It is accepted by the great majority that the definitive proof of the role of the pancreas in diabetes mellitus was the publication by O. Minkowski, in 1893, demonstrating that pancreatectomy in dogs induced experimental glycosuria. Minkowski made glycosuria disappear in depancreatised dogs by subcutaneous implants of pancreatic tissue, and he was the first to try, unsuccessfully, to restore the antidiabetic action of the pancreas by the administration of pancreatic extracts either orally and parenterally1.
Nevertheless, prior to Minkowski's discovery, other scientists had provided evidence on the relationship between the pancreas and diabetes. In 1709, Johan Conrad Brunner removed the pancreas from dogs, and noted that they displayed extreme thirst and polyuria. His experiments could probably well be considered as the first ones on the internal secretion of the pancreas2.
In 1788, Thomas Crawley reported a singular case of diabetes in a subject who died showing stones and signs of tissue damage of the pancreas. In 1869, Paul Langerhans described in his doctoral thesis the pancreatic islets, ignoring their functional role3. Etiénne Lancereaux described in 1877 (12 years before the initial observation of J. von Mering and O. Minkowski) a form of diabetes occurring in a group of 12 subjects with pancreatic disorders; he named it pancreatic diabetes. The disorder was heterogeneous; one type was characterized by sudden onset, overt clinical manifestations, decreased weight and more severe pancreatic lesions; the other type, often inherited, was associated with obesity, usually asymptomatic, and depicted a slow progression4. Claude Bernard also investigated the hypothesis of pancreatic diabetes; for this purpose, he ligated the pancreatic ducts; after observing the atrophy of the gland without diabetes, he abandoned the hypothesis of pancreatic diabetes, and insisted on the metabolic role of the liver and the influence of central nervous system in glucose5.
It would not be until 1901 when Eugene L Opie established the relationship between the hyaline degeneration of the islets of Langerhans and the occurrence of diabetes. In 1906, Wilhelm Heiberg developed a method for counting the islets and found that the number was consistently low in diabetic individuals6. In 1909, Jean de Meyer introduced the name of insulin to describe the active substance from the islets of Langerhans7. In 1916, Sir Edward A. Sharpey-Schafer (1850-1935), Professor of Physiology at the University of Edinburgh theorised that the islets of Langerhans must secrete a substance which governed carbohydrate metabolism. For this suspected internal secretion of the pancreas, Schafer suggested the name of insuline8.
J. Rennie and T. Fraser, from Aberdeen Royal Infirmary, published in 1907 clinical experiences carried out between 1902 and 1904, investigating the effects of administering pancreatic islets from various teleostei, in particular the Lophius Piscatorious to 5 diabetic patients, with negative or inconclusive results. In these particular fishes, the islets are anatomically independent from the acinar tissue9,10.
GL Zuelzer did not observe beneficial effects on the first diabetic patient treated with a pancreatic extract. Results were more satisfactory in the following patients, including a 6 year old male child with ketonuria. However, all his patients developed adverse effects: fever, vomiting, stomatitis, profuse sweating10,11. J. Forschbach reproduced the results of Zuelzer in 1909, following Minkowski's advice, and recognised: First (Zuelzer) to produce, successfully, from the pancreas a preparation that eliminates sugar excretion in a shorter or longer period by intravenous administration12. In 1912, Zuelzer submitted an application to the USA patent office on ACOMATOL on May 6, 1908. The patent was awarded (serial number 431,226), as «pancreas preparation suitable for the treatment of diabetes» on May 28, 191210,13.
Clinical use of insulin (1922-1923)University of Toronto and Toronto General HospitalDuncan Archibald Graham was Chair of the Department of Medicine and Physician-in -Chief at the Toronto General Hospital in 1921. Fred Banting asked him for permission to treat diabetic patients in the Internal Medicine Ward of Toronto General Hospital (TGH) with the pancreatic extracts. Professor Duncan did not allow him to do it at first Nevertheless, the mediation of John Macleod made it possible to administer the first extract prepared by Banting and Best14.
Duncan Archibald Graham (1882-1974), born on a farm near Ivan, Ontario, held the first position in the British Empire of Chair of Clinical Medicine, established by John Craig Eaton at the University of Toronto in 1919. He was Chair of the Department of Medicine and Physician-in -Chief at the Toronto General Hospital, until 1947. From 1933 to 1935, he was the President of the Royal College of Physicians and Surgeons of Canada. From 1940 to 1941, he was the President of the Canadian Medical Association. He received honorary degrees from the Universities of Western Ontario, Toronto, and Queen's15.
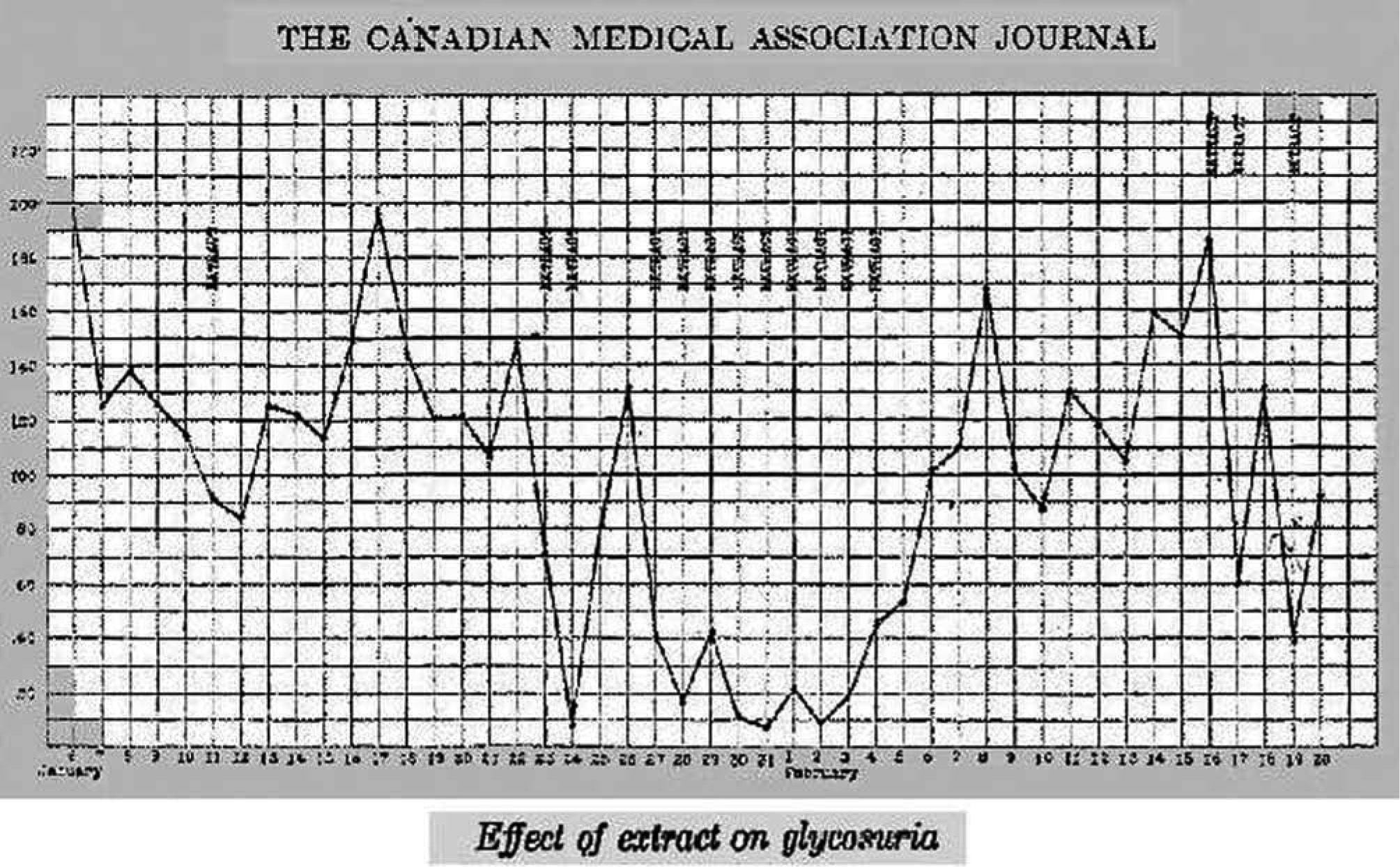
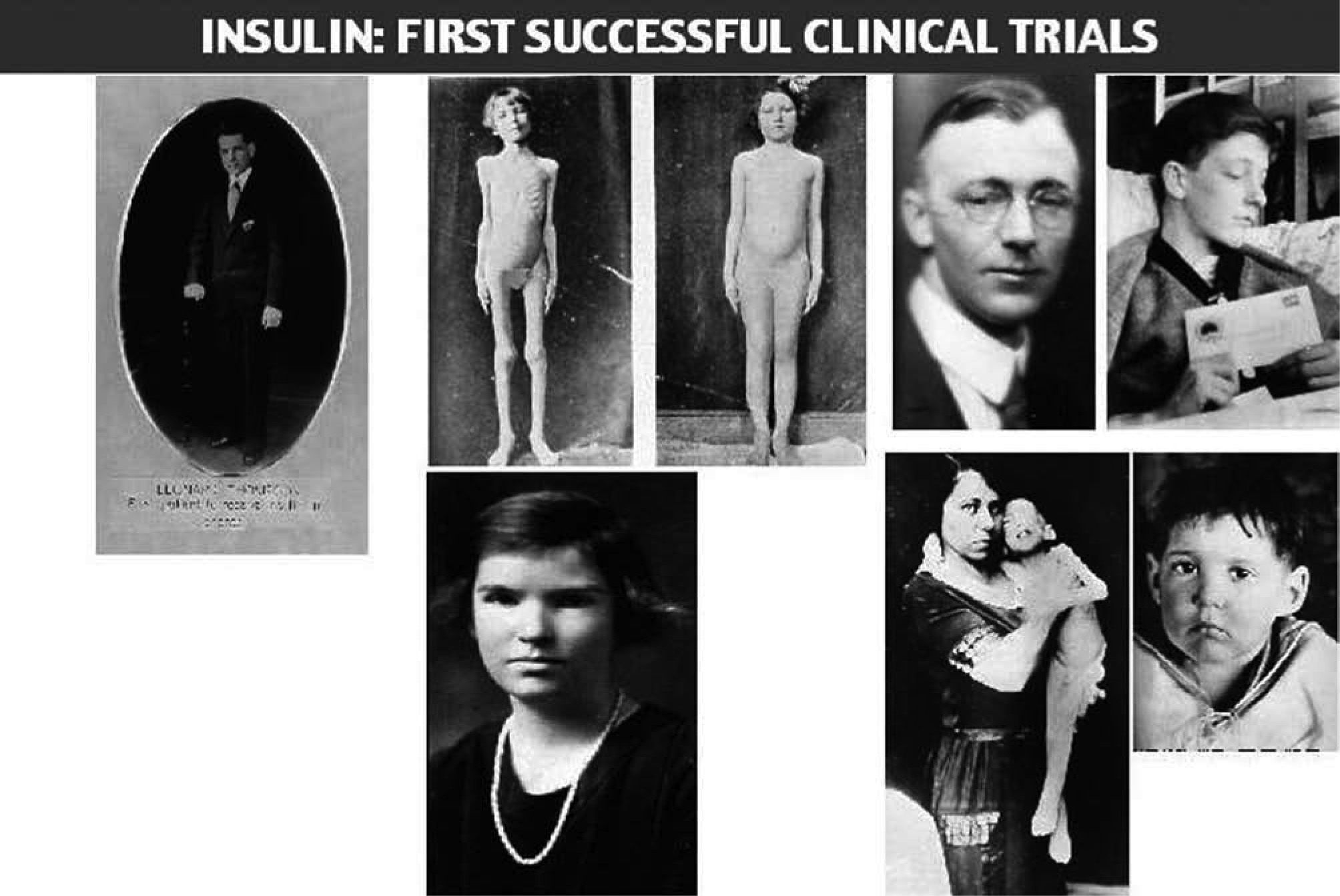
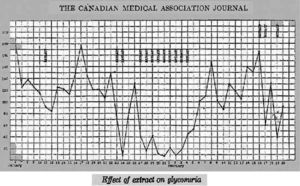
Dr. Walter R Campbell, Attending Physician of the Internal Medicine Department, TGH, obtained permission from Leonard Thomson's father to allow him to participate in the first trial of Banting's pancreatic extract16. Leonard was a 14 year-old boy with juvenile diabetes diagnosed two years before. On January 11, 1922, Dr. Campbell ordered Dr. Ed Jeffrey to administer, subcutaneously, 7.5ml of the extract into each buttock. Figure 1 depicts the daily urine glucose excretion of Leonard from his admission at TGH on December 2, 1921. The Banting's pancreatic extract was not successful; only a slight decrease in glycosuria and a 25% decrease in blood sugar could be observed, together with an aseptical abscess at one injection site17.
Banting et al17.
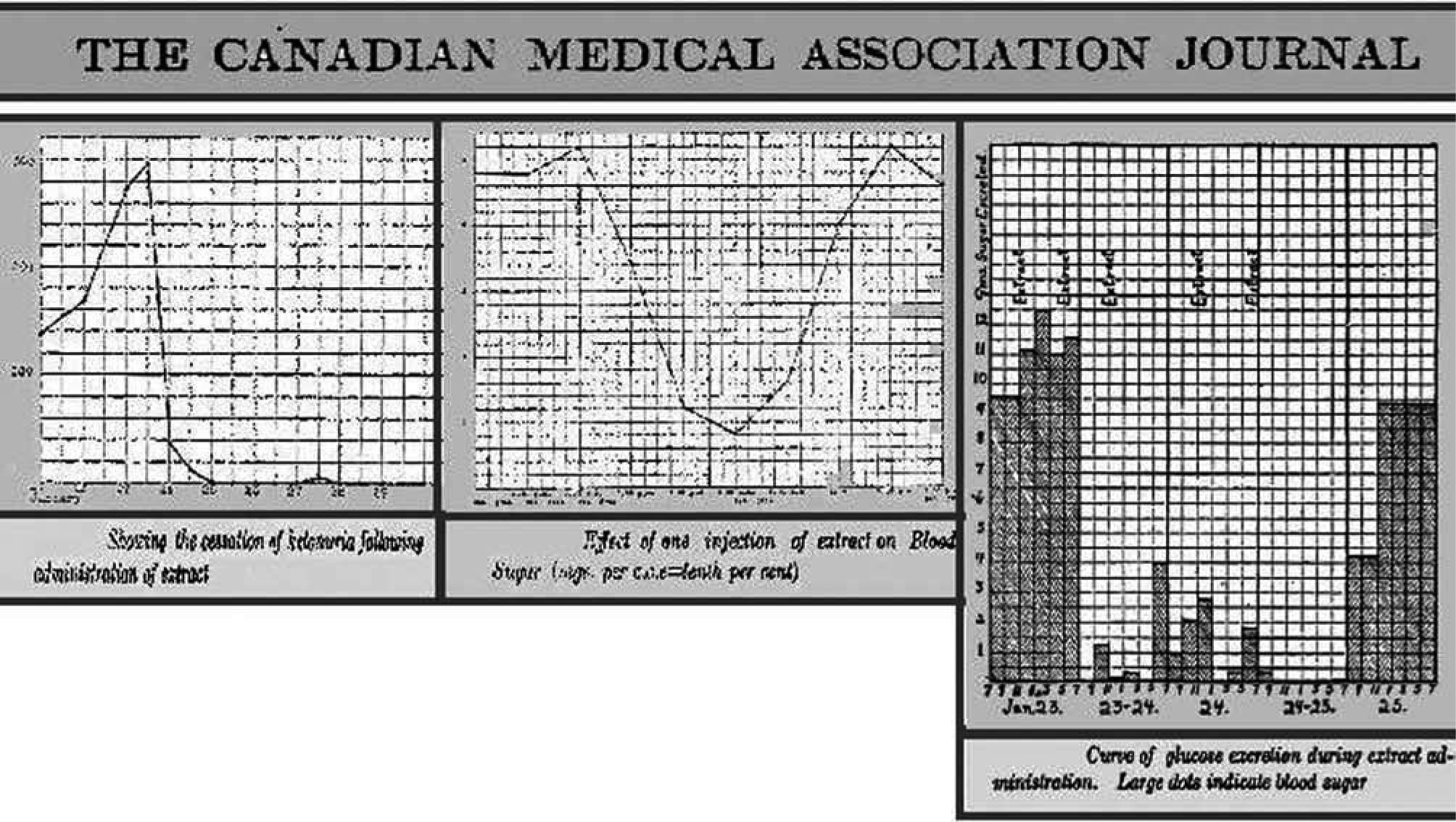
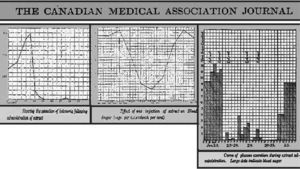
On the contrary, the graphic illustrates, from January 23 to February 4, the excellent response of the sugar excretion to the administration of the Collip's pancreatic extract18,19. Figure 2 shows, in parallel to the marked reduction in the amount of glucose excreted, a substantial reduction of blood glucose and urinary ketone bodies, together with clinical improvement. After this historical experience, Prof. Duncan Graham assigned Drs. W.R. Campbell and A. A. Fletcher to solve all features related to the trial with this new treatment in the wards of the medical Department at the TGH. The successful results achieved with the case of Leonard Thomson were reproduced quite soon. By February 1922, six more patients had been already investigated17.
Banting et al17.
The immediate consequences of these clinical observations at the Department of Medicine, were reported to the American Diabetes Association by Walter Campbell:
The situation created in the medical world by the discovery of insulin was, I think, unprecedented…The necessity for expanded clinical facilities was immediate and pressing. With Professor Graham directing, a clinic was formed to carry further the clinical investigations, with Banting, Fletcher and I taking charge of the patients. We worked together literally night and day and Professor Graham was not the least tireless of the group. It was absorbing work and each day someone in the laboratory or the clinic had a new fact to add - a new hypothesis to test. The pooling of facts and ideas was an important element in the rapid progress in both laboratory and clinic…16
The investigators from Toronto University and TGH decided to present the results of the successful clinical experience at the meeting of the Association of American Physicians on May 3, 192220. The general audience was impressed after the lecture given by Macleod.
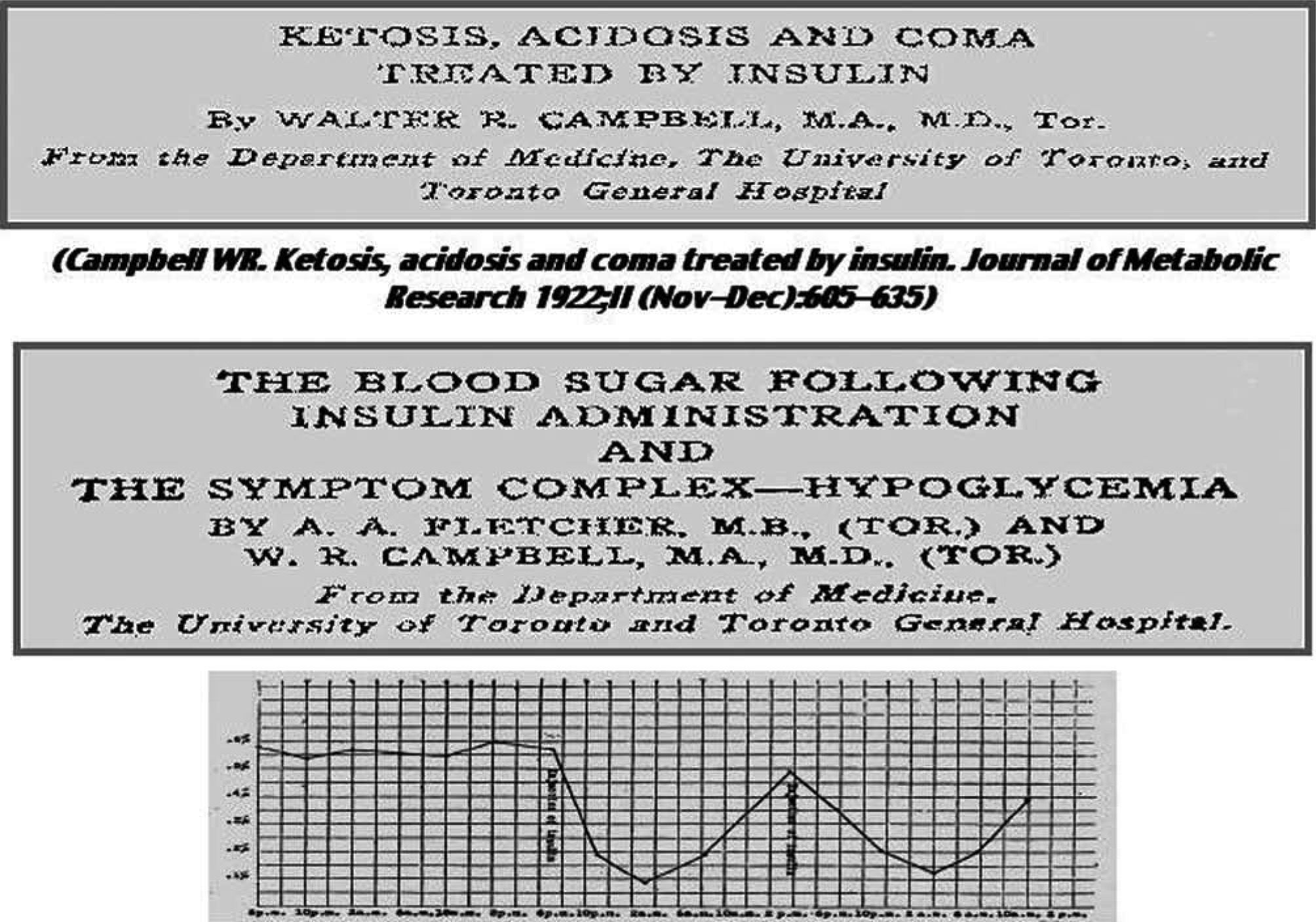
The Canadian team made a detailed presentation of nine cases of insulin-treated diabetic subjects in a special issue of the Journal of Metabolic Research (Director, F.M. Allen; Editor, FG Banting)21 (fig. 3).
Fletcher et al23.
In the same issue of the Journal of Metabolic Research), Walter R Campbell reported careful clinical observations of the first fourteen cases of diabetic coma treated in the Medicine Clinic, Toronto General Hospital (fig. 3). In one of the reported cases, a woman with glycosuria, acetone odour and dyspnoea, ketoacidosis was complicated by a six month pregnancy. Insulin was given subcutaneously and intravenously at high doses. Large amounts of ketones in in the urine were reduced to negligible values. Ketone bodies in blood also disappeared. Seven cases survived. With the exception of two patients, all those who have died have had an associated infection, sufficiently severe to result fatally apart from the diabetic condition. One of these died from the toxic effects of a sloughing gangrenous foot ten days after being brought out of coma; three had pneumonia and one, pyemic abscess in the kidney. In all but one case, where perhaps insufficient insulin was used, the comatose condition was improved clinically and chemically.
In the article, Campbell provided an outstanding lesson about the treatment of diabetic ketoacidosis, which, in many aspects remains as valid today. He pointed out the extraordinary tolerance to insulin in coma, and the key role of water, insulin glucose, and alkali, in the treatment22.
Apparently, the ordinary carbohydrate equivalent value of the insulin, as found in other patients, bears no relation to the requirement during severe acidosis and coma… large quantities of carbohydrate are useful in the treatment of coma with insulin for three reasons: (1) it prevents hypoglycaemia when large doses of insulin are used; (2) it furnishes energy and reduces to a minimum the incomplete combustion of fat and protein, and thus limits ketone production; (3) it aids in the combustion of ketones already present in the blood and tissues. In the severe cases, glucose may be given intravenously… Fluids are valuable in the treatment as a vehicle for carbohydrate, for promoting diuresis and for relieving dehydration. Water, broths and various fruit juices may be used… The judicious use of alkali may prove of value in some cases of diabetic coma. As a therapeutic measure, alkali, if necessary at all, should be given as early as possible, and… the administration should be by the vein… At the same time, the harmful effect of an overdose to patients not really requiring alkali must be guarded against… One might introduce in all cases 20 gm of sodium bicarbonate per 84lb. body weight with safety… Further amounts of alkali might be introduced later if chemical evidence of its necessity is obtained.
In general, intravenous insulin was more effective than subcutaneous injection. Interesting clinical observations are depicted in the article (The clinical points on which this requirement for insulin should be based are: acetone odor on the breath; prolonged or irregular breathing; general evidence of dehydration - dry skin, loss of tissue turgor, nausea, emaciation and, particularly, a dry, glazed tongue. A patient with tongue moist at the edges is relatively safe for several hours at least-).
Pros and cons of the administration of sodium bicarbonate were discussed. The administration of 20g. of sodium bicarbonate per 84lb of body weight was considered safe. It was also argued that large quantities of carbohydrate with insulin were useful in the treatment of coma. Fluids were considered valuable for promoting diuresis and relieving dehydration, as well as a vehicle for carbohydrate22.
Finally, in the November-December issue of the Journal of Metabolic Research, Almon Fletcher and Walter Campbell made an excellent clinical description of insulin-induced hypoglycaemia23:
... The initial symptom may be a feeling of nervousness or tremulousness, sometimes a feeling of excessive hunger, at other times a feeling of weakness or a sense of goneness… The reaction may go no further than this… More usually it is rapidly followed by objective signs-most frequently a sweat which may be very profuse; pallor and flushing is common; sometimes a change in the pulse rate… At times there is a feeling of heat or cold, sometimes of faintness.
Some have complained of vertigo; others of diplopia… Much more severe manifestations are observed with further lowering of the blood sugar. Marked excitement, emotional instability, sensory and motor aphasia, dysarthria, delirium, disorientation, confusion… Syncope or collapse may occur, rarely going on to a state of unconsciousness… Bradycardia has occurred… loss of sphincter control… When a single dose of insulin is given there occurs a fall in the blood sugar percentage which reaches a low point, sometimes as early as two hours, sometimes as late as twelve after the administration, and tends to return to the original level at a variable rate (fig. 3).
They observed severe reactions with a blood sugar of 60mg/dL; a blood sugar of 35mg/dL was usually accompanied by unconsciousness. The administration of glucose brought the patients out of hypoglycaemia. Different treatments were tested: glucose (5-20g), orange juice, when the patient can swallow; epinephrine (1/1000 sol., 1ml), when the patient is unconscious.
In the last paragraph of the document, the authors indicated that with the average severe diabetic, hypoglycaemia has not presented a serious problem, but special nursing precautions may well be taken if insulin is administered late in the day, to guard against the possibility of a fatal reaction occurring during sleep23.
In the University of Toronto, Best was placed in charge of Connaught's Laboratory for insulin manufacturing; Macleod and Collip to the Carnegie Corporation with a Grant to pursue experimental research and development at the Marine Biological Station in St. Andrew's, New Brunswick, conducted personally by Macleod, and at the University of Alberta by Collip. An ad hoc Committee at the University of Toronto decided that Banting would have a university appointment and facilities shared with Graham, Campbell and Fletcher at the Toronto General Hospital.
CC Sutter and JR Murlin published a case report of an adult severe diabetic subject admitted with ketosis at the Rochester General Hospital (July 1922) and treated with a pancreatic extract, able to reduce blood sugar, urine sugar, and ketone bodies, after subcutaneous administration10,24. Murlin applied for a patent on his antidiabetic pancreatic extract in July 1923 (finally granted in 1925).
In many centers in USA, prominent diabetologists were attending dying diabetic patients in their clinics. Therefore, the University of Toronto and Eli Lilly agreed that a selected group of physicians and institutions would be given the extract as soon as it became available; Macleod sent them details of the method, asking them not to divulge the procedure to those with the intention of producing the extract commercially.
Insulin manufacture was attempted by various specialists; among them Dr. Frederick M. Allen of the Rockefeller Institute of Medical Research in Morristown, New Jersey; H. Rawle Geyelin of Columbia University Presbyterian Hospital in New York; Elliot P. Joslin of Boston; Russell M. Wilder of the Mayo Clinic in Rochester, Minnesota; J.R. Williams of Rochester, New York; Roland T. Woodyatt of Chicago; William D. Sansum of the Potter Metabolic Clinic, Santa Barbara, California.
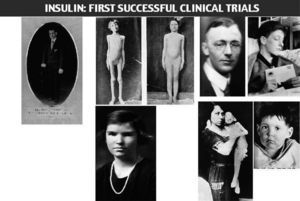
Leonard Thompson, the first patient treated with insulin at the age of 14, died at the age of 27. He worked as an assistant in a drug and chemicals factory. He was never a well-controlled patient, injecting daily an average of 85 units of insulin. In 1932 he was admitted again at Toronto General Hospital in diabetic coma. In his final illness he suffered influenza, followed by pneumonia combined with severe ketoacidosis. The autopsy revealed a small and atrophied pancreas, with only a few islets. His pancreas is displayed in the anatomical museum at the Banting Institute.
The following two photos were taken of John L, a patient of Dr. H.R.Geyelin, before and after insulin been administered.
Dr. Joseph Gilchrist, physician and patient, was a classmate of Frederick Banting.
James D. Havens, the son of an important executive of Eastman Kodak, was the first patient treated with insulin in USA. His personal physician was Dr. John R. Williams in the city of Rochester, under the supervision of Banting (Havens became a member of the National Academy of Arts; he died in 1960, due to colon cancer).
Elizabeth Hughes, daughter of Charles E. Hughes, US Secretary of State. Elizabeth. Hughes was successful in raising 3 children, all born by caesarean section. She was physically active and travelled a lot. She died suddenly after a myocardial infarction, at the age of 74, after being diabetic for 60 years.
The last 2 photos appeared in JAMA, they were taken on December 15, 1922, before initiating insulin treatment (the 3 year-old child's weight was 15 pounds (7 Kg), and on February 15, 1923, with a new weight of 29 pounds (13 Kg) (fig. 4).
Dr. Nathaniel Bowditch Potter (1869-1919) was considered one of most relevant experts in Metabolic Medicine. Initially he worked in New York, becoming Professor of Clinical Medicine at Columbia University, and Chief of the Medical Division of St. Mark's Hospital. At the age of 47, he was diagnosed with diabetes, and developed kidney disease, cardiovascular atherosclerotic disease, and pulmonary tuberculosis.
In 1917, Dr. Potter became a member of the senior staff of Cottage Hospital at Santa Barbara, CA, and the Director of a new Metabolic Clinic, integrated into the facilities of Cottage Hospital. In 1919, G. O. Knapp, founder of Union Carbide; CKG Billings, a high executive of Union Carbide; and F.F. Peabody of Cluett, Peabody & Company, made it possible to inaugurate the Potter Metabolic Wing at Cottage Hospital, equipped with the finest laboratory and clinic for research work in Medicine. Unfortunately, in June 1919, Dr. Potter's disease progressed to diabetic coma which caused his death on July 5, 1919. The Medical Director of Santa Barbara Cottage Hospital, G.O. Knapp, following the recommendation of the Chief of Staff (Dr. Franklin R. Nuzum, previously a close associate of Dr. Woodyatt, at Rush Medical College in Chicago), requested an interview with Dr. William D. Sansum to offer him the post of Director of the Potter Metabolic Wing.
William David Sansum was born on September 25, 1880 in Baraboo, Wisconsin. He was the third child in a family of four boys and four girls. His parents were English, who emigrated to USA. Sansum enrolled in the University of Wisconsin in 1910, where he took his Bachelor of Science degree in 1912. At the age of 32, was admitted to the Rush Medical College in Chicago. At the age of 35, Sansum received the degree of medical doctor. His doctoral thesis was entitled «Studies on the Theory of Diabetes Mellitus». He then became an intern at the Presbyterian Hospital in Chicago under Dr. Roland T. Woodyatt, specialist in metabolic research. He continued as junior and then senior staff at Rush, being very active clinically and academically.
On September 25, 1920, at the age of 40, William D. Sansum, received a letter from George Own Knapp, Santa Barbara, California, the multimillionaire president and founder of the Union Carbide Company, offering him, in his capacity of member of the Board of the Santa Barbara Cottage Hospital, to become the director of the Potter Metabolic Clinic, equipped with outstanding research facilities. Sansum accepted.
Dr. Norman R. Blatherwick was in charge of the laboratory; Loyal C. Maxwell was the Laboratory Technician. Sansum, Blatherwick and Maxwell decided to manufacture insulin from beef, hog, and sheep pancreas, starting on April 1922, using Banting and Best's techniques. Dr. Sansum would get help from R.N. Gehl, a leading meat seller who provided the Potter Metabolic Unit with beef pancreas free of charge. The scientist at the laboratory proceeded with an alcoholic extract, followed by filtering and concentration of the aqueous phase by vacuum distillation at low temperature; the concentrated solution was chilled, and fatty material filtered out; the fíltrate was then treated with sodium chloride, and insulin precipitated; then, the pH was adjusted to 2.5, and a new precipitation generated with alcohol; after another alcohol wash, the extract was finally dissolved in distilled water. The potency of the extract was estimated by comparison with a standard sample provided by Toronto University. The early yields contained no more than 500 units of insulin per pound of pancreas tissue.
Dr. Sansum had under his care 7 diabetic patients beyond hope of survival. The second patient to receive insulin in USA was Charles E. Cowan, a 51 year old patient from Anaheim with severe diabetes developed in 1918, following a bad case of Spanish influenza. His physician was Dr. William David Sansum of the Potter Metabolic Clinic in Santa Barbara, California. Cowan was injected on May 31, 1922, with three cubic centimetres of a potent pancreatic extract, developed by the Potter group («Santa Barbara insulin»), just ten days after the Canadian-made insulin was administered to James Haven at the office of his physician, Dr. John R. Williams in Rochester. Cowan had been already hospitalized in Santa Barbara for 6 months, treated with an 850-calorie diet; his weight was 95 pounds (43 Kg). On the third day after the first dose, glycosuria became negative. One year after the first injection, Cowan's weight was 125 pounds (57 Kg), and he maintained a diet with 2,400 calories. Cowan continued on insulin for the remaining 39 years of his life. For many years, his insulin treatment was kept steady at 40 units of U-80 NPH insulin before breakfast, and, occasionally, 5 units of regular insulin before one of the meals. He was 90 years old when he died at Anaheim on February 10, 1958; he did not suffer retinal disease, kidney failure, neuropathy, hypertension or ischemic foot.
The first case of juvenile-onset diabetes, treated by Dr. Sansum, was Carl Klass, a boy from San Dimas, who became sugar free in collected urine within 24 hours after the first «Santa Barbara insulin» administration. Then insulin was maintained associated to a normal diet. The boy gained weight at the rate of half a pound (250g) per day in the first weeks, looking like a normal happy child in two months.
Dr. Sansum submitted a report to Prof. Macleod in Toronto, with the details of the first 20 diabetic subjects treated in Santa Barbara. Macleod replied advancing the last findings in his lab regarding improvements in the technical production of insulin. When Santa Barbara Hospital Superintendent asked Sansum's permission to bill patients for insulin, his answer was «Never-so long as it is classified as an experimental drug». In fact, Sansum paid over $1,400 from his own pocket to pay for free insulin administered to his patients. Additional equipment was acquired for manufacturing of insulin, including copper distillation stills and a large press.
Dr. Sansum was also a pioneer in the field health education for diabetic patients. Starting in 1922, a program of diabetes education was implemented at Potter Metabolic Unit, and a handbook was provided to instructed patients, along with a hypodermic syringe and a supply of insulin at home for some time.
After visiting Santa Barbara in the summer of 1922, Dr. Henry S. Pritchett, President of the Carnegie Corporation convinced the members of the Executive Board of the Company to provide a cash gift of $15,000 to the Potter Metabolic Unit for future developments. The improved efficiency allowed unlimited supply of insulin, for the local needs, and to reduce the daily cost of insulin per patient in Santa Barbara from $100 to less than $20, before the end of 1923.
The Carnegie Corporation support helped to increase the staff members working at the laboratory. The Potter Clinic became overloaded with diabetic men, women and children to be admitted and receive insulin treatment. Dr. Sansum felt obliged to accept only cases when diabetes coma appeared imminent. The insulin manufacture at Potter laboratory operated day and night, including Sunday. On March 1, 1923, the Potter Metabolic Clinic announced that although the fundamental principle of Dr. Sansum was that no one would ever be turned away for lack of money to pay for treatment, the Clinic was not in the situation to continue supplying free insulin, with the exception of the needy people. The annual budget of the Clinic was $40,000 but the expenses were $60,000. Therefore, from now on the Clinic would charge for its experimental insulin at the rate of 3 cents per unit. At that time, Lilly insulin cost was 5 cents a unit. By the end of 1923, insulin production at Potter's research lab was offering unlimited supply. The cost of manufacturing had been drastically reduced, and the quality of insulin had also improved25 (fig. 5).
Frederick M. Allen and Elliot P. Joslin: First insulin treated patientsThe earliest large clinical trials with insulin in USA were carried out by Joslin, Allen, and Woodyatt26–29.
Frederick Madison Allen (1874-1964) was born in Iowa. Studied Medicine in California. Later, he worked at Harvard University and the Rockefeller Institute. On April, 1921, he opened the Physiatric Institute in Morristown, New Jersey. Along with Elliot Joslin, he was one of the leading diabetologists. Before insulin, Allen became famous through his diet treatment of diabetes, consisting of a drastic reduction of calories, particularly, carbohydrate intake (as low as 8% of total calories). This treatment reduced the incidence of glycosuria and prolonged life for a few years.
Allen visited Toronto before starting to use insulin in Morristown. In August 1922, Allen administered insulin to six severe patients. On August 16, he wrote to Banting expressing his enthusiasm after observing the disappearance of hyperglycaemia, glycosuria and ketonuria, and improved well-being. One of his desperate cases was Elizabeth Hughes. Allen decided, following the wishes of her family, to bring Elizabeth (15 years old) to Toronto. At the end of her first 2 weeks of insulin therapy, Elizabeth was following a normal diet of 2,200 calories; five weeks after arriving in Toronto, Elizabeth had gained over 10 pounds (4.5 Kg); a few months later she had also increased her height.
Allen gave insulin to 161 diabetic patients in the first year of clinical trials.
In addition to Allen and Joslin, the leading group of clinicians involved in early clinical trials with insulin in USA included Dr. H. Rawle Geyelin of New York City; John R Williams from Rochester, New York, who treated the first patient in USA with insulin, Jim Havens; Dr. Russell Wilder from the Mayo Clinic, and Dr. Rollin T. Woodyatt, who worked at the Presbyterian Hospital in Chicago.
Dr. Elliot Proctor Joslin (1869-1962) was born in Oxford, Massachusetts, on the sixth of June, 1869. His father, Allen, was a partner in the Joslin Shoe Factory, and his mother, Sarah Proctor, was related to the founders of the corporate giant Proctor and Gamble. He attended Yale College, and finally Boston in the Medical School at Harvard. Joslin became interested in diabetes while attending Yale, when his aunt developed the disease.
Joslin embarked for the great medical clinics of Germany and Austria, from Freiburg to Vienna and back to Berlin. Upon returning home in the summer of 1897, he served as an intern at the Massachusetts General Hospital for a year, and joined the staff of the Boston City Hospital and the recently founded New England Deaconess Hospital (NEDH), and worked part-time in the Boston Dispensary. He was a man imbued with Protestant morals and socially progressive ideas. Joslin began his private practice in Boston in 1898, at the age of 29 (fig. 6). In 1899 his 60 year old mother also became his patient; she died in 1913, at the age of 74.
In 1908, cooperating with physiologist Francis G. Benedict, Joslin intensively investigated the effect of carbohydrate and calorie restricted diets in diabetic patients admitted to the New England Deaconess Hospital. His monograph The treatment of Diabetes Mellitus (1916) included the main findings from 1,000 cases; Joslin observed a 20% reduction in mortality with the combination of diabetes education, diet and exercise. In 1918 he published the first edition of Diabetic Manual- for the Doctor and Patient, the pioneering patient handbook; there have been 14 editions; its current version is published by the Joslin Diabetes Center as The Joslin Guide to Diabetes.
Elizabeth Mudge was the first insulin-treated patient by Elliott P. Joslin (fig. 7). She received the first injection on August 7, 1922. Elizabeth was a nurse and her diabetes was diagnosed in July 1917. Under Joslin's care, she lost weight, so that by August 1922 she weighed 71 pounds (32 Kg). Elliot P. Joslin received the first insulin supply on August 6, 1922. He felt too nervous to make the first injection himself; therefore, he asked his associate Dr. Howard Root to administer the first dose to Miss Mudge, a 42 year-old former nurse, and now an invalid. Six weeks after receiving insulin daily, she was able to walk four miles a day. A similar experience was the case of Dorothy Z., a 5-year old girl who could not climb stairs before insulin treatment; and also the case of Annie N, a Finnish child who after two days of treatment played and would not stay in bed.
Joslin realized that insulin meant the end of an era in diabetes history, but, unfortunately, not the end of diabetes30. Joslin created the Diabetes Teaching Unit, where he started the whole educational aspect of diabetes. He was the first physician to have diabetes teaching nurses to visit, treat and educate diabetic children at their homes and to develop special care for diabetic children at summer camps (fig. 8).
First European cases of insulin treatmentRomania (N.C. Paulescu)In Bucharest, Paulescu intensively engaged himself in purifying the pancreatic extract; in addition, he carried out limited clinical experiments. Under the successive influence of hydrochloric acid and caustic soda, he obtained a clear aqueous pancreatic extract, with a lower protein content. He also observed that 96% ethanol partly precipitated the proteins of the extract; he then evaporated the alcohol of the extract at low temperature over 50ºC and destroyed the active ingredient of the extract, which later reconstituted with physiological saline21. He was afraid of using the parenteral delivery of the pancreatic extract in diabetic subjects, because of previously observed side effects; therefore, he used (without success) the administration of the pancreatic extract by an enema in two clinical observations (one case of slim diabetes in a 43-yearold man, on February 25, 1922, and several times afterwards, and another case of fat diabetes in a 52-year-old woman, on March 3)32.The observed results led him to the following conclusions13:
The introduction into the organism of a diabetic patient of a pancreatic extract, rendered free from proteins using acids and alkalis, using the intravenous route, must certainly be very efficient in attenuating or temporary suppressing metabolic emergencies. But this administration determines the appearance of bouts of fever, which make it inapplicable in medical practice. The introduction of this extract using the subcutaneous route, must also be efficient. But it produces fever and sometimes abscesses…
The introduction of an aqueous pancreatic extract via the oral or intestinal (enemas) route is not efficacious. However, this kind of treatment, repeated for many times, appears to produce in time, a significant fall in the level of urinary glucose.
In his book The Discovery of Insulin, Michael Bliss pointed out that, with the exception of the pioneer experience of Georg. Zuelzer, the first European to use insulin was Dr. R. Carrasco-Formiguera, who spent one year at the Department of Physiology, directed by Professor B. Cannon, in Harvard (1921-1922). He was present in New Haven when Banting made his first presentation on pancreatic extracts33.
In September 1922, Carrasco-Formiguera and his associate, Dr. Pere González, were able to produce an impure pancreatic extract containing insulin in the Municipal Laboratory of Barcelona. They used dogs and rabbits as experimental animals34.
On October 3, 1922, Carrasco (fig. 9) administered 10ml. of the extract to the patient Francesc Pons. Although the results of this experiment were positive, the patient died when no additional extract was available. Finally, Carrasco was able to treat other patients when he undertook to supervise the manufacture and distribution of insulin in Spain35,36. No other Europeans were in the condition to use insulin until 1923. In a document published in 1924, Carrasco explained that at the end of 1923 he had already treated 80 diabetic patients with insulin36; in 22 of them, surgical complications were associated (fig. 10).
EnglandIn June 1922, JJR Macleod asked the Medical Research Council (MRC) of England to accept the donation, from the University of Toronto, of the complete rights to the patent of pancreatic extracts33. The MRC appeared sceptical about the miracle of insulin. Drs. Henry H. Dale and Harold Dudley visited Toronto in September 2002, as well as Eli Lilly headquarters in Indianapolis and several American Hospitals. After their return, they reported to the MRC with high enthusiasm and recommended to accept the control of the British patent. Clinical trials started in England in the month of December, 1922. Approximately 50 diabetic patients received insulin in Britain in the winter of 1922-2333. One of the first people in England to receive insulin was the Treasurer of the Royal College of Physicians of Edinburgh, Norman Purvis Walker (1862-1942), who would become President of both this College and the General Medical Council. In April, 1932, the supplies of Lilly and Burroughs Wellcome made insulin available to most insulin-treated patients in England.
ScandinaviaTorsten Deckert wrote that insulin came to Denmark thanks to Birthe Marie Krogh (1874-1943), the wife of August Krogh. She was a physician, and became a Doctor of Medical Sciences in 191438.
Schack August Steenberg Krogh received the Nobel Prize in 1920. He was invited to lecture at the Mayo Clinic, Johns Hopkins, and several other universities in USA. Marie developed diabetes, probably diagnosed in 1921. Dr. Hans Christian Hagedorn accepted her care. He prescribed a starvation diet, and glycosuria disappeared. In September 1922, August and Marie Krogh went to USA, arriving in Boston. Elliott P. Joslin told them he was conducting clinical experiments with an insulin preparation provided by the University of Toronto. Marie and August were guests at JJR Macleod's home on November 23-25, 1922. With the assistance of Macleod, Krogh was able to negotiate an agreement with the Governors of the University of Toronto to produce and use insulin in Scandinavia. Immediately, after returning to Copenhagen, Krogh and Hagedorn decided to work as a team. Hagedorn would produce the pancreatic extracts from pancreas of cattle and fish in his private laboratory; Krogh would measure the biological activity of the extracts in the Laboratory of Zoo physiology. On December 21, they achieved an active preparation from ox pancreas, successfully tested in rabbits. They also developed a new standardisation method for insulin, using mice as experimental animals. Before the end of 1922 Krogh and Hagedorn reached an agreement for manufacturing insulin in Denmark with August Kongsted, the owner of Lovens Kemisk Fabrik38. Kongsted decided to be responsible for the expenses related to the experiments, production and distribution; once an insulin market was created, he would claim to cover all expenses but would not ask for any profit from sales to the Scandinavian countries. One condition was the Loven trademark (lion, Leo in Latin) to be displayed on the ampoules. Hagedorn and Krogh carried out the experimental work without payment. Therefore, at the end of 1923, the price of 100 units of insulin in the USA was 4 kroner; 3.5 in England, and 2.5 in Denmark38.
A significant advance was the achievement by George Walden, Eli Lilly's first Chemist, that the precipitation of insulin at its isoelectric point yielded a method of purification by isoelectric fractionation which allowed largescale production of insulin. Therefore, early in 1923 the supply of insulin was adequate to meet the requirements of many institutions selected for its clinical use39. The purity of insulin was increased, and the duration of insulin solutions greatly improved, these advances were immediately incorporated by the Danish investigators.
In the morning on March 13, 1923, the first subcutaneous injection of insulin was given to a diabetic patient admitted with diabetic coma in the Municipal Hospital of Copenhagen, under the responsibility of the Consultant, Dr. Sophus Bang. After administering several doses, the blood sugar fell from 740mg/dL to 390, the Kussmaul respiration stopped and the consciousness recovered. Nevertheless, the patient developed heart failure in the afternoon and died during the night. In the month of April, seven severe patients were successfully put on insulin. Hagedorn presented a report on these clinical experiences to the Danish Society for Internal Medicine on June 23, and to the 11th Nordic Congress for Internal Medicine in July. In May 1923, Krogh, Hagedorn and Kongsted created the Nordisk Insulinlaboratorium, ready to make insulin available to Scandinavian Centers, with no more limitations on supplies38.
Germany and FranceIn Germany, the effects of the war, and the uncontrolled inflation delayed the manufacturing of insulin until 1924. In France, the situation was equally inadequate in 1922 and 1923.
The authors thank Dr. Lois Jovanovic, Chief Officer, Sansum Research Diabetes Institute (Santa Barbara, CA, USA), for her kind help, facilitating reports, written documents and pictures from her own research files.