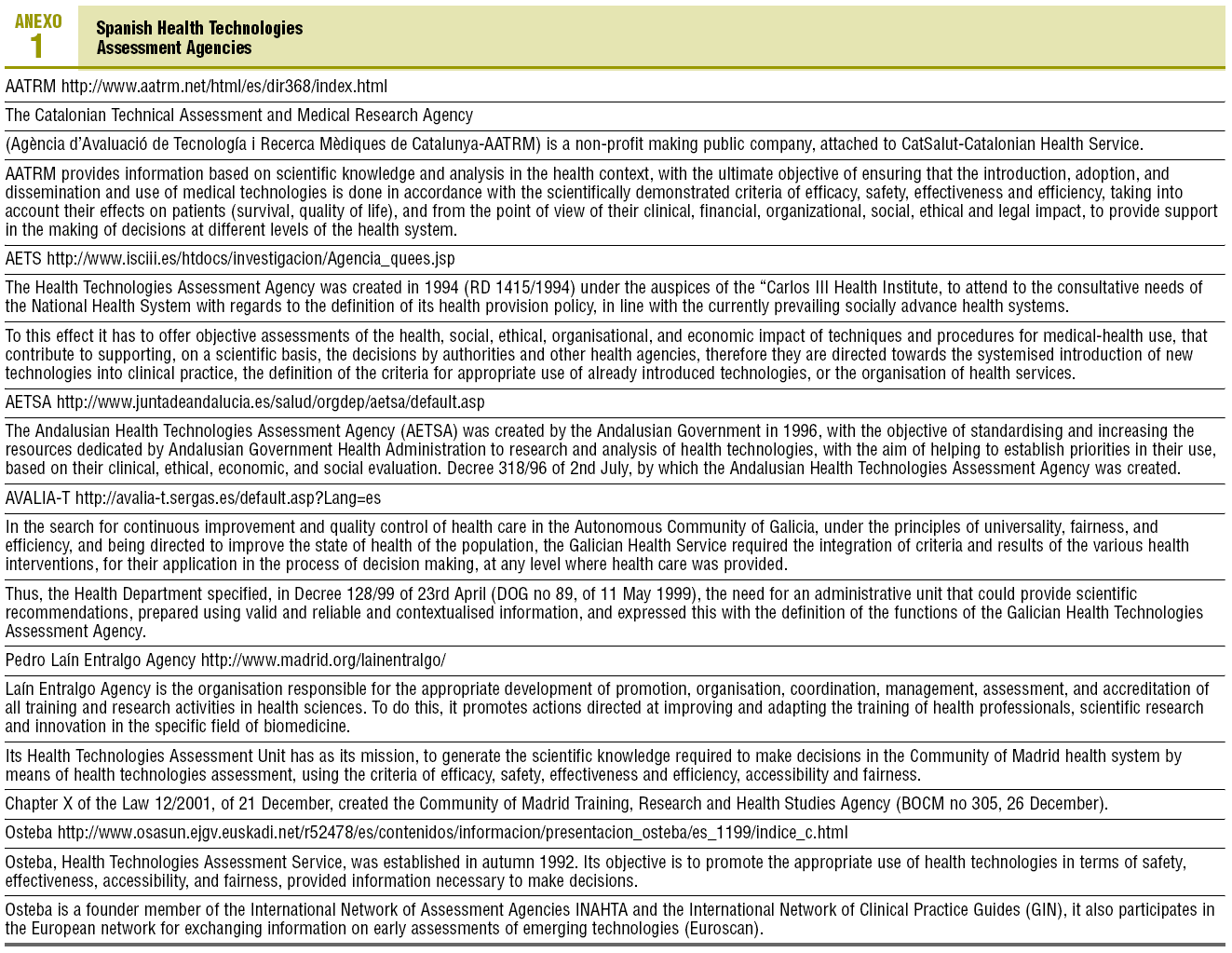
For the technology assessment agencies in Spain,7 their objectives and main activities are summarised in Table 1.
The literal responses of the professionals were grouped into 7 new technology categories (which were subsequently compared with the categories identified in the assessments by the agencies). These categories, together with the 2 new ones that were included from those assessed by agencies but were not mentioned by the doctors, as well as explanatory examples, are shown in Table 1.
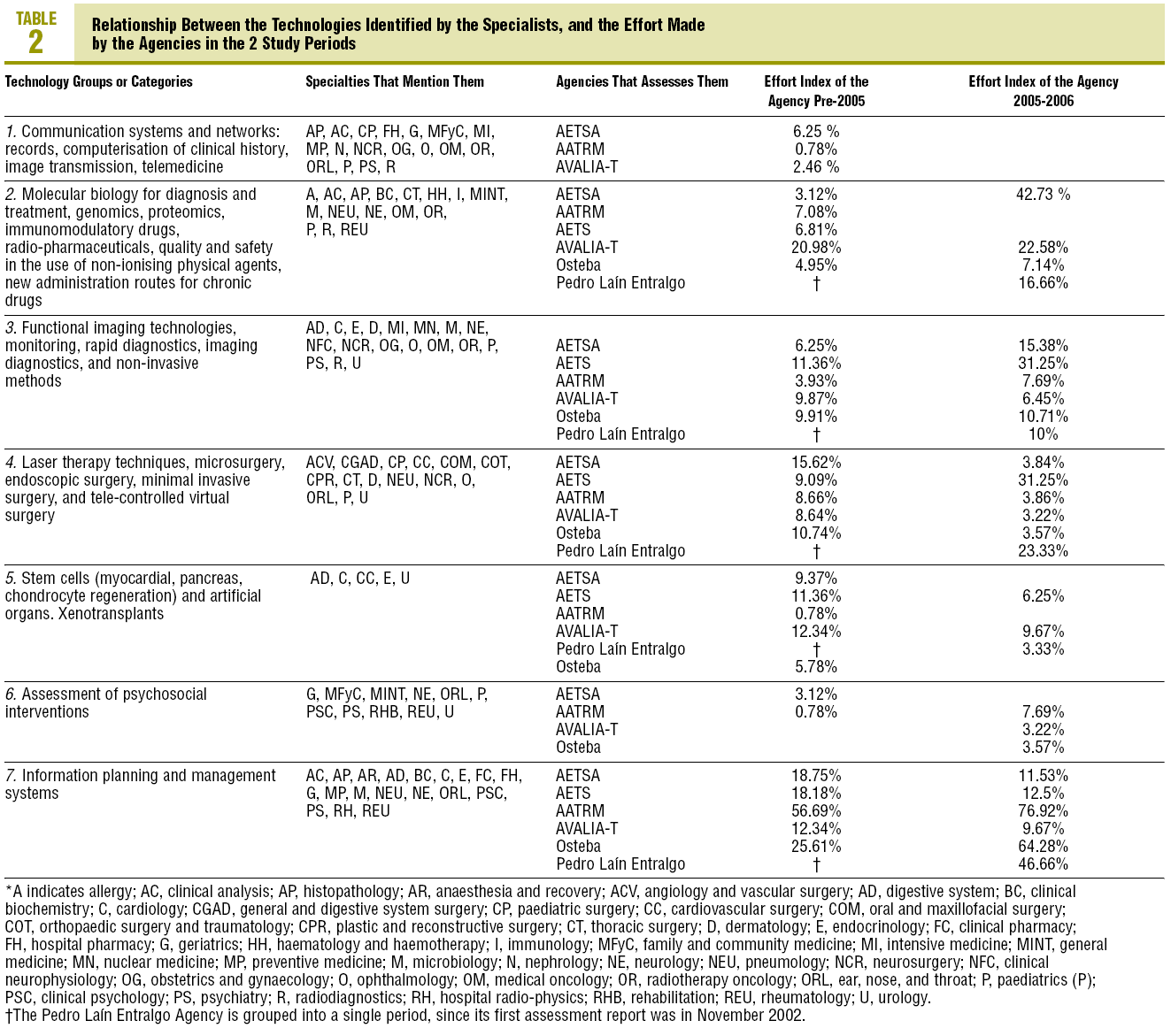
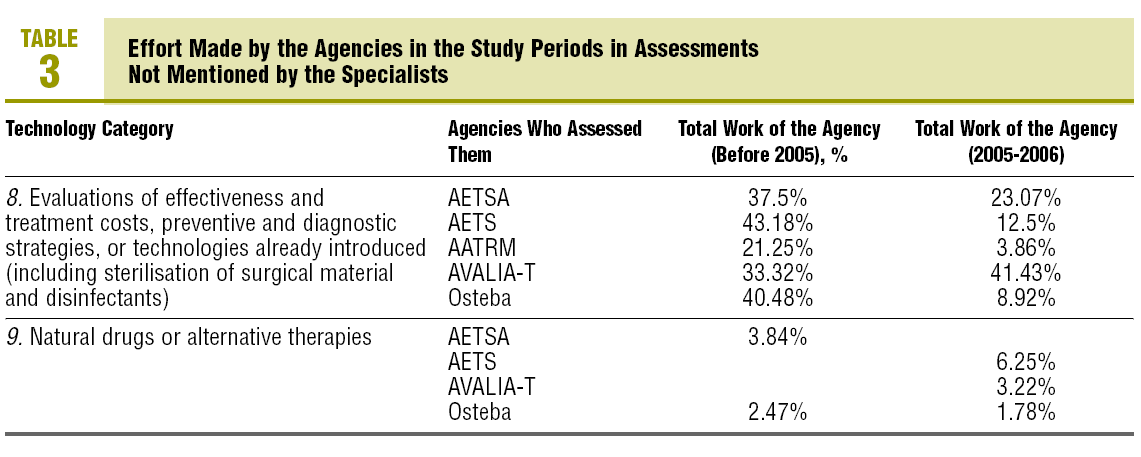
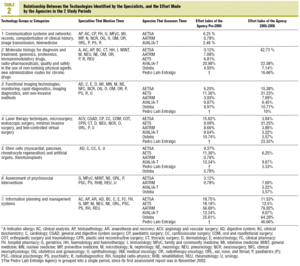
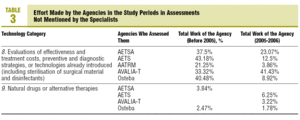
In the 7 related categories, an index of "effort dedicated" by the agencies to the technologies mentioned by the professionals was calculated. This index was arrived at by calculating the percentage of coincident reports in each category over the total assessment reports published by each agency. The results are shown in tables 2 and 3.
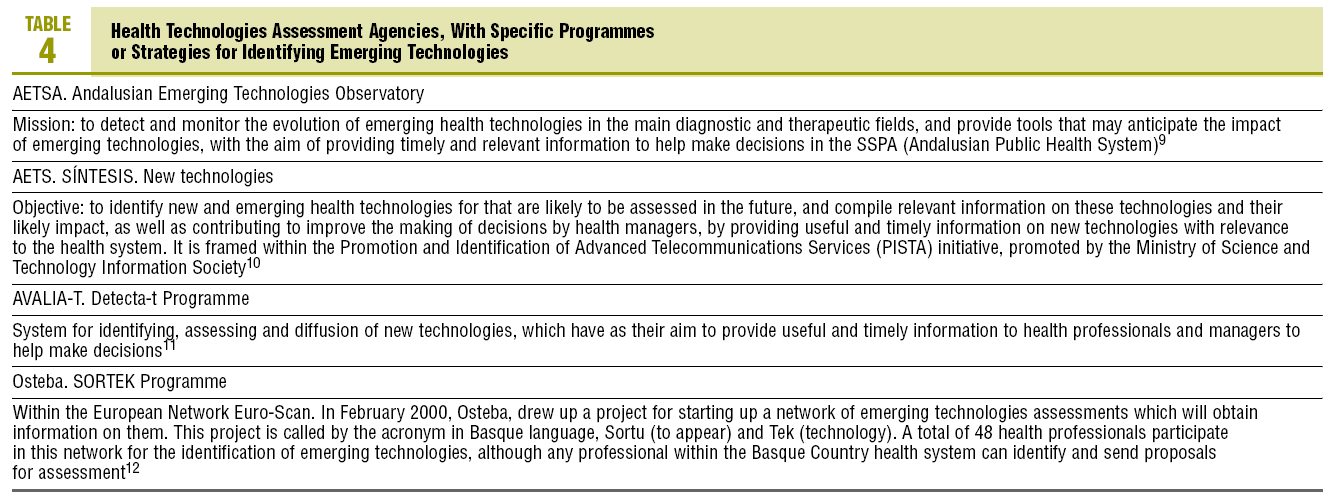
In 4 of the 6 agencies included in this study, they have their own specific programmes and structures, for detecting new and emerging technologies. These are summarised in Table 4.8-11
Discussion
The assessment of health technologies is gaining increasing importance nowadays, but there is still little evidence of their true impact on health care. Articles have appeared recently that demonstrate the important role of technologies in the increase in health spending,12 along with others which highlight problems of the agencies adapting to the real needs of the professionals.13
In the Ministry of Health and Consumer Affairs Quality Plan of 2006, it places special emphasis on the need for making use of technologies, by promoting knowledge and information on emerging technologies, to prepare technique practice guides and support the continuing education of the professionals in the use of the new technologies.14
The Spanish Federation of Health Technology Companies, aware of the importance of taking into account the profile of the users of new information technologies among the patients, with a clear increase in their level of information and their demands.15,16
This work attempts to provide a new element for reflection, by finding out the concerns of professionals as regards technologies to develop and the assessments by the agencies, by providing some data to improve coordination between both health protagonists.
The professional sample was not randomly selected as they were chosen due to their leadership and involvement in the development of their specialty, looking for, among other things, their knowledge and involvement (heads of units, members of scientific societies, participants in expert groups, advisors to the SSPA [Safety, Health and Environmental Protection Programme], involvment in assessment projects and/or health research).
The high response rate of the survey and its diversity suggests that the professionals are keen to give their opinions on future health aspects that are likely to be introduced into health care.
From the professional perspective, it is very likely that the development of new technologies may give rise to new sub-specialties and health and non-health posts.
Among the opinions detected in this study, the tendency for individualising of treatments, specific pioneering techniques, and the need to apply technological advances to connectivity, communication networks and management, are noteworthy.
A high percentage of agreement between the professionals and the agencies is found in technologies related to: a) molecular biology; b) functional imaging technology; c) new surgical techniques; and, above all, d) planning, management, and information systems. Perhaps the inclusion of clinical practice guides in this last group may justify--at least partly--a significant volume in the production of documents by the agencies.
The low percentages of agreement obtained in the remainder, could be explained because the question that was used in the NESPECIALIST study referred to future technologies related to the specialty, while the reports by the agencies basically assess already introduced technologies or in the introduction phase.
Some emerging technologies, for example, Telemedicine, will still need time to really become new technologies,17 although it is perceived as a fundamental step for the development of the rest of many other advances. The speed with which scientific advances arise, does not always follow the pace demanded by the professionals for adopting them, but it would be advisable to be able continue evaluating their economic and technical feasibility, effectiveness, and efficiency before their introduction, as well as the need for training the health professionals in their use and performance when they are introduced.
Another limitation could be the mean age of some professional groups and the difficulty and the cost of generalised and permanent training of new generations in the use of technologies in constant development. We have found 16 specialties with 20% of the professionals over 55 years (data not published) among the specialists who work in the SSPA.
A final interesting element for discussion is the influence of different factors in the upward movement of health spending. Although the influence of technological progress and the demographic increase in the aging population, sometimes changes in the health services offered (services menu, prescribing trends, etc) explain better the upward trend in health costs than other factors. It has been shown recently that those changes are responsible for 63.22% of the growth, compared to 27.96% associated to demographic changes.18
It is one argument more for coordinating efforts and assessing that the "new technologies being developed will really be useful for patients, and can be financed in our health system, before increasing the health services on offer."
What Is Known About the Subject
• The opinions of the professionals can be a good indicator of the technologies that will be developed in the health field in the future.
• There is a need to have an exhaustive system available to detect new health technologies so that they can be assessed by the agencies.
• The effectiveness and safety of new health technologies should be detected before their introduction.
What This Study Contributes
• Knowing the opinions of Andalusian professionals on foreseeable new health technologies that will be developed.
• Identifying a complementary information source for the detection of future health technologies.
• Highlighting the importance of the role of agencies in assessing the safety and efficacy of the technologies already introduced in clinical practice.
Spanish version available
www.doyma.es/202.315
Funding: Health Research Fund (evaluation of health technologies) and Andalusia Regional Government. FIS PI FIS 06/90109PI and SAS 0199/2005.
The present article uses some of the partial results of the NESPECIALIST study (Analysis of the Needs for Specialised Training in the Andalusia Public Health System).
Correspondence:
J.D. Prados Torres.
Unidad Docente de Medicina de Familia y Comunitaria de Málaga, pab. C. Hospital Carlos Haya.
Plaza del H. Civil, s/n. 29009 Málaga.
E-mail: juand.prados.sspa@juntadeandalucia.es